Abstract
Signal transducers and activators of transcription 3 (STAT3) are activated by various cytokines and oncogenes; however, the activity and pathogenesis of STAT3 in diffuse large B cell lymphoma of the central nervous system have not been thoroughly elucidated. We investigated the phosphorylation levels of STAT3 in 40 specimens of primary central nervous system diffuse large B-cell lymphoma (PCNS DLBCL) and analyzed the association between phsopho-STAT3 (pSTAT3) expression and cerebrospinal fluid (CSF) concentration of interleukin-10 (IL-10) or IL-6. Immunohistochemistry and Western blot analysis revealed that most of the specimens in PCNS DLBCL expressed pSTST3 protein, and a strong phosphorylation levels of STAT3 was statistically associated with high CSF IL-10 levels, but not with CSF IL-6 levels. Next, we demonstrated that recombinant IL-10 and CSF containing IL-10 induced the phosphorylation of STAT3 in PCNS DLBCL cells. Furthermore, molecular subtype classified by Hans’ algorithm was correlated with pSTAT3 expression levels and CSF IL-10 levels. These results suggest that the STAT3 activity is correlated with CSF IL-10 level, which is a useful marker for STAT3 activity in PCNS DLBCLs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary central nervous system diffuse large B-cell lymphoma (PCNS DLBCL) is an aggressive extra-nodal non-Hodgkin lymphoma (NHL) that involves the brain, spinal cord, and eyes. PCNS DLBCL occurs in both immunocompetent and immunocompromised patients, as well as in approximately 4 % of all brain tumors [1]. High-dose methotrexate-based treatment has elicited good responses in PCNS DLBCL patients, and combined modality therapy has produced response rates of up to 80–90 % and a median overall survival of up to 4 years; however, PCNS DLBCL has a poor prognosis compared to other extranodal NHLs [1].
Several cytokines such as interleukin-10 (IL-10), IL-6, and interferons activate signal transducers and activators of transcription (STAT) proteins [2]. After a cytokine is engaged with its receptor, members of the Janus kinases (JAKs) family are phosphorylated, leading to the recruitment and phosphorylation of signal transducers and activators of transcription (STAT) proteins on tyrosine residues. Phosphorylated STAT proteins then dimerize, translocate to the nucleus, and trigger the transcription of the target genes involved in cell proliferation, survival, angiogenesis, and immunity [2]. Of all STAT-related proteins, STAT3 is most frequently activated in tumors. The most common mechanism causing abnormal STAT3 activation in malignant cells is dysregulated cytokine signaling [3]. The persistent activation of the STAT3 pathway has been identified in a variety of both solid and hematologic malignancies, such as colon cancer [4, 5], lung cancer [6], prostate cancer [7], and leukemia [8, 9]. In addition, nasal NK-cell lymphomas contain high levels of total and phosphorylated STAT3 proteins [10]. A subset of systemic DLBCLs has been reported to have high STAT3 activity [11], but STAT3 activity in PCNS DLBCLs has not been analyzed sufficiently.
It is well known that IL-10 and IL-6 are key activators of STAT3 [2]. We previously reported that the CSF level of IL-10 and IL-6 was significantly increased in PCNSL patients compared with patients suffering from other brain tumors [12]. However, the levels of CSF IL-10 and IL-6 were quite different among the patients with PCNS DLBCL. In the present study, we examined the STAT3 activation in the tumor of PCNS DLBCLs and analyzed the association of STAT3 activation with CSF IL-10 or IL-6 levels in 40 patients with PCNS DLBCLs. Furthermore, we analyzed the association of STAT3 activation with molecular subtype and prognosis in patients with PCNS DLBCLs.
Materials and methods
Cell culture and measurement of IL-10
HKBML, a human brain malignant lymphoma cell line, was purchased from RIKEN BioResource Center (Tsukuba, Japan) and maintained in culture in Ham’s F-12 (Nakalai tesque, Kyoto, Japan) supplemented with 10 % heat-inactivated fetal bovine serum (FBS).
Antibodies and reagents
Antibodies to STAT3 and pSTAT3 (tyrosine 705) were purchased from Cell Signaling Technologies (Danvers, MA, USA). Additionally, the following antibodies were used: IL-10 (Abcam, Cambridge, MA, USA), IL-10 for neutralization (BD Biosciences, San Jose, CA, USA), IL-10 Receptor-A (Millipore, Billerica, MA, USA), CD10 (56C6; AbD Serotec, Oxford, UK), Bcl-6 (P1F6; Leica microsystems, Wetzlar, Germany), MUM1, CD20, and CD3 (Dako Cytomation, Glostrup, Denmark). The recombinant human IL-10 (rIL-10) was obtained from R&D Systems (Minneapolis, MN, USA). IL-10RA siRNA was obtained from Santa Cruz Biotechnology (Dallas, TX, USA).
Patients and clinical specimens
The patients with PCNS DLBCL were treated at the Department of Neurosurgery, Kobe university hospital, between January 2004 and December 2013. All patients were histologically newly diagnosed with PCNS DLBCL. Patients with HIV-associated lymphoma were excluded from this study. Furthermore, patients who were treated with steroids prior to surgery were excluded from this study. For the pre-operative diagnosis, 2–6 ml of CSF was withdrawn from the patients via lumbar puncture after obtaining informed consent when not contraindicated. The CSF samples were immediately centrifuged and stored at −70 °C. The CSF concentrations of IL-10 were measured using a human enzyme-linked immunosorbent assay. The IL-6 levels were measured using a human chemiluminescent enzyme immunoassay. This study was approved by the ethical review board of our institutions (Nos. 1111 and 1312).
Immunocytochemistry
Archived paraffin blocks of PCNSL patients from the Department of Pathology of our hospital were used. The serial sections were deparaffinized, immersed in methanol with 3 % hydrogen peroxide, and heated in 0.01 M citrate buffer (pH 6.0) for 15 min by autoclaving (121 °C, 2 atm.). The sections were then incubated with primary antibodies at 4 °C overnight. The sections were allowed to react with either a peroxidase-conjugated anti-rabbit or anti-mouse IgG polyclonal antibody (Histofine Simple Stain MAX-PO; Nichirei, Tokyo, Japan) for 60 min, and the reaction products were visualized by immersing the sections in 0.03 % diaminobenzidine solution containing 2 mM hydrogen peroxide for 5 min.
Assessment of staining
Imunohistochemical staining was evaluated and scored by the neurooncologist (K.T) and reviewed by the pathologist (T.H.) without knowledge of clinical data and patients’ outcome. The pSTAT3 expression was semi-quantitatively scored using the following system: a score of 1–6 represented the sum of the staining intensity (1 = negative/weak, 2 = moderate, and 3 = strong) (Fig. 1B) and the percentage of positively stained cells (0 = 0 %, 1 = 1 ~ 30 %, 2 = 31 ~ 60 %, and 3 = 61–100 %). At least 500 cells per specimens were counted and moderate to strong immunoreactivity were considered positive. The cell counting method for immunohistochemistry (pSTAT3) has advantages over manual cell counting by microscopy. The slides were randomly selected and photographed using color films, and the photographs were then enlarged to adequate size for observation. The total cells and cells with positive staining were counted, and the percentage of positive cells in each high power field was calculated.
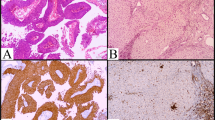
A Immunohistochemistry of pSTAT3, and STAT in 3 cases with PCNS DLBCL. Clinical data were shown in Table 1. (Original magnification: × 200). B Staining intensity of pSTAT3. Left panel negative or weak stain, middle panel moderate stain, right panel strong stain (original magnification: × 400). C Comparison of CSF IL-10 (left panel) or CSF IL-6 (right panel) levels in each pSTAT3 scoring group. Staining scores were calculated based on both the staining intensity and number of positive cells (“Materials and methods” section). Highest pSTAT3 score group (score 6) has significantly higher CSF IL-10 levels. D Comparison of staining score of pSTAT3 between higher CSF IL-10 group (>60 pg/ml, n = 18) and lower CSF IL-10 group (<60 pg/ml, n = 22). The pSTAT3 staining score was statistically higher in the higher CSF IL-10 group than in the lower CSF IL-10 group. (# p = 0.010), E (a) STAT3 and pSTAT3 expression in 17 frozen samples of PCNS DLBCL we obtained. The CSF IL-10 concentrations in each case are displayed above the panels. Lower graphs comparison of the relative value of the pSTAT3 and STAT3 expression between the higher CSF IL-10 group (>60 pg/ml, n = 7) and the lower CSF IL-10 group (<60 pg/ml, n = 10). The relative values of pSTAT3 were obtained using densitometric evaluation of the immunoblots normalized to β-actin. The pSTAT3 expression was statistically higher in the higher CSF IL-10 group than in the lower CSF IL-10 group. (# p = 0.040)
siRNA experiment
Small interfering RNAs (siRNA) were transfected into HKBML cells using Lipofectamine RNAiMAX (Invitrogen), IL-10RA siRNAs (sc-35646, Santa-Cruz Biotechnology, Dallas, Texas, USA) and control siRNAs. siRNA transfection was performed according to the manufacturer’s instructions.
Neutralizing assay
IL-10 antibody (BD Biosciences, San Jose, CA, USA) was diluted with PBS and added into a solution of rIL-10 or CSF for 1 h at 4 °C. The solution containing IL-10 antibody was then added to serum-starved HKBML cells and incubated for 1 or 6 h at 37 °C. Subsequently, the cells were harvested and analyses were performed.
Western blot analysis
The cells or tissue were lysed and homogenized using Lysis buffer AM1 (Active Motif). Equal amounts of protein extracts were separated by electrophoresis on a 4–12 % NuPAGE Bis–Tris Mini Gel (Invitrogen) and then transferred to a nitrocellulose membrane (GE Healthcare) using the XCell II Blot Module (Invitrogen). The membrane was blocked for 1 h in Tris-buffered saline containing 0.1 % Tween 20 and 5 % bovine serum albumin and then probed with various primary antibodies, followed by secondary antibodies conjugated to horseradish peroxidase (HRP). The immunoblots were developed as recommended in the ECL Advance Western blot detection kit (Amersham Biosciences).
Real-time RT-PCR for IL-10
Reverse transcription was performed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s instructions. The expression levels of IL-10 mRNA were quantitatively assayed by real-time RT-PCR using TaqMan® Gene Expression assays according to the manufacturer’s instructions (Applied Biosystems). The 18S ribosomal RNA transcript was used as the endogenous control. The quantitative mRNA expression data were acquired and analyzed via the ΔΔ−Ct method using an Applied Biosystems 7500 real-time PCR system (Applied Biosystems).
In vivo study
The animal study was conducted according to a protocol approved by the Institutional Animal Care and Use Committees. Male 5-week-old nude mice (BALB-c nu/nu) (Clea Japan, Inc., Osaka, Japan) were housed within an approved specific pathogen-free barrier facility maintained at the Kobe University in accordance with Laboratory Animal Resources Commission standards. HKBML PCNSL cells were concentrated to 5 × 106 per 200 μl and injected s.c. into the right flank of each mouse. The nude mice were classified into the following three treatment groups according to tumor size approximately 14 days after the implantation: (a) control group (b) rIL-10 of 0.1 μg group (c) rIL-10 of 1 μg group. Two-hundred microliters of rIL-10 reagent were injected into the center of the tumor, and the tumors were removed 2 h after the injections and analyzed by Western blot analysis or immunohistochemistry.
Statistical analysis
A Mann–Whitney U test was used to analyze the differences between two groups. Survival was estimated using the Cox’s proportional hazards model and Kaplan–Meier method, and the significance was determined using the log-rank test. p < 0.05 was considered to be statistically significant. The statistical analysis was performed using the JMP® 11 package (SAS Institute Japan, Tokyo, Japan).
Results
The expression of STAT3 and phospho-STAT3 protein in PCNS DLBCLs
To examine the expression of STAT3 and phospho-STAT3 (pSTAT3: tyrosine 705) in PCNS DLBSLs, we performed immunohistochemical analyses. Most of the specimens have strong expression of STAT3; however, pSTAT3 expression levels were various among the specimens (Fig. 1A). We scored the phosphorylation levels of STAT3 according to the positive cell rate and staining intensity (“Materials and methods” section). The mean score was 4.3, the maximum score was 6 (13 patients) and the minimum score was 2 (9 patients) (Table 1).
pSTAT3 expression levels are associated with CSF IL-10 levels, but not with CSF IL-6
We analyzed the association between pSTAT3 staining score and CSF IL-10 and IL-6 levels. The highest pSTAT3 staining score group (score 6) had significantly higher CSF IL-10 level than the lowest score group (score 2) (Fig. 1C, left). However, there was no significant difference in CSF IL-6 levels (Fig. 1C, right). Additionally, the higher CSF IL-10 group had a statistically higher pSTAT3 staining score than the lower CSF IL-10 group (p = 0.010) (Fig. 1D left). In contrast, the CSF IL-6 levels did not associate with the pSTAT3 staining score (Fig. 1D, right). Next, we analyzed the association between the CSF IL-10 levels and pSTAT3 expression in a Western blot using 17 frozen samples of the patients (Fig. 1E). Although the total STAT3 protein expression levels did not differ between the higher CSF IL-10 group and the lower CSF IL-10 group, the pSTAT3 expression levels were significantly increased in the higher CSF IL-10 group compared with that in the lower CSF IL-10 group (Fig. 1E, lower graphs). These results suggest that STAT3 activity may be associated with CSF IL-10 level in PCNS DLBCLs.
IL-10 phosphorylates STAT3 in PCNS DLBCL cells
To examine whether IL-10 activates STAT3 in PCNSL cells, HKBML cells and primary cultured PCNS DLBCL cells were treated with recombinant IL-10 (rIL-10), and a Western blot was performed. As shown in Fig. 2A, rIL-10 stimulated the phosphorylation of STAT3 at the tyrosine 705 residue (Fig. 2A). rIL-10 also phosphorylated STAT3 in a dose-dependent manner (Fig. 2B). rIL-10 increased the IL-10 mRNA level in HKBML cells, which is known to be one of the target genes of STAT3 (Fig. 2C). Next, we examined whether IL-10 could phosphorylate STAT3 in a mouse model. The inoculated HKBML tumors were positive for CD20, MUM1, IL-10 and IL-10 receptor-A, and negative for CD3, CD10 and Bcl-6 (Supplementary Fig. 1). After injection of rIL-10 into tumors, the STAT3 phosphorylation was increased compared to the control (Fig. 2D, E).
A Serum-starved PCNSL cells were treated with 50 ng/ml of recombinant IL-10 (rIL-10) for 15 and 30 min, and the phosphorylation of STAT3 was assessed by Western blot analysis. rIL-10 stimulates the phosphorylation of STAT3 in all PCNSL cells. PCNSL-1 and PCNSL-2 are primary cultured PCNS DLBCL cells from the patients. B Serum-starved HKBML cells were treated with the indicated dose of rIL-10 for 30 min, and the phosphorylation of STAT3 was assessed by Western blot analysis. C Serum-starved HKBML cells were treated with the indicated dose of rIL-10 for 12 h, and the relative mRNA level of IL-10 was assessed by real-time PCR via the ΔΔ−CT method.(Bar mean value, # p < 0.01), D STAT3 and pSTAT3 protein expression in the tumors injected with rIL-10 in mouse model. HKBML PCNSL cells (5 × 106/200 μl) were inoculated into the right flank of nude mice (BALB-c nu/nu). Two weeks later, 200 μl of rIL-10 reagent (0.1 or 1 μg/tumor) were injected into the center of the tumor. The tumors were then removed 2 h after the injection, and the expression levels of pSTAT3 and STAT3 were analyzed using a Western blot analysis. The expression of pSTAT3 was higher in rIL-10-injected tumors than in the control tumors. (control mice (n = 2), 0.1 μg of rIL-10 injected mice (n = 2), 1 μg of rIL-10 injected mice (n = 4)), E pSTAT3, and STAT3 expression in tumor injected with rIL-10 in mouse model. HKBML PCNSL cells were inoculated into the right flank of nude mice, and 2 weeks after the implantation, 200 μl of rIL-10 reagent (1 μg/tumor) were injected into the center of the tumor. Two hours after the injections, the tumors were removed, fixed, and analyzed by immunohistochemistry. (original magnification ×400)
We performed a siRNA experiment to ascertain whether the induction of IL-10 was responsible for the phosphorylation of STAT3 in HKBML cells. The knockdown of the IL-10 receptor-A (IL-10 RA) could inhibit STAT3 phosphorylation (Fig. 3A). In addition, neutralizing anti-IL-10 antibody decreased the IL-10-induced phosphorylation of STAT3 in a dose-dependent manner (Fig. 3B), and neutralizing anti-IL-10 antibody inhibited the CSF IL-10-induced phosphorylation of STAT3 (Fig. 3C). Interestingly, higher levels of CSF IL-10 could induce a stronger phosphorylation of STAT3 (Fig. 3D). The mRNA level of IL-10, which is one of the STAT3 targets, decreased in response to treatment with a neutralizing antibody to IL-10 (Fig. 3E). These results suggest that CSF IL-10 phosphorylates and activates the STAT3 pathway in PCNS DLBCL cells.
A Knockdown of IL-10 receptor-A (IL-10RA) by siRNA decreases the expression of pSTAT3. Normal cultured HKBML cells were transfected with IL-10RA siRNA or control siRNA for 72 h, and the expression levels were analyzed by Western blot analysis. B Serum-starved HKBML cells were treated with rIL-10 and/or neutralizing IL-10 antibody (IL-10Ab, 5 or 25 μg/ml), and the STAT3 and pSTAT3 expression levels were analyzed. C Serum-starved HKBML cells were treated with CSF from a patient containing 1.4 ng/ml of IL-10 with/without neutralizing IL-10Ab (25 μg/ml), and the pSTAT3 and STAT3 expression was analyzed. D Serum-starved HKBML cells were treated with CSF from a patient containing 1340 or 7 pg/ml of IL-10 with/without neutralizing IL-10Ab, and the pSTAT3 expression was analyzed. Notably, CSF from a patient containing higher levels of IL-10 induced a higher expression of pSTAT3. E Serum-starved HKBML cells were treated with rIL-10 with/without neutralizing IL-10Ab (5 or 25 μg/ml) for 6 h, and the IL-10 mRNA expression was analyzed by real-time PCR. The level of IL-10 mRNA, which is one of the targets of STAT3, increased in response to rIL-10 and was inhibited by the neutralizing IL-10Ab. (Bar mean value, # p < 0.01)
High STAT3 activity is associated with non–GCB type of PCNS DLBCL
Because Gupta et al. have reported that there was a clear trend toward higher pSTAT3 positivity in non-GCB DLBCL tumors [13], we examined the association between pSTAT3 staining score and DLBCL subtype. The subtype was determined by the Hans’ algorithm according to the previous reports [14]. As shown in Fig. 4A, pSTAT3 staining score of the non-GCB type was significantly higher than that of the GCB type (p = 0.021) (Fig. 4A). In addition, the CSF IL-10 levels, but not the CSF IL-6 levels were significantly higher in the non-GCB type than in the GCB type (p = 0.02) (Fig. 4B). These results indicate that non-GCB tumor is associated with high activity of STAT3 and high CSF IL-10 levels in PCNS DLBCLs.
A A comparison of the pSTAT3 staining scores between GCB (n = 10) and non-GCB (n = 30) types of PCNS DLBCLs. The staining score of pSTAT3 was significantly higher in the non-GCB type. (# p = 0.021). B A comparison of the CSF IL-10 (left panel) and IL-6 (right panel) levels between the GCB and non-GCB types of PCNS DLBCLs. The CSF IL-10 level was significantly increased in the non-GCB type compared with that in the GCB type. (# p = 0.020). C Comparison of Kaplan–Meier curves according to pSTAT3 staining score, CSF IL-10 levels and CSF IL-6 levels. a, b Comparison of Kaplan–Meier PFS and OS curves according to the pSTAT3 staining scores (low group (score 2–4, n = 17) vs. high group (score 5–6, n = 20)). There were no significant differences. c, d Comparison of Kaplan–Meier PFS and OS curves according to CSF IL-10 levels (low group (<60 pg/mL, n = 19) vs. High group (>60 pg/mL, n = 18)). The PFS of the patients with higher CSF IL-10 was significantly shorter. (HR 2.52; 95 % CI 1.039–6.130; log-rank, p = 0.032). e, f Comparison of Kaplan–Meier PFS and OS curves according to CSF IL-6 levels (low group (<7 pg/mL, n = 22) vs. high group (>7 pg/mL, n = 15)). There were no significant differences
Elevated CSF IL-10 were significantly associated with poorer prognosis
Thirty-seven of the 40 patients received chemo-radiotherapy (Table 1). Of these, 20 patients survived and 17 patients died. The median survival time of all these patients was 40 months, and the median time to progression was 30 months. We analyzed the association between the pSTAT3 expression levels and PFS or OS, but there was no statistically significant relationship between these indicators (Fig. 4C-a, b). In contrast, the elevated CSF IL-10 was significantly associated with shorter PFS [hazard ratio (HR) 2.52; 95 % confidence interval (CI) 1.039–6.130; log-rank, p = 0.032] (Fig. 4C-c). In addition, there was a trend towards shorter OS in patients with higher CSF IL-10 (Fig. 4C-d). However, CSF IL-6 level was not associated with OS and PFS (Fig. 4C-e, f).
Discussion
Many reports have investigated the expression of STAT3 or pSTAT3 in cancer [5]; however, only two reports focused on PCNSLs. Vajpayee et al. retrospectively examined the expression of pSTAT3 in 17 patients with PCNSL, and all of the cases were negative for pSTAT3 [15]. In contrast, Komohara et al. have shown that most of the PCNSLs expressed pSTAT3, and the phosphorylation levels of STAT3 were significantly higher in patients with a high infiltration of CD163-positive tumor associated macrophages [16]. Interestingly, they have also shown that IL-10 production increased in macrophages cultured in a supernatant of malignant lymphoma cells compared with a control medium [16]. In the present study, most of the tumors of PCNS DLBCL were positive for STAT3 and pSTAT3. However, the pSTAT3 stain levels varied among the samples. We found that strongly expressed pSTAT3 was significantly positively correlated with higher CSF IL-10 levels, but not with CSF IL-6 levels.
The present study demonstrated that IL-10 induced the phosphorylation of STAT3 in PCNSL cells both in vitro and in vivo. The phosphorylation levels of STAT3 were stronger in the higher CSF IL-10 group than in the lower CSF IL-10 group according to the immunohistochemical analysis and the Western blot experiment. To our knowledge, this is the first report showing the relationship between the CSF IL-10 concentration and the activation of the STAT3 in PCNS DLBCLs. We speculate that IL-10 protein is secreted from both lymphoma cells and infiltrative immune cells into the CSF, and that an increased CSF IL-10 activates the JAK/STAT3 pathway in PCNS DLBCLs. Recent studies have identified STAT3 as an important molecule that mediates tumor-induced immunosuppression [17]. STAT3 is not only a potent negative regulator of T helper 1 (Th1)-cell-mediated inflammation but also an important activator of many genes that are crucial for immunosuppression [17, 18]. Because IL-10 is widely known to act as an immunosuppressive cytokine, STAT3 seems to be strongly associated with IL-10. Additionally, because STAT3 is crucial for tumor cell proliferation, survival angiogenesis, and invasion [19], the IL-10-STAT3 pathway may constitute a direct link between oncogenesis and immunosuppression [2, 17].
In the present study, the CSF IL-10 level was negatively correlated with shorter PFS. However, the pSTAT3 expression levels did not correlate with survival, although the CSF IL-10 levels were positively correlated with the frequency of strong staining for pSTAT3. One reason for this finding might be that some biopsy tissues of the tumors did not reflect the pSTAT3 expression of the total tumor because these samples were too small. Another possible reason is that seven patients with low CSF IL-10 levels exhibited high expression levels of pSTAT3 (score 4 ~ 6) and had relatively better prognoses. This indicates that STAT3 is activated by other factors. Several studies have reported that increased pSTAT3 expression does not predict a poor prognosis in patients with DLBCL, although it is recognized as a strong predictor of a poor prognosis in various other cancers [5, 20–22]. Komohara et al. found no relationship between pSTAT3 levels and survival time in the patients with PCNSLs [16]. More recently, Gupta et al. reported that pSTAT3 expression status did not predict event-free survival time in systemic DLBCL patients treated with epratuzumab/R-CHOP [13]. These results are consistent with our results. It is possible that several other pathways, such as the NF-κB and c-myc pathways, may affect the prognosis of DLBCL.
Although CSF IL-10 levels showed a relationship with PFS, they did not show a significant relationship with OS. This lack of a significant relationship may due to a deviation of the patients who could not receive salvage therapy for relapse. Jahnke et al. reported that salvage therapy for relapse (versus no treatment) was independent prognostic factor for survival after relapse [23]. Actually, of seven patients who could not receive salvage therapy, 5 patients belonged to the lower CSF IL-10 group. When the patients who could not receive salvage therapy were excluded in the survival analysis, high CSF IL-10 was significantly associated with shorter OS. Our survival study has intrinsic limitations given that it is a retrospective, single-institution analysis. In addition, the number of the patients was small, because PCNS DLBCL is a rare disease. Our results of survival study should be confirmed in a larger prospective analysis.
We found that the staining level of pSTAT3 was stronger in the non-GCB type than in the GCB type. Similar findings were reported in systemic DLBCLs [3]. Lam et al. reported that the STAT3 mRNA levels in non-GCB DLBCL were approximately two-fold higher than in GCB DLBCLs, and the pSTAT3 protein expression in non-GCB DLBCLs was stronger than in GCB DLBCLs [24]. Most recently, Gupta et al. reported a clear trend towards high pSTAT3 positivity in the non-GCB type of systemic DLBCL [13]. Our findings suggest that cytokine-stimulated STAT3 protein contributes to the pathogenesis of a subset of non-GCB type of PCNS DLBCLs.
Based on the results of our study, we propose that patients with higher levels of CSF IL-10 should be considered for more extensive chemo-radiotherapy, rather than more dogmatic statement. In addition, IL-10 and its downstream molecules may be targets for the treatment of PCNS DLBCL. The JAK2 inhibitor blocks IL-10/STAT3 signaling and oncogenesis in solid tumors [25]. Moreover, the JAK2 inhibitor can effectively inhibit tumor angiogenesis and metastasis in tumor cells [26]. Because the IL-10 pathway is activated in most patients with PCNS DLBCL, inhibition of the IL-10 pathway may offer a promising therapeutic approach for these patients.
Conclusions
Our results indicate that the STAT3 activity in PCNS DLBCL is associated with elevated CSF levels of IL-10, and elevated CSF levels of IL-10 may be a predictive marker of STAT3 activity and molecular subtype.
References
Prodduturi P, Bierman PJ (2012) Current and emerging pharmacotherapies for primary CNS lymphoma. Clin Med Insights Oncol 6:219–231
Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9:798–809
Gupta M, Han JJ, Stenson M et al (2012) Elevated serum IL-10 levels in diffuse large B-cell lymphoma: a mechanism of aberrant JAK2 activation. Blood 119:2844–2853
Lee SW, Ahn YY, Kim YS et al (2012) The Immunohistochemical expression of STAT3, Bcl-xL, and MMP-2 proteins in colon adenoma and adenocarcinoma. Gut liver 6:45–51
Morikawa T, Baba Y, Yamauchi M et al (2011) STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer 17:1452–1462
Seki Y, Suzuki N, Imaizumi M et al (2004) STAT3 and MAPK in human lung cancer tissues and suppression of oncogenic growth by JAB and dominant negative STAT3. Int J Oncol 24:931–934
Barton BE, Karras JG, Murphy TF et al (2004) Signal transducer and activator of transcription 3 (STAT3) activation in prostate cancer: direct STAT3 inhibition induces apoptosis in prostate cancer lines. Mol Cancer Ther 3:11–20
Lin TS, Mahajan S, Frank DA (2000) STAT signaling in the pathogenesis and treatment of leukemias. Oncogene 19:2496–2504
Vainchenker W, Constantinescu SN (2013) JAK/STAT signaling in hematological malignancies. Oncogene 32:2601–2613
Tsutsui M, Yasuda H, Suto H et al (2010) Frequent STAT3 activation is associated with Mcl-1 expression in nasal NK-cell lymphoma. Int J Lab Hematol 32:419–426
Wu ZL, Song YQ, Shi YF et al (2011) High nuclear expression of STAT3 is associated with unfavorable prognosis in diffuse large B-cell lymphoma. J Hematol Oncol 4:31
Sasayama T, Nakamizo S, Nishihara M et al (2012) Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL). Neuro-Oncology 14:368–380
Gupta M, Maurer MJ, Wellik LE et al (2012) Expression of Myc, but not pSTAT3, is an adverse prognostic factor for diffuse large B-cell lymphoma treated with epratuzumab/R-CHOP. Blood 120:4400–4406
Hans CP, Weisenburger DD, Greiner TC et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275–282
Vajpayee N, Hussain J, Tolocica I et al (2010) Expression of signal transducer and activator of transcription 3 (STAT3) in primary central nervous system diffuse large B-cell lymphoma: a retrospective analysis of 17 cases. J Neurooncol 100:249–253
Komohara Y, Horlad H, Ohnishi K et al (2011) M2 macrophage/microglial cells induce activation of Stat3 in primary central nervous system lymphoma. J Clin Exp Hematopathol 51:93–99
Yu H, Kortylewski M, Pardoll D (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7:41–51
Williams LM, Ricchetti G, Sarma U et al (2004) Interleukin-10 suppression of myeloid cell activation–a continuing puzzle. Immunology 113:281–292
Frank DA (2007) STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett 251:199–210
Ding BB, Yu JJ, Yu RY et al (2008) Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood 111:1515–1523
Scuto A, Kujawski M, Kowolik C et al (2011) STAT3 inhibition is a therapeutic strategy for ABC-like diffuse large B-cell lymphoma. Cancer Res 71:3182–3188
Xu YH, Lu S (2014) A meta-analysis of STAT3 and phospho-STAT3 expression and survival of patients with non-small-cell lung cancer. European J Surg Oncol 40:311–317
Jahnke K, Thiel E, Martus P et al (2006) Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol 80:159–165
Lam LT, Wright G, Davis RE et al (2008) Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-κB pathways in subtypes of diffuse large B-cell lymphoma. Blood 111:3701–3713
Hedvat M, Huszar D, Herrmann A et al (2009) The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell 16:487–497
Xin H, Herrmann A, Reckamp K et al (2011) Antiangiogenic and antimetastatic activity of JAK inhibitor AZD1480. Cancer Res 71:6601–6610
Conflict of interest
None.
Funding
This study was supported in part by a Grant-in-Aid for Scientific Research to Eiji Kohmura (25293309), Takashi Sasayama (25462258), and Kazuhiro Tanaka (24791501) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 842 kb)
Supplementary Fig. 1 Histological examinations of HKBML tumors implanted into nude mice. HKBML PCNSL cells (5 x 106/200 μl) were injected s.c. into the right flank of nude mice (BALB-c nu/nu). After 2 weeks, the tumor was removed, fixed, and analyzed by immunohistochemistry. The HKBML tumor was identified as a non-GCB type tumor. (Original magnification: × 400)
Rights and permissions
About this article
Cite this article
Mizowaki, T., Sasayama, T., Tanaka, K. et al. STAT3 activation is associated with cerebrospinal fluid interleukin-10 (IL-10) in primary central nervous system diffuse large B cell lymphoma. J Neurooncol 124, 165–174 (2015). https://doi.org/10.1007/s11060-015-1843-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1843-9