Abstract
In this study, neoplastic perfusion abnormalities were investigated by computed tomography perfusion (CTP) scanning in 38 patients with solitary intra-axial brain tumors (19 with high grade gliomas, 7 with low grade gliomas and 12 with brain metastasis). Regional cerebral blood flow (rCBF), cerebral blood volume (rCBV), mean transit time (rMTT) and permeability surface flow (rPSF) levels were measured in two different regions of interest: (1) enhancing or non-enhancing tumor tissue and (2) a mirror area of apparently normal brain tissue located in the contralateral hemisphere. rCBF mean levels were greater in tumoral tissue than in the contralateral area for high-grade gliomas (p < 0.02). rCBV and rPSF mean values were higher in tumoral tissue than in the contralateral area for high-grade gliomas (p < 0.01 and p < 0.05, respectively) and metastasis (p < 0.05 and p < 0.001, respectively). rCBV mean values of tumoral tissue were greater in high-grade than in low-grade gliomas (p < 0.05). rPSF mean levels of tumoral tissue were higher in metastasis than in low-grade gliomas (p < 0.02). These findings indicate that multi-parametric CTP mapping may contribute to differential diagnosis of solitary intra-axial brain tumors.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Conventional Magnetic Resonance Imaging (MRI) is used widely in the diagnosis and follow-up treatment of brain tumors due to its ability to describe morphological characteristics and anatomic relationships (12). However, conventional MRI is often non-specific and is limited in the determination of tumor angiogenesis, which is strongly associated with malignant potential of intracranial neoplasms and patient survival (9). For these reasons, increasing attention has been focused on perfusion-weighted imaging (PWI), an advanced MRI technique that detects hemodynamic abnormalities associated with brain tumors (2). In particular, PWI can identify increased cerebral blood volume (CBV) and blood–brain barrier permeability, which reflect underlying tumor angiogenesis (2, 9). This methodological approach seems useful in predicting tumor grading and could assist in surgical, radiation and chemotherapeutic planning as well as monitoring response to treatment. Emerging evidence from clinical studies indicates that dynamic computed tomography perfusion (CTP) scanning is a rapid, feasible and readily available imaging modality that provides a quantitative assessment of cerebral hemodynamic disturbances in patients with acute ischemic stroke (11). More important, recently CTP imaging has been shown to provide information about the physiology of cerebral neoplasms; thus, this technology may be a promising tool for the analysis of brain tumor microcirculation. In fact, it has been reported that cerebral blood flow (CBF), CBV and Permeability Surface flow (PSF) indicated by CTP seem to correlate well with tumor grade (4) and can help to distinguish high-grade gliomas from tumor-mimicking lesions such as subacute infarction (7) as well as to identify isolated brain metastasis (10). In addition, CTP multi-parametric maps have proven helpful in differentiating between high-grade and low-grade gliomas (3, 5) and between tumor recurrence and radiation necrosis (6). As PWI is not always available, CTP could be an alternative method for assessing the hemodynamics of brain tumors with their extension, type and grade and for discriminating tumor from post-radiation enhancement. In this study, we investigated by CTP imaging perfusion disorders occurring in solitary intra-axial brain tumors in order to further understand the role of CTP in differentiation between high-grade and low-grade gliomas and between cerebral gliomas and metastasis.
2 Materials and Methods
2.1 Patients
Thirty-eight patients (25 male and 13 female; mean age ± SD = 61.6 ± 12.1 years) with suspected solitary intra-axial brain tumor at admission CT and/or MRI and having a Karnofsky performance status (8) at entry ranging from 60% to 95% were prospectively included in the study. All patients underwent surgical biopsy or tumor removal within 7 days after CTP examination. The neoplasms were histopathologically classified as: glioblastoma multiforme (n = 10), anaplastic astrocytoma (n = 9), diffuse astrocytoma (n = 5), oligodendroglioma (n = 2) and brain metastasis (n = 12). According to the World Health Organization system, our series included 19 high-grade gliomas (WHO grade IV = 10; WHO grade III = 9) and 7 low-grade gliomas (WHO grade II). Moreover, cerebral metastases were secondary to lung carcinoma (n = 6), breast carcinoma (n = 4), renal carcinoma (n = 1) and colon carcinoma (n = 1).
2.2 CTP Technique
CTP studies were performed using a single-section CT scanner (CT Hispeed ZX/i; GE Medical System, Milwaukee, Wis.) equipped for CT perfusion imaging (CT Perfusion; GE Medical System, Milwaukee, Wis). The CT perfusion protocol consisted of a series of 45 CT scans acquired in a single slice (10 mm slice thickness, 80 kVp; 200 mAs; matrix 512 × 512; FOV 25 cm; total scan time 50 s) located at tumor level containing the largest volume of neoplastic tissue during the automatic injection of 50 mL of non-ionic contrast agent at the rate of 3.5 mL/s, starting 5 s before the initial image. According to Cenic (1), perfusion maps were generated for CBF, CBV, mean transit time (MTT) and PSF for each patient with a deconvolution-based algorithm and a two-compartmental model by using an imaging workstation (Advantage Windows; GE Medical System, Milwaukee, Wis) with commercial dedicated software (CT Perfusion 2, GE Medical System, Milwaukee, Wis). To minimize inaccuracies in the measured perfusion parameter values, care was taken to exclude large blood vessels from perfusion parameter calculation. As depicted in Fig. 1, regional CBF (rCBF), CBV (rCBV) MTT (rMTT) and PSF (rPSF) levels were measured in two different regions of interest (ROI) larger than 1 cm2 and manually outlined on the baseline diagnostic CT scan: (1) enhancing or non-enhancing tumor tissue and (2) an area of apparently normal brain tissue that mirrored the tumor region and was located in the contralateral hemisphere. More precisely, tumor ROI was drawn in the solid portion of the neoplastic mass by avoiding necrotic and cystic areas of the tumor. In case of limited visibility of the solid part of the lesion on CT scan, the corresponding T1-enhanced MRI imaging was utilized to correctly guide tumor ROI placement. CBF, CBV, MTT and PSF values were expressed in mL/100 g/min, mL/100 g, s and mL/100 g/min, respectively.
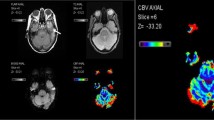
Tumor location and perfusional mapping in patients with anaplastic astrocytoma located parasagittally in left parietal lobe. Image A shows tumor location on admission enhanced CT scan. Images B, C, D and E illustrate the corresponding maps of cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT) and permeability surface flow (PSF). Hand-drawn regions of interest (ROIs) placed within tumor tissue and in the contralateral hemisphere are shown
2.3 Statistical Analysis
After checking data for normality using the Kolmogorov–Smirnov test, statistical analysis was performed by t-test and Mann–Whitney U test with Bonferroni correction for multiple comparisons.
A value of p < 0.05 was considered statistically significant.
3 Results
3.1 CTP Parameters Between Neoplastic Tissue and Contralateral Area in Each Tumor Subtype
As illustrated in Fig. 2, for high-grade gliomas, rCBF mean levels were significantly greater (p < 0.02) in tumor tissue (111.5 ± 52.9 mL/100 g/min) than in the contralateral area (71.2 ± 38.3 mL/100 g/min) whereas low-grade gliomas and metastases had similar rCBF values between tumor tissue (71.5 ± 32.9 and 106 ± 73.7 mL/100 g/min, respectively) and the contralateral area (69.5 ± 11.8 and 71.9 ± 28.9 mL/100 g/min, respectively). For high-grade gliomas, rCBV mean levels were significantly higher (p < 0.01) in tumor tissue (5.4 ± 2.3 mL/100) than in the contralateral area (3.2 ± 1.6 mL/100 g). For metastases, rCBV levels were also significantly higher (p < 0.05) in tumor tissue (4.6 ± 2.2 mL/100 g) than in the contralateral area (3.0 ± 1.1 mL/100 g). Conversely, rCBV mean values were equivalent between tumor tissue (71.5 ± 32.9 mL/100 g) and the contralateral area (69.5 ± 11.8 mL/100 g) in low-grade gliomas. rPSF mean values were significantly increased (p < 0.05) in tumor tissue (6.9 ± 8.7 mL/100 g/min) compared to the contrala-teral area (1.7 ± 4.2 mL/100 g/min) for high-grade gliomas and were significantly more prominent in tumor tissue (9.9 ± 7.9 mL/100 g/min) than in the contralateral area (0.7 ± 0.5 mL/100 g/min) for metastases as well. On the contrary, comparable rPSF mean levels were observed between tumor tissue (2.5 ± 3.3 mL/100 g/min) and the contralateral area (0.8 ± 0.9 mL/100 g/min) in low-grade gliomas. No statistical difference was found for rMTT mean levels between tumor tissue and the contralateral area in the three tumor subtypes examined (data not shown).
Regional cerebral blood flow (rCBF), cerebral blood volume (rCBV) and permeability surface flow (rPSF) between neoplastic tissue and contralateral area in patients with high grade glioma (n = 17) and brain metastases (n = 12) expressed as median and interquartile range (IQR). rCBF mean levels were significantly greater (p < 0.02) in tumor tissue than in contralateral area for high grade gliomas (Panel A). rCBV mean values were higher in tumor tissue than in contralateral area for high grade gliomas (p < 0.01; Panel B ) and metastasis (p < 0.05; Panel C). rPSF mean levels were more elevated in tumor tissue than in contralateral area for high grade gliomas (p < 0.05; Panel D) and in metastasis (p < 0.001; Panel E). The boundaries of the box represent the 25th–75th quartile. The line within the box indicates the median. The whiskers above and below the box correspond to the highest and lowest values, excluding outliers
3.2 CTP Parameters in Neoplastic Tissue Among the Different Tumor Subtypes
rCBF mean levels did not statistically differ among the three tumor subtypes analyzed. In contrast, as shown in Fig. 3, while tumor tissue rCBV mean levels were significantly higher in high-grade than in low-grade gliomas (p < 0.05), tumor tissue rPSF mean values were greater in metastases than in low-grade gliomas (p < 0.02). There was no additional statistical difference in tumor tissue rCBV and rPSF mean values between high-grade gliomas and metastases; in addition, there was no statistical difference in tumor tissue rMTT mean levels among the three tumor subtypes evaluated.
Regional cerebral blood volume (rCBV) and permeability surface flow (rPSF) in neoplastic tissue from 28 patients with solitary intra-axial brain tumor (high grade gliomas = 17; low grade gliomas = 7; brain metastases = 12) expressed as median and interquartile range (IQR). Tumor tissue rCBV mean values were higher (p < 0.05) in high grade than in low grade gliomas (Panel A). Tumor tissue rPSF mean levels were greater (p < 0.02) in metastases than in low grade gliomas (Panel B). The boundaries of the box represent the 25th–75th quartile. The line within the box indicates the median. The whiskers above and below the box correspond to the highest and lowest values, excluding outliers
4 Discussion
It is generally assumed that tumor angiogenesis is related to the biological aggressiveness of intracranial neoplasms (2, 9). PWI is currently considered the most reliable imaging method in providing an accurate assessment of brain tumor microcirculation, as there is a strong correlation between tumor angiogenesis and an elevation in CBV and microvascular permeability (2, 9). However, the use of PWI technology is hampered by its limited availability, long scan-time, cost and patient tolerance. Thus, the identification of a faster and more easily accessible imaging modality for the recognition of perfusion changes associated with brain tumors would be helpful. Recently, CTP has been proposed as an improved method for the detection of perfusion abnormalities associated with high-grade gliomas (4) and isolated brain metastasis (10), as well as in the discrimination between high-grade and low-grade gliomas (3, 5) and between recurrent tumor and radionecrosis (6). In this study we tested the multi-parametric CTP mapping in the determination of tumor grading in various neoplastic brain lesions. According to previous studies (3–5), high-grade gliomas had increased intralesional levels of rCBF, rCBV and rPSF compared to contralateral normal appearing brain tissue, whereas no difference was observed between tumor tissue and contralateral non-injured area in low-grade gliomas; for rMTT levels, no difference was observed in all neoplastic subtypes explored. In addition, rCBV levels obtained from the solid portion of the tumor were more elevated in high-grade than in low-grade gliomas. These findings confirm that CTP is a powerful tool in establishing tumor grade in brain neoplasms since it can reliably recognize perfusion alterations promoted by tumor angiogenesis. On the other hand, here we also show that intralesional rCBV and rPSF values were greater than those measured in the contralateral non-damaged tissue in brain metastases. Interestingly, tumor tissue rPSF levels, but not rCBV values, were more pronounced in metastases than in low-grade gliomas. These data were partially in agreement with a prior preliminary investigation (10) and suggest that, compared to low-grade gliomas, high-grade gliomas were characterized by an increase in rCBV levels, whereas metastases were marked by an elevation in rPSF values. Overall, our results indicate that increased vascularity and microvascular permeability, which are both consistent with tumor angiogenesis, seem to be selectively restricted to high-grade brain tumors and brain metastases. Moreover, whereas a vascular proliferation may be associated with high-grade gliomas, in metastases a vascular leakage may predominate. Thus, rCBV and rPSF levels could serve to distinguish high-grade gliomas and metastases from low-grade gliomas, whereas rCBF values could be utilized to differentiate between high-grade gliomas and brain metastasis. Further studies with large numbers of patients are needed to explore the effectiveness of the multi-parametric analysis offered by dynamic CTP scanning in differential diagnosis of solitary intra-axial brain tumors.
Conflict of interest statement We declare that we have no conflict of interest.
References
Cenic A, Nabavi DG, Craen RA, Gelb AW, Lee T-Y (2000) A CT method to measure hemodynamics in brain tumors: validation and application of cerebral blood flow maps. AJNR Am J Neuroradiol 21:462–470
Cha S (2006) Update on brain tumor imaging: from anatomy to physiology. AJNR Am J Neuroradiol 27:475–487
Ding B, Ling HW, Chen KM, Jiang H, Zhu YB (2006) Comparison of cerebral blood volume and permeability in preoperative grading of intracranial glioma using CT perfusion imaging. Neuroradiology 48:773–781
Eastwood JD, Provenzale JM (2003) Cerebral blood flow, blood volume, and vascular permeability of cerebral glioma assessed with dynamic CT perfusion imaging. Neuroradiology 45:373–376
Ellika SK, Jain R, Patel SC, Scarpace L, Schultz LR, Rock JP, Mikkelsen T (2007) Role of perfusion CT in glioma grading and comparison with conventional MR imaging features. AJNR Am J Neuroradiol 28:1981–1987
Jain R, Scarpace L, Ellika S, Schultz LR, Rock JP, Rosenblum ML, Patel SC, Lee TY, Mikkelsen T (2007) First-pass perfusion computed tomography: initial experience in differentiating recurrent brain tumors from radiation effects and radiation necrosis. Neurosurgery 61:778–786
Lee R, Cheung RTF, Hung KN, Au-Yeung KM, Leong LLY, Chan FL, Lee TY (2004) Use of CT perfusion to differentiate between brain tumor and cerebral infarction. Cerebrovasc Dis 18:77–83
Mor V, Laliberte L, Morris JN, Wiemann N (1984) Karnofsky performance status scale. An examination of its reliability and validity in a research setting. Cancer 53:2002–2007
Provenzale JM, Mukundan S, Barboriak DP (2006) Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology 239:632–649
Roberts HC, Roberts TPL, Lee T-Y, Dillon WP (2002) Dynamic, constrast-enhanced CT of human brain tumors: quantitative assessment of blood volume, blood flow, and microvascular permeability: report of two cases. AJNR Am J Neuroradiol 23:828–832
Wintermark M, Essay M, Barber E, Bodily K, Dillon WP, Eastwood JD, Glenn CT, Gradin CB, Pederast S, Susie JF, Maria T, Anarchic G, Camille JM, Doused V, Yonas H (2005) Comparative overview of brain perfusion imaging techniques. Stroke 36:e83–e99
Young RJ, Knopp EA (2006) Brain MRI: tumor evaluation. J Magn Reson Imaging 24:709–724
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag/Wien
About this paper
Cite this paper
Fainardi, E. et al. (2010). Potential Role of CT Perfusion Parameters in the Identification of Solitary Intra-axial Brain Tumor Grading. In: Czernicki, Z., Baethmann, A., Ito, U., Katayama, Y., Kuroiwa, T., Mendelow, D. (eds) Brain Edema XIV. Acta Neurochirurgica Supplementum, vol 106. Springer, Vienna. https://doi.org/10.1007/978-3-211-98811-4_53
Download citation
DOI: https://doi.org/10.1007/978-3-211-98811-4_53
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-211-98758-2
Online ISBN: 978-3-211-98811-4
eBook Packages: MedicineMedicine (R0)