Abstract
The role of diffusion weighted imaging and apparent diffusion coefficient in intracranial germ cell tumors has not been fully elucidated. The aim of this study was to evaluate whether the ADC correlates with the histologic subtypes of germ cell tumors. We also aimed to investigate whether the ADC values can predict treatment response. The authors retrospectively analyzed the ADC values of the enhancing and solid regions of germ cell tumors. The absolute ADC values and the normalized ADC values were compared among different histologic diagnoses. The ADC values before and after the first course of chemotherapy were also compared between the different prognostic groups. Ten patients were included in the study. The median age at diagnosis was 9.3 years (range 5.3–13.8 years). There were four patients with germinoma and six patients with nongerminomatous germ cell tumor (NGGCT) including five mixed germ cell tumors and one immature teratoma. The mean absolute and normalized ADC values (×10−3 mm2/s) were significantly lower in germinomas [0.835 ± 0.065 (standard deviation) and 1.11 ± 0.096, respectively] than in NGGCTs (1.271 ± 0.145 and 1.703 ± 0.223, respectively) (p = 0.01). The ADC values before and after the first course of chemotherapy were available in four patients. The ADC value after the first chemotherapy had a tendency to increase more in patients who eventually demonstrated complete response with chemotherapy than in patients who required second-look surgery. Assessment of the ADC values of germ cell tumors is considered to facilitate differentiation of histological subtypes of germ cell tumors. Evaluation of the ADC may also be useful for predicting treatment response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intracranial germ cell tumors are relatively rare and a biologically diverse group of neoplasms. They account for approximately 2 % of pediatric brain tumors in Western countries and 10 % in Far East Asian countries [1–4]. Prognosis has been demonstrated to correlate with histopathological diagnoses as either pure germinoma or nongerminomatous germ cell tumors (NGGCTs). High relapse risk and poor outcome are associated with NGGCTs, whereas improved outcome is associated with pure germinoma. Therefore, more intensified therapeutic regimens are planned for NGGCTs. It is beneficial to predict the presurgical diagnosis since the prognosis and treatments are different in the two groups [5–7].
Diffusion weighted imaging (DWI) assesses microscopic water diffusion within tissues and apparent diffusion coefficient (ADC) maps represent average diffusion for each voxel. Diffusion of water molecules is determined by the microstructure and decreases in densely-packed cerebral tissue with high cellularity, small extracellular space, and high nuclear-to-cytoplasmic ratio [8]. Accordingly, the ADC values in brain tumors are inversely related to cellularity and the ratio of nuclear area to cytoplasm. The role of DWI and ADC for differentiation of various types of brain tumors has been evaluated [9–19]. However, the variation of ADC amongst different types of intracranial germ cell tumors has not been fully investigated. We aimed to assess whether the ADC correlates with the histologic subtypes of germ cell tumors.
DWI and ADC have also been used to predict treatment response by comparing the pre- and posttreatment ADC values, with increased ADC correlating with a more favorable clinical course [20–24]. We also aimed to investigate whether the ADC values of pre- and posttreatment of the first course of chemotherapy can differentiate patients who eventually demonstrated complete response with chemotherapy and patients who required second-look surgery.
Methods
We reviewed 10 patients with histologically confirmed germ cell tumors who were treated at National Center for Child Health and Development, Tokyo, over the period of March 2009–July 2013. Retrospective analysis for patients’ information was performed using medical charts and PACS. The inclusion criteria for the study were a histologically proved intracranial germ cell tumor and the availability of pretreatment ADC map in addition to conventional magnetic resonance imaging (MRI) including axial T1-weighted, axial T2-weighted, axial fluid-attenuated inversion recovery (FLAIR), axial, coronal, and sagittal postcontrast T1-weighted, and diffusion-weighted images.
All MR studies were performed on 1.5T MR units. DWI sequences were acquired prior to contrast injection using b values of 0 and 1,000 s/mm2 applied in the X, Y, and Z directions. ADC maps were calculated automatically on the MR scanners.
The imaging analysis was performed at the PACS workstation with supervision of an experienced neuroradiologist who was blinded to the tumor histology. For each patient, the enhancing solid component of the tumor was identified on postcontrast T1-weighted images and matching ADC maps. Then, three non-overlapped regions of interest (ROIs) of 20–100 mm2 were manually positioned in the enhancing solid portion. The ADC values were automatically calculated on the PACS workstation, and three lesion ROIs were averaged and used as the tumor ADC value. For the control, three ROIs were placed in the normal-appearing cerebellum and bilateral centrum semiovale and the ADC value was calculated. The average ADC value was used as the control ADC value. Finally, the normalized ADC value, which was calculated as the ratio of the tumor ADC value to the control ADC value, was obtained.
Comparison of the tumor ADC values and ratios between histological subtypes, and comparison of the ratios of the tumor ADC values of post-treatment to that of pre-treatment between different prognostic groups were done by using a two-sample Mann–Whitney test. A value of p < 0.05 was considered significant.
Results
DWI and pretreatment ADC map was available in 10 patients with germ cell tumors. A summary of patient data is shown in Table 1. The median age at pre-treatment MRI was 9.3 years (range 5.3–13.8 years). Histopathological examination demonstrated four germinomas and six NGGCTs including five mixed germ cell tumors and one immature teratoma.
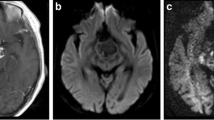
Germinoma and NGGCT could be differentiated by both absolute and normalized ADC values (Figs. 1, 2, 3). The mean absolute and normalized ADC values (×10−3 mm2/s) were significantly lower in germinoma (0.835 ± 0.065 and 1.11 ± 0.096, respectively) than in NGGCT (1.271 ± 0.145 and 1.703 ± 0.223, respectively) (p = 0.01 for both the absolute and normalized ADC values).
The minimum ADC values averaged over each group were 0.663 ± 0.13 for germinoma and 1.03 ± 0.145 for NGGCT. These values were also significantly different among the groups (p = 0.018).
The ADC values before and after the first course of chemotherapy were available in four patients (Table 2). Of those, two patients with germinoma eventually demonstrated radiographic complete response with chemotherapy. In these patients, the pre-treatment tumor ADC values were 0.87 and 0.9, respectively. The tumor ADC values after the first course of chemotherapy (with carboplatin and etoposide) were 2.06 and 2.14, respectively. The ratios of the tumor ADC values of post-treatment to that of pre-treatment were 2.37 and 2.36, respectively. In another two patients with NGGCT, residual tumor remained or increased after several courses of chemotherapy and necessitated second-look surgery. In these patients, the pre-treatment tumor ADC values were 1.06 and 1.4, respectively. The tumor ADC values after the first course of chemotherapy (with carboplatin and etoposide) were 1.34 and 1.42, respectively. The ratios of the tumor ADC values of post-treatment to that of pre-treatment were 1.26 and 1.1, respectively. Although it was not statistically significant, the ADC value after the first chemotherapy had a tendency to increase more in patients who eventually demonstrated complete response with chemotherapy than in patients who required second-look surgery (p = 0.12) (Fig. 4).
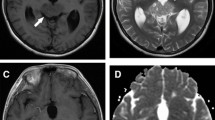
Magnetic resonance (MR) imaging from a 12-year-old boy with germinoma. Axial T1-weighted postcontrast (a) and apparent diffusion coefficient (ADC) (b) images obtained prior to initiation of chemotherapy demonstrated an enhancing pineal mass with restricted diffusion. MR images obtained after one course of chemotherapy revealed pineal enhancing mass with decreased diffusion restriction (c, d)
Discussion
The role of DWI and ADC in intracranial germ cell tumors has not been fully investigated, while prognostic difference associated with histologic germ cell tumor subtypes necessitated classification into either germinomas or NGGCTs. In our study, germinoma and NGGCT were differentiated by both the absolute and normalized ADC values. The ADC values were significantly lower in germinoma than in NGGCT (p = 0.01). Douglas-Akinwande et al. studied the ADC values of 11 germinoma [25]. They reported that evaluation of the solid components revealed that 36 % of germinomas (4 of 11) had predominantly restricted diffusion (the ADC value: 0.694 ± 0.145 × 10−3 mm2/s), the majority [55 % (6 of 11)] had normal diffusion (the ADC value: 0.947 ± 0.054 × 10−3 mm2/s), 9 % (1 of 11) had increased diffusion (the ADC value: 1.172 ± 0.048 × 10−3 mm2/s). The mean absolute ADC value of their 11 germinomas was 0.838 ± 0.152, which was comparable to our result (0.835 ± 0.065). In our study, six NGGCTs included one immature teratoma, two mixed germ cell tumors composed of immature teratoma and germinoma, two mixed germ cell tumors composed of yolk sac tumor and germinoma, and one mixed germ cell tumor composed of embryonal carcinoma and yolk sac tumor. The histological appearance of immature teratomas consists of primitive embryonal mesenchymal tissue, neuroectodermal tissue with canalicular structures resembling neural tube, and fetal-type glands consisting of columnar epithelial cells, immature cartilages, and rhabdomyoblasts. Yolk sac tumors are composed of reticular architecture with numerous cysts lined by neoplastic cells. Embryonal carcinomas consist of pleomorphic immature cells arranged in sheets with abortive papillae [26, 27]. Thus, in those NGGCTs, especially in yolk sac tumors and immature teratomas, tumor cells were not densely packed and cellularity is not considerably high, while histopathology of classic germinomas demonstrates densely packed tumor cells interspersed by fibrous tissue with lymphocytes. Such characteristic hypercellularity of germinomas is considered to result in the lower ADC values than those of NGGCTs (Fig. 5).
a Photomicrograph from a 11-year-old boy with germinoma demonstrated densely packed tumor cells in a solid, sheet-like arrangement with lymphocytic infiltration. (hematoxylin-eosin stain, ×20), b Photomicrograph from a 5-year-old girl with immature teratoma demonstrated canalicular structures resembling neural tube. (hematoxylin-eosin stain, ×20)
DWI and ADC have also been demonstrated to predict treatment response in several types of brain tumors by comparing the pre- and posttreatment ADC values, with increased ADC correlating with a more favorable clinical course [20–24]. Growing teratoma syndrome has been reported to develop in 6.5–11.5 % of germ cell tumors during the course of chemotherapy. Early recognition and timely surgical excision is crucial for growing teratoma syndrome since surgical resection is the only effective treatment [28, 29]. We hypothesized that DWI and ADC may have role for prediction of treatment response by chemotherapy in germ cell tumors. Prediction of necessity for second-look surgery at an earlier stage of the treatment would be beneficial for timely surgical treatment for growing teratoma syndrome. In our study, the ADC value after the first chemotherapy had a tendency to increase more in patients who eventually demonstrated complete response with chemotherapy than in patients who required second-look surgery (p = 0.12). This was considered due to early decrease of cellularity of the tumor in the treatment-responder group, even though the lesion was still enhanced. On the other hand, in non-responder group, the ADC value after the first course of chemotherapy does not increase considerably, whereas the tumor markers after the chemotherapy were nearly-normalized as shown in Table 2. The second-look surgery may be required in the course of treatments when the ADC value after the first course of chemotherapy does not demonstrate considerable increase despite nearly-normalized tumor markers. DWI and ADC could be useful to distinguish responder and non-responder groups at an early stage of chemotherapy in germ cell tumors.
There are several limitations to this study. The number of patients is rather small, especially for patients whose ADC values before and after the first course of chemotherapy was available. Therefore, our findings have to be evaluated in larger number of cohorts. The prospective studies will be required to evaluate whether ADC can predict treatment response. Also, there can be the variation between radiologist interpretations of ADC measurements. However, we tried to minimize such variation by accurate placing of the ROIs only in the enhancing solid portion.
Conclusions
Assessment of the ADC values of germ cell tumors is considered to facilitate differentiation of histological subtypes of germ cell tumors into germinomas and NGGCTs. Evaluation of the ADC may also be useful for predicting treatment response in germ cell tumors.
References
Cho KT, Wang KC, Kim SK et al (2002) Pediatric brain tumors: statistics of SNUH, Korea (1959–2000). Childs Nerv Syst 18:30–37
Dearnaley DP, A’Hern RP, Whittaker S et al (1990) Pineal and CNS germ cell tumors: royal Marsden Hospital experience 1962–1987. Int J Radiat Oncol Biol Phys 18:773–781
Matsutani M, Sano K, Takakura K et al (1997) Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg 86:446–455
Weiner H, Finlay J (1999) Surgery in the management of primary intracranial germ cell tumors. Childs Nerv Syst 177:1–4
Calaminus G, Kortmann R, Worch J et al (2013) SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol 15:788–796
Carlos Chung KH, Owler BK et al (2013) Paediatric germ cell tumours of the central nervous system: results and experience from a tertiary-referral paediatric institution in Australia. J Clin Neurosci 20:514–519
Kim JW, Kim WC, Cho JH et al (2012) A multimodal approach including craniospinal irradiation improves the treatment outcome of high-risk intracranial nongerminomatous germ cell tumors. Int J Radiat Oncol Biol Phys 84:625–631
Huisman TA (2003) Diffusion-weighted imaging: basic concepts and application in cerebral stroke and head trauma. Eur Radiol 13:2283–2297
Bull JG, Saunders DE, Clark CA et al (2012) Discrimination of paediatric brain tumours using apparent diffusion coefficient histograms. Eur Radiol 22:447–457
Gauvain KM, McKinstry RC, Mukherjee P et al (2001) Evaluating pediatric brain tumor cellularity with diffusion-tensor imaging. Am J Roentgenol 177:449–454
Jaremko JL, Jans LB, Coleman LT et al (2010) Value and limitations of diffusion-weighted imaging in grading and diagnosis of pediatric posterior fossa tumors. Am J Neuroradiol 31:1613–1616
Kan P, Liu JK, Hedlund G et al (2006) The role of diffusion-weighted magnetic resonance imaging in pediatric brain tumors. Childs Nerv Syst 22:1435–1439
Pillai S, Singhal A, Byrne AT et al (2011) Diffusion-weighted imaging and pathological correlation in pediatric medulloblastomas—“They are not always restricted!”. Childs Nerv Syst 27:1407–1411
Poussaint TY, Rodriguez D (2006) Advanced neuroimaging of pediatric brain tumors: mR diffusion, MR perfusion, and MR spectroscopy. Neuroimaging Clin N Am 16:169–192
Provenzale JM, Mukundan S, Barboriak DP (2006) Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology 239:632–649
Rumboldt Z, Camacho DL, Lake D et al (2006) Apparent diffusion coefficients for differentiation of cerebellar tumors in children. Am J Neuroradiol 27:1362–1369
Schneider JF, Confort-Gouny S, Viola A et al (2007) Multiparametric differentiation of posterior fossa tumors in children using diffusion-weighted imaging and short echo-time 1H-MR spectroscopy. J Magn Reson Imaging 26:1390–1398
Yamasaki F, Kurisu K, Satoh K et al (2005) Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 235:985–991
Yeom KW, Mobley BC, Lober RM et al (2013) Distinctive MRI features of pediatric medulloblastoma subtypes. AJR Am J Roentgenol 200:895–903
Hamstra DA, Chenevert TL, Moffat BA et al (2005) Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proc Natl Acad Sci USA 102:16759–16764
Huang CF, Chiou SY, Wu MF et al (2010) Apparent diffusion coefficients for evaluation of the response of brain tumors treated by Gamma Knife surgery. J Neurosurg 113:97–104
Li Y, Lupo JM, Polley M-Y et al (2011) Serial analysis of imaging parameters in patients with newly diagnosed glioblastoma multiforme. Neuro Oncol 13:546–557
Mardor Y, Pfeffer R, Spiegelmann R et al (2003) Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J Clin Oncol 21:1094–1100
Gutierrez Rodriguez, Manita M D et al (2013) Serial MR diffusion to predict treatment response in high-grade pediatric brain tumors: a comparison of regional and voxel-based diffusion change metrics. Neuro Oncol 15:981–989
Douglas-Akinwande AC, Ying J, Momin Z et al (2009) Diffusion-weighted imaging characteristics of primary central nervous system germinoma with histopathologic correlation: a retrospective study. Acad Radiol 16:1356–1365
Hirato J, Nakazato Y (2001) Pathology of pineal region tumors. J Neurooncol 54:239–249
Sato K, Takeuchi H, Kubota T (2009) Pathology of intracranial germ cell tumors. Prog Neurol Surg 23:59–75
Kim CY, Choi JW, Lee JY et al (2011) Intracranial growing teratoma syndrome: clinical characteristics and treatment strategy. J Neurooncol 101:109–115
Kong DS, Nam DH, Lee JI et al (2009) Intracranial growing teratoma syndrome mimicking tumor relapse: a diagnostic dilemma. J Neurosurg Pediatr 3:392–396
Conflict of interest
There are no conflicts of interest and there is no financial disclosure.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogiwara, H., Tsutsumi, Y., Matsuoka, K. et al. Apparent diffusion coefficient of intracranial germ cell tumors. J Neurooncol 121, 565–571 (2015). https://doi.org/10.1007/s11060-014-1668-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1668-y