Abstract
Chordomas are rare, slow-growing neoplasms, characterized by locally aggressive growth patterns and high local recurrence rates. To the best of our knowledge, the MGMT promoter methylation status has not been studied in a population of patients with chordomas to determine if a biologic rationale exists to support the use of temozolomide. We here show for the first time that methylation of MGMT promoter is present in a significant portion or recurring clival chordomas; on the contrary in clival chordomas without recurrence MGMT promoter was always unmethylated (p = 0.0317). Although these observations need to be confirmed in a larger study population, our results (1) indicate that methylation of MGMT promoter is present in a significant portion of recurring chordomas, and (2) prompt further investigation into the potential role of temozolomide as an adjuvant treatment of these tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chordomas are rare, slow-growing neoplasms, characterized by locally aggressive growth patterns and high local recurrence rates. Chordomas are believed to arise from vestigial or ectopic notochordal remnant [1–3] and involving the axial skeleton from clivus to sacrum. In the clivus these tumors destroy bone, incorporating vital neurovascular structures, therefore a wide curative surgical excision is rarely allowed. Nevertheless surgery remains the best standard treatment [3, 4], because chordomas are typically largely resistant to conventional chemo- and radiotherapy. The endoscopic endonasal approach is a competitive alternative to transcranial approaches with minimal morbidity and high success rates of gross total resection when performed by experienced cranial base surgeons [5].
Outcome of chordomas is often poor and recurrent disease is a common event [1].
The molecular and genetic events involved in the development and progression of chordomas are not well understood and some common cancer associated genes, like KRAS and BRAF, failed to show a consistent genetic profile [6].
IDH1/2 mutations have not been observed in intracranial chordomas; on the contrary they are frequent in chondrosarcomas and may constitute a diagnostic clue for differential diagnosis [7].
DNA methylation is a tightly regulated process during normal development and it becomes deregulated during neoplastic transformation and disease development [8]. DNA methylation of cytosine at CpG islands can function as transcription repressor, which subsequently leads to the silencing of the associated genes. It is well known that promoter methylation of the MGMT gene represents a well established molecular feature in gliomas, with prognostic and predictive significance: in fact the efficaciousness of temozolomide (TMZ) is linked to the presence of methylated MGMT, whereas the absence of such feature would predict a poor response [9]. The assessment of MGMT promoter methylation status is usually achieved using methylation-specific PCR [10].
To the best of our knowledge, the MGMT promoter methylation status has not been studied in a population of patients with chordomas. In this paper we assessed the MGMT promoter methylation status in a group of chordomas patients, to determine if it may play a role and if a biologic rationale could suggest the use of TMZ for treatment of chordomas.
Materials and methods
Patients
Cases of patients operated for clival chordoma between 1997 and 2011 were retrieved from the files of the Section of Pathology of the Department of Biomedical and Neuromotor Sciences of the University of Bologna at Bellaria Hospital. The same neurosurgeons (GF, DM) operated on all the patients using the endoscopic extended trans-sphenoidal approach.
The cases meeting the following criteria were selected: (1) the availability of enough material to allow molecular characterization, (2) total/subtotal surgical excision, (3) cases of chondroid subtype were excluded, (4) no radiation therapy before and after surgery, (5) no treatment with imatinib after surgery, and (6) the availability of follow-up information. A comprehensive written informed consent was signed for the surgical treatment that produced the tissue samples and the related diagnostic procedures. All information regarding the human material used in this study was managed using anonymous numerical codes and samples were handled in compliance with the Helsinki declaration. Data about age, sex and recurrence were collected.
Histology and immunohistochemistry
Tissue was fixed in 10 % buffered formalin and embedded in paraffin. Blocks were serially cut and underwent haematoxylin and eosin (H&E). For immunohistochemical analysis the avidin–biotin complex immunohistochemical method was followed. An epitope retrieval procedure was used. Before immunostaining, sections were steamed in citrate buffer for 5 min and cooled for 5 min. All specimens were immunostained with anti-cytokeratin (Cell Marque, clone AE1+AE3, prediluted), anti-S100 (Ventana, clone 4C4.9, prediluted) and anti–EMA (Cell Marque, clone E29, prediluted) antisera. The Ki 67 (Ventana, clone 30-9, prediluted) labelling index was calculated as the percentage of positive cells per 10 HPF (400×; Zeiss NeoFluar 0.25 mm2). The number of mitoses was measured in each case per 10 HPF.
MGMT status assessment
Tissue blocks were selected for DNA extraction after careful examination on H&E staining of corresponding sections to rule out the presence of contaminating necrotic debris. Molecular genetic analyses were performed on samples showing an estimated tumor cell content of at least 90 % from five sections of 10 μm from paraffin embedded tissue blocks. Two incubations with xylene at 60 °C and two incubations with absolute ethanol at room temperature for 10 min each were used to eliminate paraffin. DNA was purified by High Pure PCR Template Preparation kit (Roche Applied Science, Mannheim) according to the manufacturer’s instructions and quantified by Quant-iT™ dsDNA BR kit (Invitrogen, Carlsbad, California). At least 200 ng of DNA was then treated with bisulfite using the EpiTect Bisulfite kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. MGMT methylation status was evaluated by means of the methylation sensitive—quantitative locked nucleic acid PCR (MS-qLNAPCR) protocol described by Morandi et al. [10].
In brief, Real Time PCR analysis was performed using an SDS-ABI Prism 7000 (Applied Biosystems, Foster City, CA) with 3′-locked nucleic acid (LNA) modified primers recognizing the methylated and unmethylated alleles of MGMT (Fig. 2b) and SNURF promoter as a reference for normalization. Relative quantification of the methylated and unmethylated allele ratio (M/U ratio) was calculated according to ΔΔCt method [11] using an equal amount of SssI-treated wild type DNA (fully methylated) mixed with the same amount of untreated DNA as a calibrator [10].
Amplicons were detected by SeaKem LE agarose gel (3 %, Lonza, Basel, Switzerland) by the use of GelStar (Lonza, Basel, Switzerland) as intercalator.
IDH1 mutation analysis
Isocitrate dehydrogenase 1 (IDH1) analysis for the p.R132H mutation was performed on paraffin sections, using allele specific locked nucleic acid quantitative PCR (ASLNAqPCR), according to previously described protocol [12] from all cases. The forward primers used for the analysis were designed for the wild type allele (5′-TTGATCCCCATAAGCATGA[+C]-3′) and for the p.R132H allele (5′-TTGATCCCCATAAGCATGA[+T]-3′) (LNA bases are preceded by +); the reverse primer (5′-GTGGCACGGTCTTCAGAGA-3′) allowed to amplify a segment of 104 bp long. The ASLNAqPCR IDH1 results were confirmed by sequencing using the 454 GS-Junior next generation sequencer (Roche, Branford, CT, USA).
Statistical analysis
For statistical evaluation features of the 2 groups were compared using Fisher exact test (sex), Mann–Whitney test (Ki67) and Student’s t test (age, recurrence time).
Correlation of recurrence with MGMT promoter methylation status was evaluated using χ 2-test.
A p value less than .05 was considered statistically significant.
Results
Forty-nine patients with diagnosis of clival chordomas were operated during the 15-year considered period. Thirty cases met the inclusion criteria and were subdivided in two groups, those without recurrences (Group 1) and those with recurrences (Group 2). Clinico-pathological data are summarized in Table 1 (Group 1) and in Table 2 (Group 2). Follow-up ranged from 24 to 198 months.
Group 1
The study population contained 7 male and 8 female patients. Mean age at surgery was 51 years (range 30–73 years). None of these patients developed recurrence after a mean follow-up of 8.5 years (range 2–15 years).
On histology, the lesions were composed of mucin-rich lobules, consisting of ribbons and cords of epithelioid cells, associated with a fibrous stroma. Tumor samples were positive for cytokeratin, EMA and S100 protein. All cases were diagnosed as conventional chordoma. None case presented dedifferentiated or sarcomatous features. In all cases necrosis was absent. Mitotic figures were rare, in 13 cases less than 1 mitosis per 10 HPF was observed, in 2 cases 1 per 10 HPF. Mean Ki67 label index (LI) was 2.8 %.
In no case MGMT promoter was found to be methylated. All cases did not harbor IDH1 mutations.
Group 2
The study population contained 9 male and 6 female patients. Mean age at surgery was 52.1 years (range 31–79 years). Mean follow-up was 9.1 years (range 2–16.5 years). All these patients presented tumor recurrence and thus were submitted to a second surgery. Mean recurrence time was 22.6 months (range 5–56 months).
On histology, the lesions showed the same histological and immunohistochemical findings observed in Group 1, and such features were similar in first tumors and recurrences, except for case no. 30.
All first tumors were diagnosed as conventional chordoma (Fig. 1a). In the first lesion 11 cases showed less than 1 mitosis per 10 HPF, while in 4 cases 1 mitosis per 10 HPF was present. In the recurrences 9 cases presented less than 1 mitosis per 10 HPF, 5 cases 1 mitosis per 10 HPF, while case no. 30 showed 3 mitoses per 10 HPF. Mean Ki67 LI was 3 % in first tumors, and 4.73 % in recurrences.
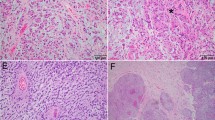
a Case no. 16 (methylated recurring chordoma). The tumor is composed of mucin-rich lobules, consisting of ribbons and cords of epithelioid cells (H&E, ×200 magnification). b Case no. 27 (unmethylated recurring chordoma). Neoplastic cells are similar to those of the case no. 16, but an area of necrosis is evident on the left side (H&E, ×200 magnification)
Case no. 27 showed foci of necrosis in both first tumor (Fig. 1b) and recurrence, while in case no. 30 necrosis appeared in recurrent specimen. In all the remaining cases necrosis was absent. Only case no. 30 showed dedifferentiated features in recurrent lesion. All cases did not harbor IDH1 mutations.
Analysing the first tumors MGMT promoter was found to be methylated in 4 samples (26.7 %) (Fig. 2) and unmethylated in 11 samples (73.3 %). Also the tumor recurrences in those 4 cases were found to be methylated.
Correlations
Comparison of age, sex and Ki67 LI between Groups 1 and 2 did not evidence any statistically significant difference.
On the contrary, the association between promoter methylation of MGMT gene and recurring chordomas (Group 2) resulted statistically significant (p = 0.0317).
Inside the Group 2, methylated cases and unmethylated cases did not show significant differences in age, sex, recurrence time and Ki67 LIs of first tumor.
Comparing Ki67 LIs observed in recurrence specimens, the mean value in was 2.25 in methylated cases and 5.63 in unmethylated cases (p = 0.0386).
Discussion
We here show for the first time that methylation of MGMT promoter is present in a significant portion or recurring clival chordomas; on the contrary in clival chordomas without recurrence MGMT promoter was always unmethylated (p = 0.0317).
Malignancy of chordomas is due to local invasiveness, tendency of recurrence and potential to metastasize. They have two distinct biological behaviors: the first group consists of slow growing lesions which, in rare cases, may not even grow at all. These tumors remain locally confined rather than metastasize to other areas; the second group is composed by tumors with a more aggressive behavior, rapid local recurrence, spread to other areas of the neuroaxis or metastasis to the lung, liver or bone [13].
Clival chordomas destroy bone, incorporating vital neurovascular structures, therefore a wide curative surgical excision is rarely allowed and recurrent disease is a common event [1]. Nevertheless surgery remains the best standard treatment [3, 4], because chordomas are typically largely resistant to conventional chemo- and radiotherapy. Therapeutic advances are therefore urgently required for improving the prognosis.
Molecular and genetic events involved in pathogenesis of chordomas have been explored in the last years. Recent studies evidenced loss of CDKN2A and PTEN expression [14] and significant changes of the DNA methylation pattern [6]. Furthermore patients harboring skull base chordomas were found to demonstrate positive expression of PDGFRB [15]. Inhibition of PDGFRB is likely to mediate antitumour activity of imatinib in chordoma [16, 17].
In the present study, as expected [7], we did not find any case with IDH1 mutations, confirming that none case of chondrosarcoma was present in our series.
Assessment of MGMT promoter methylation status evidenced promoter methylation of the MGMT gene in 26.6 % of recurring chordomas; all observed methylated cases recurred.
The results of this study suggest a prognostic role of promoter methylation of the MGMT gene, as this molecular feature seems to label cases prone to show a more aggressive behaviour and could help clinicians in predicting the postoperative outcome and planning a better follow-up schedule.
Furthermore it is well known that promoter methylation of the MGMT gene may predict the efficaciousness of TMZ (9). Chordomas with an aggressive behaviour, which are a higher priority for chemotherapy, could benefit from TMZ, as a biologic rationale exists to support the use of such treatment.
In conclusion, although these observations need to be confirmed in a larger study population, our results (1) indicate that methylation of MGMT promoter is present in a significant portion of recurring chordomas, and (2) findings prompt further investigation into the potential role of TMZ as an adjuvant treatment of these tumors.
References
Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ (2012) Chordoma: current concepts, management, and future directions. Lancet Oncol 13:e69–e76. doi:10.1016/S1470-2045(11)70337-0
Scheil S, Brüderlein S, Liehr T, Starke H, Herms J, Schulte M, Möller P (2001) Genome-wide analysis of sixteen chordomas by comparative genomic hybridization and cytogenetics of the first human chordoma cell line, U-CH1. Genes Chromosom Cancer 32:203–211
Brüderlein S, Sommer JB, Meltzer PS, Li S, Osada T, Ng D, Möller P, Alcorta DA, Kelley MJ (2010) Molecular characterization of putative chordoma cell lines. Sarcoma 2010:630129. doi:10.1155/2010/630129
Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH (2007) Chordoma: the nonsarcoma primary bone tumor. Oncologist 12:1344–1350
Frank G, Sciarretta V, Calbucci F, Farneti G, Mazzatenta D, Pasquini E (2006) The endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomas. Neurosurgery 59(1 Suppl 1):ONS50–ONS57 discussion ONS50-7
Rinner B, Weinhaeusel A, Lohberger B, Froehlich EV, Pulverer W, Fischer C, Meditz K, Scheipl S, Trajanoski S, Guelly C, Leithner A, Liegl B (2013) Chordoma characterization of significant changes of the DNA methylation pattern. PLoS One 8:e56609. doi:10.1371/journal.pone.0056609
Arai M, Nobusawa S, Ikota H, Takemura S, Nakazato Y (2012) Frequent IDH1/2 mutations in intracranial chondrosarcoma: a possible diagnostic clue for its differentiation from chordoma. Brain Tumor Pathol 29:201–206. doi:10.1007/s10014-012-0085-1
Deniz ML, Kiliç T, Almaata I, Kurtkaya O, Sav A, Pamir MN (2002) Expression of growth factors and structural proteins in chordomas: basic fibroblast growth factor, transforming growth factor alpha, and fibronectin are correlated with recurrence. Neurosurgery 51:753–760
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Morandi L, Franceschi E, de Biase D, Marucci G, Tosoni A, Ermani M, Pession A, Tallini G, Brandes A (2010) Promoter methylation analysis of O6-methylguanine-DNA methyltransferase in glioblastoma: detection by locked nucleic acid based quantitative PCR using an imprinted gene (SNURF) as a reference. BMC Cancer 10:48. doi:10.1186/1471-2407-10-48
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods 25:402–408
Morandi L, de Biase D, Visani M, Cesari V, De Maglio G, Pizzolitto S, Pession A, Tallini G (2012) Allele specific locked nucleic acid quantitative PCR (ASLNAqPCR): an accurate and cost-effective assay to diagnose and quantify KRAS and BRAF mutation. PLoS One 7(4):e36084. doi:10.1371/journal.pone.0036084
Gagliardi F, Boari N, Riva P, Mortini P (2012) Current therapeutic options and novel molecular markers in skull base chordomas. Neurosurg Rev 35:1–13. doi:10.1007/s10143-011-0354-1
Le LP, Nielsen GP, Rosenberg AE, Thomas D, Batten JM, Deshpande V, Schwab J, Duan Z, Xavier RJ, Hornicek FJ, Iafrate AJ (2011) Recurrent chromosomal copy number alterations in sporadic chordomas. PLoS One 6:e18846. doi:10.1371/journal.pone.0018846
Orzan F, Terreni MR, Longoni M, Boari N, Mortini P, Doglioni C, Riva P (2007) Expression study of the target receptor tyrosine kinase of Imatinib mesylate in skull base chordomas. Oncol Rep 18:249–252
Casali PG, Messina A, Stacchiotti S, Tamborini E, Crippa F, Gronchi A, Orlandi R, Ripamonti C, Spreafico C, Bertieri R, Bertulli R, Colecchia M, Fumagalli E, Greco A, Grosso F, Olmi P, Pierotti MA, Pilotti S (2004) Imatinib mesylate in chordoma. Cancer 101:2086–2097
Stacchiotti S, Longhi A, Ferraresi V, Grignani G, Comandone A, Stupp R, Bertuzzi A, Tamborini E, Pilotti S, Messina A, Spreafico C, Gronchi A, Amore P, Vinaccia V, Casali PG (2012) Phase II study of imatinib in advanced chordoma. J Clin Oncol 30:914–920
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marucci, G., Morandi, L., Mazzatenta, D. et al. MGMT promoter methylation status in clival chordoma. J Neurooncol 118, 271–276 (2014). https://doi.org/10.1007/s11060-014-1445-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1445-y