Abstract
To evaluate, through a systematic review of the literature, the antitumoral effects of cannabinoids on gliomas. Research included the following electronic databases: PUBMED, EMBASE, LILACS and The Cochrane Collaboration Controlled Trials Register. All published studies involving the antitumoral effects (cellular and molecular mechanisms) of cannabinoids were considered for this review. The bibliography search strategy included all publications of each of these databases until December 31, 2012. From 2,260 initially identified articles, 35 fulfilled the inclusion criteria for this review. All the studies included in this systematic review were experimental (in vivo and/or in vitro), except for one pilot clinical trial phase I/II involving humans. In all experimental studies included, cannabinoids exerted antitumoral activity in vitro and/or antitumoral evidence in vivo in several models of tumor cells and tumors. The antitumor activity included: antiproliferative effects (cell cycle arrest), decreased viability and cell death by toxicity, apoptosis, necrosis, autophagy, as well as antiangiogenic and antimigratory effects. Antitumoral evidence included: reduction in tumor size, antiangiogenic, and antimetastatic effects. Additionally, most of the studies described that the canabinnoids exercised selective antitumoral action in several distinct tumor models. Thereby, normal cells used as controls were not affected. The safety factor in the cannabinoids’ administration has also been demonstrated in vivo. The various cannabinoids tested in multiple tumor models showed antitumoral effects both in vitro and in vivo. These findings indicate that cannabinoids are promising compounds for the treatment of gliomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The antineoplastic activity of THC and its analogues was first observed in the 1970s [1] prior to the discoveries of cannabinoid and endocannabinoid receptors. Nevertheless, more in-depth research on the subject was pursued only in the 1990s.
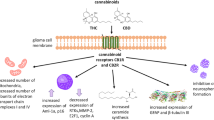
Cannabinoids present two well established endocannabinoids: anandamide (AEA), first described by Dr. Mechoulam’s group [2] and 2-arachidonoylglycerol (2-AG) [3, 4]. To date, endocannabinoids have been described as interacting with five receptors: receptor CB1, CB2, vanilloid receptor 1 (TPRV1), GPR55 and PPARα (although little is known about the functional role of endocannabinoids’ interactions with the last two receptors in the list) [5].
The so-called endocannabinoid system is constituted by endogenous cannabinoids, their receptors and their synthesis, reuptake and degradation processes [6]. Many of the pharmacological actions of the endocannabinoid system are a result of its modulatory properties with other neurotransmission systems [7], which offers great potential for the discovery of therapeutic drugs that act within the system.
In addition to endocannabinoids, there are three primary structural classes of cannabinoid agonist ligands: (i) classical cannabinoid analogues of THC; (ii) (bicyclic and tricyclic) nonclassical cannabinoid analogues of THC; and (iii) aminoalkylindoles [8]. Synthetic agonists [8] and antagonists [9] were created for the CB1 and CB2 receptors through the selected modification of the chemical structures of the cannabinoid molecules.
Several studies suggest that drugs that mimic the endocannabinoid system can be used to hinder or block cancer development [10, 11]. Other studies show the effects of cannabinoids on various cellular and molecular mechanisms directly related to the control of cell proliferation and survival.
The objective of this systematic review was to find all the scientific information available on the antitumor effects of cannabinoids in international literature and then to group these results in order to provide an overview of the medicinal effects of cannabinoids in the treatment of gliomas.
Methodology (systematic review)
Inclusion criteria for this review
Types of studies
All published studies involving the antitumor effects (cellular and molecular mechanisms) of cannabinoids were included:
-
Methodologically appropriate, randomized and non-randomized clinical trials involving human beings who used cannabis (and/or cannabinoids) as antitumor treatment of gliomas.
-
Experimental studies that assessed the antitumor mechanisms of cannabinoids in any type of gliomas in laboratory animals as well as the use of cannabinoids in these tumor cells.
Types of participants (sample)
-
Patients with gliomas, independent of sex, age and area of treatment.
-
Laboratory animals with any type of glioma.
-
Glioma cells in in vitro experiments.
Types of intervention
Pharmacological interventions based on derivatives of cannabis (cannabinoids) and/or smoked cannabis, independently of the duration of intervention and independently of the association with other types of antitumor therapy in the following cases: (i) in patients with gliomas; (ii) in laboratory animals with gliomas; (iii) in tumor cells (gliomas) in vitro experiments.
Search strategy for study identification
Searches were conducted using the electronic databases of MEDLINE (PUBMED), EMBASE, LILACS and the Cochrane Collaboration Controlled Trials Register. Initially, a bibliographic search was conducted across all databases through December 2012. A further search of all references from the relevant articles found (which included review articles) was also performed in order to select items that might have been overlooked during the initial electronic search. There was no language restriction, but only complete papers published in peer-reviewed journals were considered. Data not related to the cellular and molecular effects of the antitumor actions of cannabinoids were not considered. There was no need to contact the authors of the included studies for clarification of the their study data. The search was based on the following Medical Subject Heading terms and categories: “cannabis”, “cannabinoids”, “endocannabinoids”, “cannabinoid receptors”, “gliomas”, “cultured tumor cells”, “antineoplastic drugs”, “fatty acid synthesis inhibitors”, “antimitotic drugs”, “cancer treatment protocol”. Adjustments were made to the terms used according to the electronic database consulted.
Methodological quality of the studies included
This systematic review did not find any randomized studies for the outcomes proposed. The only study included on the antitumor effects of cannabinoids in human beings was a pilot phase I/II clinical trial. All other studies included were experimental. Consequently, the methodological quality was intrinsic since the authors included a detailed description of all the experimental procedures carried out. Thus, the methodological assessment criteria contained in the Cochrane Collaboration Manual [12, 13] were not used to assess methodological quality.
Based on the inclusion criteria, the authors of this review included all published studies that, despite the limitations inherent to experimental studies, currently comprehend the body of scientific evidence regarding these outcomes.
Study selection process
2,260 articles were identified using the aforementioned search strategy. The titles of these articles were then sifted to exclude all articles that did not meet the objectives of this review. Subsequently, 735 abstracts were assessed in detail. Up to this point, assessment was conducted by a single reviewer. Complete articles detailing randomized clinical trials for possible inclusion in this review were assessed by two independent reviewers (co-reviewer: JGSJr). The reviewers were not blind to the authors’ names, institutions and publication journals. Finally, 81 complete articles were assessed. Bear in mind that the bibliographic references in all of the articles deemed important for this review (articles as well as reviews) were searched. The assessment was carried out in accordance with the inclusion criteria, and 35 articles met the inclusion criteria for this review. There was no disagreement between the reviewers regarding article inclusion.
Results
Of the 2,260 articles initially identified, 35 met all of the inclusion criteria for this review. Table 1 of the included studies and the section entitled “Discussion” describe the cellular and molecular mechanisms involved in cannabinoids’ antitumor action. It is noteworthy that when researchers conducted more specific experiments, the sequence of events mediators of these antitumor action compounds was described.
Characteristics of the included studies in the systematic review
This review covered 35 studies on the antitumor effects of cannabinoids in gliomas. Only one study has been carried out on humans [14]. The remaining studies were experimental. Sixteen studies covered the antitumor effects of cannabinoids in vivo [14–29]. Different dosages of cannabinoids as well as different types of glioma cells were used in the studies. Furthermore, some studies used immunodeficient rats to assess the effect of cannabinoids on immunity [15, 17–19].
The studies in this review covered the effects of cannabinoids on the death of tumor cells. The angiogenesis–inhibitor and metastasis-inhibitor effects on the different types of cells investigated were also analyzed.
Discussion
Gliomas
Gliomas are classified as primary tumors originating in the glial cells, and astrocytomas are their most frequent representation (being approximately half of all brain tumors) [30]. Astrocytomas are considered highly aggressive, generally with a tumor mass exhibiting a high grade of heterogeneity and significant microvascular proliferation.
The WHO classifies astrocytomas as having either a low grade (I or II) or high grade (III and IV) of malignancy, depending on the location and rate of growth. Anaplastic astrocytomas (grade III) and glioblastoma multiforme (GBM—grade IV) represent the most aggressive primary tumors in the central nervous system and are responsible for one-third of all brain tumor diagnoses [31].
Cellular mechanisms and cannabinoid receptors
The antitumor action verified occurs through different mechanisms depending on the cannabinoid compound used and the tumor cells investigated [32]. Though a number of these actions involve the activation of receptors (e.g. cannabinoids, vanilloids) [19, 33, 34], many studies show that these effects occur through a receptor-independent mechanism [35, 36].
Apoptosis, or programmed cell death, was the most commonly described mechanism in the studies. Secondarily, cell cycle arrest through cannabinoid action was described as an important antiproliferative mechanism in tumor cells. In fact, this mechanism has been an important target in cancer management since molecular analyses of human tumors have shown that cell-cycle regulators suffer frequent mutations in the majority of common malignancies [37]. Finally, a recent study [26] showed that autophagy precedes apoptosis through delta-9-THC action in various tumor cells.
It is important to emphasize that all studies in vivo that evaluated cannabinoids action decreasing tumor size resulted in statistically significant reductions of the tumors’ volumes when comparing to controls.
Molecular mechanisms and intracellular signaling pathways
This review described some mechanisms underlying cannabinoids’ apoptotic effects and the cell cycle arrest. Galve-Roperh et al. [15] stressed the importance of the sustained activation of Raf1 and the accumulation of ceramide in apoptosis induced by delta-9-THC in cells C6.9. Raf1 is extremely important for controlling cell fate and involves activation of the biochemical cascade mediated by extracellular signal-regulated kinases (ERKs).
The accumulation of ceramide, in turn, leads to the sustained inhibition of Akt [38] and of other protein kinases, such as c-Jun N-terminal kinase (JNK) and p38 MAPK (mitogen-activated protein kinase) [15]. In his study, Gómez Del Pulgar et al. [39] proved a direct relationship between the accumulation of ceramide and ERK activation as a result of exposure to delta-9-THC in C6 cells. The sphingomyelin cycle has been shown to be critically important in regulating cell function as it is associated with apoptosis through the increase in intracellular ceramide levels [40] in tumor cells in both the central [15, 26] and the peripheral nervous systems [41].
Massi et al. [20], in turn, shows that the apoptotic effect of CBD in human glioma cells (U87 and U373) was not mediated by accumulation of ceramide, but, at least in part, by oxidative stress (ROS) [20]. Additional mechanisms involved in CBD action include lipoxygenase (LOX) modulation [23].
Other important cannabinoid mechanisms involve cyclooxygenase-2 (COX-2) activity [35] and the lipid transport mechanism [34]. Indeed, this review showed that these mechanisms were exclusive to the endocannabinoids action and of their synthetic analogues.
In terms of the mechanisms involved in cell cycle arresting, intrinsic characteristics occur in the cells depending on the phase of the cycle in which this block occurs. In a study of human astrocytoma cells (U251MG and U87MG), Galanti et al. [42] shows that delta-9-THC decreased E2F1 and cyclin A in cells, both being proteins that promote the progression of the cell cycle. Furthermore, there was an increase in the levels of p16INK4A, a cell cycle inhibitor [43], which is inactivated in gliomas in more than 50 % of cases [43].
We know that early mechanisms that precede deregulation of cell survivor mechanisms as well as other mechanisms that lead to tumor cell death are important targets of the cannabinoid action. Salazar et al. [26] showed the importance of endoplasmatic reticulum stress and of the up-regulation of the p8-TRB3 mechanism (through delta-9-THC action) in inducing autophagia and subsequent apoptosis of human glioma cells (U87MG, T98G and U373MG). The various experiments conducted in Salazar’s study [26] elucidate the sequential events involved in tumor cell death induced by delta-9-THC, which are listed in Table 1. An analysis of rat tumors marked with U87MG tumor cells as well as biopsies of GBM patients were consistent with the foregoing findings [26] suggesting an effetive action of delta-9-THC cannabinoid in human tumors.
It is important to highlight a recent study [28 ] showing the action of CBD (in vitro and in vivo) in the inhibition of Id-1 gene expression, which is related to the aggressiveness of some tumors, including GBM.
Inhibition of tumor angiogenesis
Vascular endothelial growth factors (VEGFs) and Ang2 (angiopoietin-2) are essential to generate new blood vessels in a number of tumors, including gliomas [44]. Inhibiting tumor angiogenesis via cannabinoids involves at least two mechanisms: (i) direct inhibition of migration and survival of endothelial cells; (ii) suppression of pro-angiogenic (VEGF and Ang2) factors [18] and expression of matrix metalloproteinase (MMP, primarily MMP-2) in tumors [18]. We must keep in mind that endothelial cells express functional CB1 [45] and CB2 [18] receptors and that these receptors modulate essential functions of these cells, such as migration and proliferation [18]. We know that ceramide also inhibits both VEGF production as well as activation of its receptor, which indicates that ceramide plays a central role in the anti-angiogenic action of cannabinoids [10, 19].
The migration-inhibitor (metastasis-inhibitor) effect
The action of MMPs and their inhibitors tissue inhibitors of metalloproteinases (TIMPs) play a central role in the migration and invasiveness of tumor cells [24]. A number of studies show that the majority of human cancers present an increase in the expression and activation of MMPs, including MMP-2 [46] and that this increase is associated with a poor prognosis [47]. In addition, the most proeminent TIMP, TIMP-1 [24], is being studied to determine if inhibition of the MMP, induced by the cannabinoid, is related to inhibition of the glioma cell migration, induction of cell death and inhibition of tumor angiogenesis [24].
In vitro (human astrocytomas cells) and in vivo experiments (biopsies of both rats and humans with gliomas) demonstrated the antitumor action of delta-9-THC through mediation of ceramide and stress protein p8, including the decrease of TIMP-1 and MMP-2 [24, 25]. Furthermore, they showed that expression thereof preceded apoptosis evoked through the cannabinoid. Migration-inhibitor effects were also observed in experiments involving other cannabinoids, such as AEA, JWH-133 and WIN 55,212-2 [24, 25].
Finally, we must describe that this cannabinoids’ effect has been reported in other types of tumor cells, such as SW480 colon cancer cells [48] and in thyroid cancer cells in rats [49].
Cannabinoids, immunity and the biphasic effect
Some studies in this review [15, 17–19] used immunodeficient rats that were deficient in mature T-cells and B-cells in order to assess the effect of the immunity in antitumor action of the cannabinoids. We know that, under certain circunstances, cannabinoids act as immunosuppressants on account of stimulation of CB2 receptors in immunological cells and organs. This action can inhibit antitumor immunity [17, 50] and, consequently, increase tumor growth [50]. However, in studies included in this review, the host’s antitumor immunity did not impact the in vivo experiments. It is possible that immunity inhibition has not been enough to minimize the cannabinoids’ antitumor effect.
Selectivity of cannabinoids in antitumor acticity
All of the studies in this review, except one [51], showed that cannabinoids are capable of selectively killing tumor cells. In contrast to its pro-apoptotic and antitumor effect in various types of tumors, cannabinoids protect normal cells from apoptosis.
One of the possible explanations for this paradoxical behavior in glia cells may owe itself to the different capacities of tumor and non-tumor cells to synthesize ceramide in response to cannabinoids [10]. This would act in addition to the different characteristics of their respective intracellular mechanisms and/or differences in the functionality of their cannabinoid receptors [10].
Cannabinoids, side effects and treatment strategies
Cannabinoid activation of the CB1 receptor is the main factor responsible for the known side effects of these substances. However, in some clinical situations, such as cancer patients who undergo chemotherapy, patients may consider some possible side effects tolerable.
Various non-psychoactive cannabinoids are potentially useful as antitumor agents in gliomas, including ajulemic acid [16], cannabidiol [20, 23, 32, 36] and JWH-133 [17–19]. Many experiments showed that cannabinoids acting in CB2 receptors are not psychoactive. In relation to gliomas, we must consider that not all human glioblastoma multiforme and types of glioma cells express functional CB2 receptors [17].
Studies also show that CBD and delta-9-THC differ in terms of the mechanisms of action in inducing apoptosis of tumor cells. As such, the combined treatment with these cannabinoids could significantly increase the efficacy of the cannabinoids as antitumor agents. This was shown in recent studies in which CBD and delta-9-THC had a greater effect when used in conjunction than separate use of them in inhibiting the viability of glioma cells in vitro [27, 52] and in the tumor growth in vivo [27]. Moreover, preliminary reports showed that the combination of these phytocannabinoids was better tolerated than the isolated use of delta-9-THC [53].
Another potential strategy for treatment of neoplasia would be to increase concentrations of the endocannabinoid AEA. In some types of tumors, the use of substances capable of increasing the level of endogenous AEA have shown promising results. This strategy is possible with drugs that inhibit reuptake or the intracellular degradation of AEA. In addition, use of these substances would be particularly useful in tumor tissues that superexpress AEA, 2-AG or both [54]. Other types of tumor cells that are especially sensitive to AEA action would be those that superexpress COX-2 given that, in these cells, the antiproliferative effects of AEA are mediated by COX-2 catabolism of prostaglandins [35, 55].
Finally, the concomitant use of ceramide and cannabinoids may be an interesting strategy in some types of tumors in order to maximize efficacy and minimize the side effects of some cannabinoids in some tumor models [15, 39, 41]. This association with ceramide is already being carried out in some treatment approaches together with conventional chemotherapies [56]. These ones, in turn, together with cannabinoids, could bring interesting antitumoral effects, as shown in two recent studies [27, 57]. As such, the ROS mediated anti-tumor effects of cannabinoids [20, 52], for example, make them good adjuvant therapy candidates.
In this sense, this review shows that cannabinoids can be considered an excellent treatment option, specifically on account of the present scarcity of effective resources to treat some types of cancers in medicine.
References
Munson AE, Harris LS, Friedman MA, Dewey WL, Carchman RA (1975) Antineoplastic activity of cannabinoids. J Natl Cancer Inst 55:597–602
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Mechoulam R, Ben Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90
Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215:89–97
Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K, Murphy SP, Kendall DA, Bennet AJ (2007) Cannabinoid activation of PPARα; a novel neuroprotective mechanism. Br J Pharmacol 152:734–743
Piomelli D (2003) The molecular logic of endocannabinoid signaling. Nat Rev Neurosci 4:873–878
Felder CC, Glass M (1998) Cannabinoid receptors nand their endogenous agonists. Annu Rev Pharmacol Toxicol 38:179–200
Guzmán M (2003) Cannabinoids: potential anticancer agents. Nat Rev Cancer 3:745–755
Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptor. Pharmacol Rev 54:161–202
Velasco G, Galve-Roperh I, Sánchez C, Blázquez C, Guzmán M (2004) Hypothesis: cannabinoid therapy for the treatment of gliomas? Neuropharmacology 47:315–323
Bifulco M, Laezza C, Gazzerro P, Pentimalli F (2007) Endocannabinoids as emerging suppressors of angiogenesis and tumor invasion (review). Oncol Rep 17(Suppl4):813–816
Cook DJ, Mulrow CD, Haynes RB (1997) Systematic Reviews: synthesis of best evidence for clinical decisions. Ann Intern Med 126:364–371
Jadad A, Moore A, Carrol D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Guzmán M, Duarte MJ, Blázquez C, Ravina J, Rosa MC, Galve-Roperh I, Sánchez C, Velasco G, González-Feria L (2006) A pilot clinical study of delta-9-tetrahydrocannabinoi in patients with recurrent glioblastoma multiforme. Br J Cancer 95:197–203
Galve-Roperh I, Sánchez C, Cortés ML, Del Pulgar TG, Izquierdo M, Guzmán M (2000) Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med 6(Suppl3):313–319
Recht LD, Salmonsen R, Rosetti R, Jang T, Pipia G, Kubiatowski T, Karim P, Ross AH, Zurier R, Litofsky NS, Burstein S (2001) Antitumor effects of ajulemic acid (CT3), a synthetic non-psychoactive cannabinoid. Biochem Pharmacol 62:755–763
Sánchez C, Ceballos ML, del Pulgar TG, Rueda D, Corbacho C, Velasco G, Galve-Roperh I, Huffman JW, Ramon y Cajal S, Guzmán M (2001) Inhibition of glioma growth in vivo by selective activation of the CB2 cannabinoid receptor. Cancer Res 61:5784–5789
Blásquez C, Casanova L, Planas A, del Pulgar TG, Villanueva C, Aceñero MJ, Aragonês J, Huffman JW, Jorcano JL, Guzmán M (2003) Inhibition of tumor angiogenesis by cannabinoids. FASEB J 17(Suppl3):529–531
Blásquez C, González-Feria L, Álvarez L, Haro A, Casanova ML, Guzmán M (2004) Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res 64:5617–5623
Massi P, Vaccani A, Ceruti S, Colombo A, Abbracchio MP, Parolaro D (2004) Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J Pharmacol Exp Ther 308:838–845
Duntsch C, Divi MK, Jones T, Zhou Q, Krishnamurthy M, Boehm P, Wood G, Sills A, Moore BM II (2006) Safety and efficacy of a novel cannabinoid chemotherapeutic, KM-233, for the treatment of high-grade glioma. J Neurooncol 77:143–152
Carracedo A, Lorente M, Egia A, Blázquez C, Garcia S, Giroux V, Malicet C, Villuendas R, Gironella M, Gonzáliz-Feria L, Piris MA, Iovanna JL, Guzmán M, Velasco G (2006) The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell 9:301–312
Massi P, Valenti M, Vaccani A, Gasperi V, Marras E, Fezza F, Macarrone M, Parolaro D (2008) 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J Neurochem 104:1091–1100
Blázquez C, Carracedo A, Salazar M, Lorente M, Egia A, González-Feria L, Haro A, Velasco G, Guzmán M (2008) Down-regulation of tissue inhibitor of metalloproteinases-1 in gliomas: a new marker of cannabinoid antitumoral activity? Neuropharmacology 54:235–243
Blázquez C, Salazar M, Carracedo A, Lorente M, Egia A, González-Feria L, Haro A, Velasco G, Guzmán M (2008) Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression. Cancer Res 68(Suppl6):1945–1952
Salazar M, Carracedo A, Salanueva IJ, Hernández-Tiedra S, Lorente M, Egia A, Vázquez P, Blázquez C, Torres S, Garcia S, Nowak J, Fimia GM, Piacentini M, Cecconi F, Pandolfi PP, González-Feria L, Iovanna JL, Guzmán M, Boya P, Velasco G (2009) Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest 119:1359–1372
Torres S, Lorente M, Rodríguez-Fornés F, Hernández-Tiedra S, Salazar M, Garcia-Taboada E, Barcia J, Guzmán M, Velasco G (2011) A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol Cancer Ther 10(Suppl1):90–103
Soroceanu L, Murase R, Limbad C, Singer E, Allison J, Adrados I, Kawamura R, Pakdel A, Fukuyo Y, Nguyen D, Khan S, Arauz R, Yount GL, Moore DH, Desprez PY, McAllister SD (2013) Id-1 is a key transcriptional regulator of glioblastoma aggressiveness and a novel therapeutic target. Cancer Res 73:1559–1569
Gurley SN, Abidi AH, Allison P, Guan P, Duntsch C, Robertson JH, Kosanke SD, Keir ST, Bigner DD, Elberger AJ, Moore BM II (2012) Mechanism of anti-glioma activity and in vivo efficacy of the cannabinoid ligand KM-233. J Neurooncol 110(Suppl2):163–177
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neurooncology 14(Suppl5):v1–v49
Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavence WK (2002) The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 61:215–225
Massi P, Vaccani A, Bianchessi S, Costa B, Macchi P, Parolaro D (2006) The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell Mol Life Sci 63:2057–2066
Jacobsson SOP, Wallin T, Fowler CJ (2001) Inhibition of Rat C6 glioma cell proliferation by endogenous and synthetic cannabinoids. Relative involvement of cannabinoid and vanilloid receptors. J Pharmacol Exp Ther 299(Suppl3):951–959
Bari M, Battista N, Fezza G, Finazzi-Agrò A, Macarrone M (2005) Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J Biol Chem 280(Suppl13):12212–12220
Hinz B, Ramer R, Eichele K, Weinzierl U, Brune K (2004) Up-regulation of cyclooxygenase-2 expression is involved in R(+)-methanandamide-induced apoptotic death of human neuroglioma cells. Mol Pharmacol 66:1643–1651
Vaccani A, Massi P, Colombo A, Rubino T, Parolaro D (2005) Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br J Pharmacol 144:1032–1036
Molinari M (2000) Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif 33:261–274
Gómez del Pulgar T, de Ceballos ML, Guzmán M, Velasco G (2002) Cannabinoids protect astrocytes from ceramide-induced apoptosis through the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem 277:36527–36533
Gómez Del Pulgar TG, Velasco G, Sánchez C, Haro A, Guzmán M (2002) De novo-synthesized ceramide is involved in cannabinoid-induced apoptosis. Biochem J 363:183–188
Guzmán M, Galve-Roperh I, Sánchez C (2001) Ceramide: a new second messenger of cannabinoid action. Trends Pharmacol Sci 22:19–22
Gustafsson K, Sander B, Bielawski J, Hannun YA, Flygare J (2009) Potentiation of canabinoi-induced cytotoxicity in mantle cell lymphoma through modulation of ceramide metabolism. Mol Cancer Res 7(Suppl7):1086–1098
Galanti G, Fisher T, Kventsel I, Shoham J, Gallily R, Mechoulam R, Lavie G, Amariglio N, Rechavi G, Toren A (2008) 9-Tetrahydrocannabinol inhibits cell cycle progression by downregulation of E2F1 in human glioblastoma multiforme cells. Acta Oncol 47(Suppl6):1062–1070
Costello JF, Berger MS, Huang H-JS, Cavenee WK (1996) Silencing of p16/CDKN2 expression in human gliomas by methylation and chromatin condensation. Cancer Res 56:2405–2410
Casanova ML, Larcher F, Casanova B, Murillas R, Fernández-Aceñero MJ, Villanueva C, Martinez-Palacio J, Ullrich A, Conti CJ, Jorcano JL (2002) A critical role for ras-mediated, EGFR-dependent angiogenesis in mouse skin carcinogenesis. Cancer Res 62:3402–3407
Ros J, Clària J, To-Figueiras J, Planagumá A, Cejudo-Martín P, Fernández-Varo G, Martín-Ruiz R, Arroyo V, Rivera F, Rodes J, Jiménez W (2002) Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology 122:85–93
Nakada M, Nakada S, Demuth T, Tran NL, Hoelzinger DB, Berens ME (2007) Molecular targets of glioma invasion. Cell Mol Life Sci 64:458–478
Deryugina EI, Quigley JP (2006) Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25:9–34
Joseph J, Niggemann B, Zaenker KS, Entschladen F (2004) Anandamide is an endogenous inhibitor for the migration of tumor cells ant T lymphocytes. Cancer Immunol Immunother 53:723–728
Pisanti S, Borselli C, Oliviero O, Laezza C, Gazzerro P, Bifulco M (2006) Antiangiogenic activity of the endocannabinoid anandamide: correlation in its tumor-suppressor efficacy. J Cell Physiol 211:495–503
Zhu LX, Sharma S, Stolina M, Gardner B, Roth MD, Tashkin DP, Dubinett SM (2000) Delta-9-Tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J Immunol 165:373–380
Widmer M, Hanemann CO, Zajicek J (2008) High concentrations of cannabinoids activate apoptosis in human U373MG glioma cells. J Neurosci Res 86:3212–3220
Marcu JP, Christian RT, Lau D, Zielinski AJ, Horowitz MP, Lee J, Pakdel A, Allison J, Limbad C, Moore DH, Yount GL, Desprez P-Y, McAllister SD (2010) Cannabidiol enhances the inhibitory effects of delta-9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol Cancer Ther 9(Suppl1):180–189
Russo E, Guy GW (2006) A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses 66(Suppl2):234–246
Ligresti A, Bisogno T, Matias I, De Petrocellis L, Cascio MG, Cosenza V, D’Argenio G, Scaglione G, Bifulco M, Sorrentini I, Di Marzo V (2003) Possible endocannabinoid control of colorectal cancer growth. Gastroenterology 125:677–687
Eichele K, Ramer R, Hinz B (2009) R(+)-methanandamide-induced apoptosis of human cervical carcinoma cells involves a cyclooxygenase-2-dependent pathway. Pharm Res 26:346–355
Morales A, Lee H, Goni FM, Kolesnick R, Fernandez-Checa JC (2007) Sphingolipids and cell death. Apoptosis 12:923–939
Nabissi N, Morelli MB, Santoni M, Santoni G (2013) Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis 34(Suppl1):48–57
End DW, Thoursen K, Dewey WL, Carchman RA (1977) A comparative study of the disposition of (-)-delta9-tetrahydrocannabinol in neuroblastoma and glioma cells in tissue culture: relation to cellular impairment. Mol Pharmacol 13:864–871
Sánchez C, Galve-Roperh I, Casanova C, Brachet P, Guzmán M (1998) Delta9-tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett 436(Suppl1):6–10
Macarrone M, Pauselli R, Di Rienzo M, Finazzi-Agró A (2002) Binding, degradation and apoptotic activity of stearoylethanolamide in rat C6 glioma cells. Biochem J 366:137–144
Fowler CJ, Jonsson KO, Andersson A, Juntunen J, Järvinen T, Vandevoorde S, Lambert DM, Jerman JC, Smart D (2003) Inhibition of C6 glioma cell proliferation by anandamide, 1-arachidonoylglycerol, and by a water soluble phosphate ester of anandamide: variability in response and involvement of arachidonic acid. Biochem Pharmacol 66:757–767
Contassot E, Wilmotte R, Tenan M, Belkouch MC, Schnüriger V, Tribolet N, Bourkhardt K, Dietrich PY (2004) Arachidonylethanolamide induces apoptosis of human glioma cells through vanilloid receptor-1. J Neuropathol Exp Neurol 63(Suppl9):956–963
Goncharov I, Weiner L, Vogel Z (2005) Delta9-tetrahydrocannabinol increases C6 glioma cell death produced by oxidative stress. Neuroscience 134:567–574
McAllister SD, Chan C, Taft RJ, Luu T, Abood ME, Moore DH, Aldape K, Yount G (2005) Cannabinoids selectively inhibit proliferation and induce death of cultured human glioblastoma multiforme cells. J Neurooncol 74:31–40
Ellert-Miklaszewska A, Kaminska B, Konarska L (2005) Cannabinoids down-regulate P13K/Akt and Erk signaling pathways and activate proapoptotic function of bad protein. Cell Signal 17:25–37
Eichele K, Weinzierl U, Ramer R, Brune K, Hinz B (2006) R(+)-methanandamide elicits a cyclooxygenase-2-dependent mitochondrial apoptosis signalling pathway in human neuroglioma cells. Pharm Res 23(Suppl1):90–94
Acknowledgments
Coordination of Improvement of People of Higher Level (CAPES), Cochrane Center.
Conflict of interest
This paper has no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocha, F.C.M., dos Santos Júnior, J.G., Stefano, S.C. et al. Systematic review of the literature on clinical and experimental trials on the antitumor effects of cannabinoids in gliomas. J Neurooncol 116, 11–24 (2014). https://doi.org/10.1007/s11060-013-1277-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1277-1