Abstract
Previous studies reported that miR-29c is significantly downregulated in several tumors. However, little is known about the effect and molecular mechanisms of action of miR-29c in human glioma. Using quantitative RT-PCR, we demonstrated that miR-29c was significantly downregulated in glioma cell lines and human primary glioma tissues, compared to normal human astrocytes and matched non-tumor associated tissues (P < 0.05, χ2 test). Overexpression of miR-29c dramatically reduced the proliferation and caused cessation of cell cycle. The reduced cell proliferation is due to G1 phase arrest as cyclin D1 and cyclin E are diminished whereas p27 and p21 are upregulated. We further demonstrated that miR-29c overexpression suppressed the glioma cell migration and invasion abilities by targeting MMP-2. In addition, we also found that overexpression of miR-29c sharply inhibited angiogenesis, which correlated with down-regulation of VEGF. The data indicate that miR-29c may be a tumor suppressor involved in the progression of glioma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas are the most common malignancies of the central nervous system in humans. Based on the histopathological and clinical criteria established by World Health Organization, glioblastoma belongs to grade IV that originates from poorly differentiated astrocytes [1]. Despite major therapeutic improvements made by combining neurosurgery, chemotherapy, and radiotherapy, the prognosis and survival rate for patients with glioblastoma is still extremely poor [2]. The poor prognosis of glioblastoma is largely attributed to their rapid growth, invasive/migratory, and high rate of recurrence [3]. Therefore, how to prolong the survival time of patients of glioblastoma is an urgent problem we are facing. The recent study of miRNAs brings us possibilities for the treatment of human glioblastoma.
MicroRNAs (miRNAs) are small, non-coding 21–23 nucleotide RNAs which regulate gene expression by binding to the 3′-untranslated regions of their target mRNA molecules, to repress transcription or induce mRNA degradation [4]. MiRNAs have been showed to play important role in a wide variety of oncogenic activities, such as proliferation, invasion, angiogenesis and metastasis [5]. Recently studies have been suggested that the expression of many miRNAs are deregulated in a variety of cancers, including glioblastoma [6–11]. Using genome-wide approaches have revealed that miRNAs, such as miR-7, miR-124, miR-128, miR-221/222, and miR-21, are globally dysregulated in glioma [12–15]. MiR-29c is the member of miR-29 family composed of miR-29a, -29b and -29c. Downregulation of miR-29c has been reported in various human malignancies including nasopharyngeal carcinoma [16, 17], bladder transitional cell carcinoma [18], esophageal cancer [19], chronic lymphocytic leukemia [20], gastric cancer [21], cervical cancer [22], hepatocellular carcinoma [23] and cutaneous melanoma [24]. In addition, a number of studies have demonstrated that deregulation of miR-29c expression is involved in the initiation and progression of cancer by affecting tumor cell function, including growth, invasion, metastasis and anti-apoptosis. However, the expression of miR-29c in human glioma patients, and its functions in human glioma cells, as well as the molecular mechanisms by which miR-29c exerts its functions, has not been fully understood.
In the present study, we report that miR-29c was significantly downregulated in glioma cells and clinical glioma tissues, compared to normal human astrocytes (NHA) and non-tumor associated tissues. Moreover, we observed that enforced overexpression of miR-29c inhibited glioma cell proliferation, invasion, migration and angiogenesis abilities. In addition, we investigated the molecular mechanisms underlying miR-29c actions in glioma cells.
Materials and methods
Cell lines and human tissue samples
Primary NHA were purchased from the Sciencell Research Laboratories (Carlsbad, CA, USA) and cultured under the conditions as instructed by the manufacturer. Human glioma cell lines (U251, U87, T98G, A172, SHG44) were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Science. All glioma cells were cultured in DMEM supplemented with 10 % fetal bovine serum (Invitrogen, Shanghai, China) at 37 °C in 5 % CO2. Ten paired glioma tissues and adjacent non-tumor tissues from the same patients were obtained from the Department of Neurosurgery, the affiliated hospital of Xuzhou Medical College.
MiRNA transfection
The has-miR-29c mimic and has-miR-negative control (miR-NC) were purchased from GenePharma (Shanghai, China). Cells at 50–70 % confluence were transfected using lipofectamine reagent (Invitrogen, Shanghai, China). Transfection complexes were prepared according to the manufacturer’s instructions. The final concentration of miR-29c mimic or miR-NC for the transfection was 40 nmol/L.
RNA extraction and real-time quantitative PCR
Total miRNA from cultured cells and fresh surgical glioma tissues was extracted using the mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA) according to the manufacturer’s instructions. cDNA was synthesized from 5 ng of total RNA using the TaqMan miRNA reverse transcription Kit (Applied Biosystems, Foster City, CA), and the expression levels of miR-29c were quantified using miRNA-specific TaqMan MiRNA Assay Kit (Applied Biosystems) on the Applied Biosystems 7500 Sequence Detection System. Relative quantification of miRNA expression was calculated with the 2−ΔΔCt method [25] and analyzed initially using Opticon Monitor Analysis Software V2.02 software (MJ Research, Waltham, MA, USA), normalized to the expression of U6.
Western blot analysis
Western blotting analysis was performed according to standard methods as previously described [26], using anti-Cyclin D1, anti-Cyclin E, anti-p27, anti-p21, anti-MMP-2, anti-VEGF antibodies (all from Cell Signaling Technology, Danvers, MA, USA). The membranes were stripped and re-probed with an anti-β-actin antibody (Zhongshan Biotech, Beijing, China) as a loading control.
Cell proliferation assay
Cell proliferation was analyzed using a WST-8 Cell Counting Kit-8 (Beyotime, Nantong, China); 3 × 103 cells suspended in 100 μL DMEM medium containing 10 % fetal bovine serum were seeded in 96-well plates and incubated for 24, 48, 72 and 96 h; 10 μL CCK-8 solution was added to each well and the cultures were incubated at 37 °C for 1 h. Absorbance at 450 nm was measured on an ELX-800 spectrometer reader (Bio-Tek Instruments, Winooski, USA).
Migration assay
Cell migration was determined by using a modified two chamber migration assay with a pore size of 8 μm. For migration assay, 1 × 105 cells were seeded in serum-free medium in the upper chamber. After 12 h incubation at 37 °C, cells in the upper chamber were carefully removed with a cotton swab and the cells that had traversed the membrane were fixed in methanol, stained with Giemsa and photographed in five independent ×200 fields for each well.
Invasion assay
Cell invasion was assessed by matrigel precoated Transwell inserts (8.0 μm pore size with polyethylene terephthalate membrane) according to the manufacturer’s protocol. To assess invasion, filters were precoated with 30 μL of 5 mg/mL matrigel (BD Biosciences, NJ, USA). 1 × 105 cells were seeded in serum-free medium in the upper chamber. After 24 h incubation at 37 °C, cells in the upper chamber were carefully removed with a cotton swab and the cells that had traversed the membrane were fixed in methanol, stained with Giemsa and photographed in five independent ×200 fields for each well.
HUVEC growth and tube-formation assay
For HUVEC growth assay, 2 × 104 HUVECs suspended in 100 μL conditioned medium from either negative control cells or miR-29c overexpressed cells. HUVECs were seeded at a density of 2 × 104 in a 96-well culture plate and incubated at 37 °C in a humidified atmosphere containing 5 % CO2 for 24 h. Then, cell proliferation was detected according to the CCK-8 manufacturer’s instructions. For tube-formation assay, U251 and U87 cells were cultured in 6-well plate with fresh complete medium for 24 h, and the medium was collected and centrifuged to remove any cells debris before its use as a conditioned medium. 48-well plate was coated with Matrigel and kept in 37 °C for 30 min. Then, 2 × 104 HUVECs were suspended in 100 μL conditional medium and applied to the pre-coated 48-well plate. After incubation at 37 °C for another 24 h, the number of capillary-like tubes from three randomly chosen fields was counted.
Gelatin zymography
5 × 105 cells were seeded in 6-well plate for 24 h and transfected with the miR-29c and negative control mimic. Thereafter, cells were incubated in serum-free for an additional 24 h. Then performed as described previous [27].
ELISA assay for VEGF
The protein levels of VEGF in the supernatant were measured using the Quantikine human VEGF ELISA kit (NeoBioscience, Shanghai, China) according to the manufacturer’s instruction. In brief, the cells were seeded in 6-well plates and cultured to 90 % confluence, and then cells were switched to fresh medium. The supernatants were collected and the number of cells in each well was counted after 24 h. VEGF in the supernatant (100 μL) was determined, and normalized to the cell number. A serial dilution of human recombinant VEGF was included in each assay to obtain a standard curve.
Statistical analysis
All experiments were performed three times, and data were analyzed with SPSS 16.0 software (SPSS, Chicago, IL). The two-tailed Student’s t test was used to evaluate the significance of the differences between two groups of data in all pertinent experiments; Differences were considered significant when P < 0.05.
Results
MiR-29c is decreased in glioma cell lines and glioma tissues
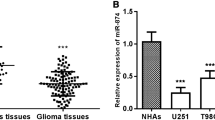
Real-time PCR analyses showed that expression of miR-29c was markedly lower in all five analyzed glioma cell lines, including U251, U87, T98G, A172, SHG44, as compared with that in NHA (Fig. 1a). To determine whether the miR-29c downregulation in glioma cell lines is also clinical relevant, we further examined the miR-29c expression in ten paired glioma tissues and adjacent non-tumor tissues from the same patients. As shown in Fig. 1b, comparative analysis showed that expression level of miR-29c was also reduced in all ten examined tumor tissues as compared to that in paired adjacent non-tumor tissues. Collectively, our results suggest that miR-29c is decreased in glioma. Interestingly, we also found miR-29a and miR-29b is decreased in glioma cell lines and glioma tissues, respectively (Supplementary Figs. S1, S2).
Analysis of miR-29c expression in glioma cell lines and tissues. a Real-time analysis of miR-29c expression in normal human astrocytes NHA and glioma cell lines, including U251, U87, T98G, A172, SHG44. The average miR-29c expression was normalized to U6 expression. b The expression of miR-29c was examined in paired primary glioma tissues (T) and glioma adjacent non-tumor tissues (ANT) from ten individual patients. The average miR-29c expression was normalized to U6 expression. Each bar represents the mean of three independent experiments. *P < 0.05
MiR-29c overexpression inhibits glioma cells proliferation and cell cycle
To investigate the biological role of miR-29c in glioma progression, we first transiently transfected miR-29c and miR-NC into U251 and U87 glioma cells. 24 h after transfection, cells were harvested for real-time PCR or subjected to cell proliferation. Real-time PCR results confirmed significant increase of miR-29c in either U251 or U87 cells transfected with miR-29c mimic (Fig. 2a). CCK-8 cell proliferation assays revealed that cell growth was reduced in miR-29c-transfected U251 and U87 cells compared with miR-NC-transfected control cells (Fig. 2b, c).
Overexpression of miR-29c reduces glioma cell proliferation in vitro. a 24 h after transfection, the expression of miR-29c in U251 and U87 glioma cells was evaluated by quantitative RT-PCR. b, c CCK-8 assays revealed that upregulation of miR-29c reduced cell proliferation of U251 and U87 glioma cells, compared to negative (NC)-transfected cells. d, e overexpression of miR-29c in U251 and U87 cells resulted in an increase of cell population at G1 phase by flow cytometry analysis. f, Western blot analysis of the relative protein of cyclin D1 cyclin E, p21 and p27 in miR-29c overexpression and NC group of U251 and U87 cells. β-Action was used as a whole cell protein loading control. g, Quantitative analysis of relative protein level of cyclin D1, cyclin E, p27 and p21 in glioma U251 and U87 cells. All experiments were carried out in triplicate. Data are shown as mean ± SE. **P < 0.01; ***P < 0.001
To determine if the reduced cell proliferation of miR-29c overexpressed cells is due to cell cycle arrest, we performed flow cytometry analysis. The results showed that overexpression of miR-29c in either U251 or U87 cells resulted in an increase of cell population at G1 phase (Fig. 2d, e). Moreover, immunoblot analysis showed increased p21 or p27 expression but decreased levels of cyclin D1 or cyclin E in glioma U251 and U87 cells that overexpression of miR-29c (Fig. 2f, g).
MiR-29c overexpression inhibits glioma cells migration, invasion and MMPs activity
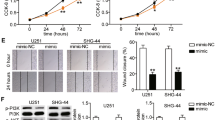
Then, we investigated the role of miR-29c in glioma cells migration and invasion. The results of cell migration assay showed that overexpression of miR-29c decreased cells migration ability of U251 and U87 cells by 49 and 47 %, respectively (Fig. 3a, b). Furthermore, overexpression of miR-29c inhibited the invasion ability of U251 and U87 cell by 77 and 61 %, respectively (Fig. 3c, d).
Effect of miR-29c overexpression on cell migration and invasion. a, b Reintroduction of miR-29c decreased cells migration ability of U251 and U87 cells by cell migration assay. c, d Reintroduction of miR-29c inhibited the invasion ability of U251 and U87 cell by matrigel cell invasion assay. e Gelatin zymography analysis of the relative enzyme activities of MMP-2 in miR-29c overexpression and NC group for both U251 and U87 cell lines. F Western blot analysis of the relative protein levels of MMP-2 in miR-29c overexpression and NC group of U251 and U87 cells. All experiments were carried out in triplicate. Data are shown as mean ± SE. **P < 0.01; ***P < 0.001
Since MMPs play a crucial role in cell migration and invasion, we then carried out the zymography assay to compare the activity of MMPs in miR-29c-overexpressing and control cells. As shown in Fig. 3e, MMP-2 gelatinolytic activity was dramatically decreased in miR-29c-overexpressing U251 and U87 cells compared with the control cells, respectively. Then, we performed Western blot to examine the MMP-2 expression in glioma cells. Western blot results showed that MMP-2 protein level was sharply decreased after miR-29c overexpressed in U251 and U87 cells (Fig. 3f).
Expression of miR-29c in glioma cells inhibited growth and tube formation of human umbilical vein endothelial cells and VEGF activity
To further determine the effect of miR-29c overexpression on angiogenic potential of human glioma cells, the angiogenic potential of the supernatant of U251 and U87 cells transfected with miR-NC or miR-29c were determined by human umbilical vein endothelial cells (HUVECs) growth assay and tube formation assay. The growth of HUVECs in conditioned medium from miR-29c-overexpression U251 and U87 cells was inhibited by 55 and 62 %, respectively, when compared with the corresponding negative control (Fig. 4a, b). The average number of complete tubular structures formed by HUVECs was significantly decreased by 72 and 68 % in conditioned medium from miR-29c-overexpressing U251 and U87 compared with negative control cells, respectively (Fig. 4c, d).
Reduction of angiogenesis in U251 and U87 cells by restoration of miR-29c expression. a, b CCK-8 cell proliferation assay was performed to detect the HUVECs proliferation. c, d representative pictures were taken in situ for tube formation in the supernatant of U251 and U87 cells. The degree of tube formation was assessed as the percentage of cell surface area versus total surface area. e Western blot analysis of the relative protein levels of VEGF in miR-29c overexpression and NC group of U251 and U87 cells. f The secretion of VEGF was determined by ELISA assay. All experiments were carried out in triplicate. Data are shown as mean ± SE. ***P < 0.001
To investigate the mechanism of miR-29c regulating angiogenesis, we performed Western blot and ELISA to detect the VEGF levels in glioma cells. Our data showed that overexpression of miR-29c was dramatically reduced VEGF protein level in U251 and U87 cells (Fig. 4e). A significant inhibition in VEGF secretion was observed in conditioned medium from U251 and U87 cells after overexpression of miR-29c (Fig. 4f).
Discussion
MiRNAs function as post-transcriptional gene regulators in regulating various physiological and pathological events. MiRNAs abnormalities are thought to play important roles in cancer development [4]. Recent studies have showed that many miRNAs are down-regulated in tumors when compared to normal tissues [28]. Several recent studies have demonstrated that the expression of miRNAs is deregulated in gliomas. Ciafre et al. [11] examined the alterations of 245 miRNAs in World Health Organization grade IV GBM, in which miR-221 was up-regulated, whereas miR-128, miR-181a, miR-181b, and miR-181c were down-regulated in the GBM specimens. Chan et al. [29] showed that expression of miR-21 was markedly up-regulated in primary GBMs and glioma cell lines compared with normal brain tissues and nontumor glial cells. In contrast, miR-124 and miR-137 were found to be significantly decreased in grade III GBMs compared with adjacent nontumor brain tissues [30]. Additionally, miR-128, miR-181a, miR-181b, and miR-451 were also found to be down-regulated in glioma tissues and glioma cell lines compared with normal brain tissues [31–33]. These findings suggest that miRNAs are involved in glioma development and progression. The key finding of the current study is that miR-29c expression is markedly downregulated in glioma cells and clinical glioma tissues as compared to NHA and normal brain tissues (Fig. 1). Consistent with previous studies, miR-29c has been reported to be downregulated in a variety of different tumor types including nasopharyngeal carcinoma [16, 17], bladder transitional cell carcinoma [18], esophageal cancer [19], chronic lymphocytic leukemia [20], gastric cancer [21], cervical cancer [22], hepatocellular carcinoma [23] and cutaneous melanoma [24]. Matsuo et al. [21] showed that miR-29c downregulation is probably not associated with DNA methylation or histone deacetylation. Chang et al. [34] has been showed that oncogene c-myc binds the promoter sequence of miR-29c and inhibits its transcription, leading to its downregulated expression in breast cancer and facilitating the tumorigenesis. This mechanism might be a cause to leading to downregulated expression of miR-29c in glioma. However, further studies will be needed to clarify these issues.
Uncontrolled cell proliferation is a hallmark of cancer. Studies showed that cancer cells ignore antigrowth signals to progress through G1 phase [35]. In gastric carcinoma cell, miR-29c has been shown to suppress cell proliferation by regulating progression of cell cycle [21]. The development of a tumor requires both the activation of oncogenes and the inactivation of tumor suppressor genes, which triggers the uncontrolled proliferation of cancer cells and prevents cancer cell apoptosis. We found that the ability of cell proliferation was drastically decreased after miR-29c overexpression in glioma cells, which is due to inhibition of cell cycle progression by arresting cell cycle at G1 phase, but not inducing apoptosis (Fig. 1b–e; Supplementary Fig. S3). Cell cycle progression is strictly controlled by cyclins and cyclin-dependent kinase (CDK) inhibitors [36]. Since the cyclins (cyclin D1, cyclin E) and CDK inhibitors (p27, p21) play key roles in G1/S phase transition during cell cycle progression [37]. Our data showed that overexpression of miR-29c increased p27 and p21 expression, which may result in glioma cells to arrest at G1 phase (Fig. 2e). Meanwhile, miR-29c overexpression decreased expression of both cyclin D1 and cyclin E (Fig. 2f). This might be due to the elevated expression of p27 and p21 which inhibits the expression of cyclins. Our finding is consistent with Ding et al. [38] who found that miR-29c induced cell cycle arrest in esophageal squamous cell carcinoma by modulating the expression of cyclin E.
Glioblastomas are able to not only proliferation but also invade the surrounding brain tissue, leading to very poor prognosis for patients suffering from glioma [39]. Previous studies demonstrated that ectopic expression of miR-29c inhibited nasopharyngeal carcinoma cell migration and invasion in vitro and suppressed the formation of lung metastases in vivo [17]. In this study, our data showed that reintroduction of miR-29c dramatically repressed the migration and invasion of glioma cells in vitro (Fig. 3a–d). MiR-29c has been reported to affect motility and migration through inhibiting the expression of ECM proteins in previous study [16]. Matrix metalloproteinase (MMPs) are a family of zinc-dependent endopeptidase that are capable of degrading components of the ECM, allowing cancer cells to migrate and invade [40]. To determine how miR-29c inhibits glioma cells migration and invasion, we focused on elucidating the relationship between miR-29C and MMPs. Here, we found that miR-29c overexpression significantly suppressed the expression and bioactivities of MMP-2 in glioma U251 and U87 cells (Fig. 3e, f). MMP2 is thought to be key enzymes involved in the degradation of type IV, which is a component of the ECM [41]. High levels of MMP2 in tissues are associated with tumor cell invasion, including glioma [42]. Hence, our studies indicate that miR-29c may suppress glioma cell invasion and migration by decreasing MMP-2 protein expression and enzyme activity. However, it remains to be elucidated how miR-29c regulates MMP-2 expression and activity and its signal pathway to regulate glioma cell invasion.
Tumor growth and metastasis formation depends on an adequate blood supply. As neoplasms grow larger, blood supply to the tumor is often ensured by new vessel formation, a process termed angiogenesis [43]. Tumor cells produce angiogenesis inducers, represented by vascular epithelial growth factors (VEGF), which play a crucial role in endothelial survival, proliferation and new vessel sprouting [44]. In the present study, we found that miR-29c inhibited HUVECs growth and tube formation in vitro (Fig. 4a–d). Then, we detected the expression and secretion of VEGF after miR-29c overexpression. Our data showed that VEGF expression and secretion was decreased by restoration of miR-29c (Fig. 4e, f). These results suggested that miR-29c suppresses blood vessel formation by regulating VEGF secretion.
In summary, we demonstrated that miR-29c plays an important role in human glioma pathogenesis. Decreased miR-29c expression may contribute to tumor progression by enhancing cell proliferation, invasion, migration and angiogenesis. Our results imply that targeting of the miR-29c pathway may constitute a potential treatment modality for glioma.
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109. doi:10.1007/s00401-007-0243-4
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups, National Cancer Institute of Canada Clinical Trials Group (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466. doi:10.1016/S1470-2045(09)70025-7
Giese A, Bjerkvig R, Berens ME, Westphal M (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21(8):1624–1636. doi:10.1200/JCO.2003.05.063
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Zaravinos A, Radojicic J, Lambrou GI, Volanis D, Delakas D, Stathopoulos EN, Spandidos DA (2012) Expression of miRNAs involved in angiogenesis, tumor cell proliferation, tumor suppressor inhibition, epithelial–mesenchymal transition and activation of metastasis in bladder cancer. J Urol 188(2):615–623. doi:10.1016/j.juro.2012.03.122
Metzler M, Wilda M, Busch K, Viehmann S, Borkhardt A (2004) High expression of precursor microRNA-155/BIC RNA in children with Burkitt lymphoma. Genes Chromosomes Cancer 39(2):167–169. doi:10.1002/gcc.10316
Michael MZ, O’ Connor SM, van Holst Pellekaan NG, Young GP, James RJ (2003) Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res 1(12):882–891
Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T (2004) Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 64(11):3753–3756. doi:10.1158/0008-5472.CAN-04-0637
Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Menard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM (2005) MicroRNA gene expression deregulation in human breast cancer. Cancer Res 65(16):7065–7070. doi:10.1158/0008-5472.CAN-05-1783
Murakami Y, Yasuda T, Saigo K, Urashima T, Toyoda H, Okanoue T, Shimotohno K (2006) Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene 25(17):2537–2545. doi:10.1038/sj.onc.1209283
Ciafre SA, Galardi S, Mangiola A, Ferracin M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM, Farace MG (2005) Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun 334(4):1351–1358. doi:10.1016/j.bbrc.2005.07.030
Chen Y, Liu W, Chao T, Zhang Y, Yan X, Gong Y, Qiang B, Yuan J, Sun M, Peng X (2008) MicroRNA-21 down-regulates the expression of tumor suppressor PDCD4 in human glioblastoma cell T98G. Cancer Lett 272(2):197–205. doi:10.1016/j.canlet.2008.06.034
Zhang C, Zhang J, Hao J, Shi Z, Wang Y, Han L, Yu S, You Y, Jiang T, Wang J, Liu M, Pu P, Kang C (2012) High level of miR-221/222 confers increased cell invasion and poor prognosis in glioma. J Transl Med 10:119. doi:10.1186/1479-5876-10-119
Wang W, Dai LX, Zhang S, Yang Y, Yan N, Fan P, Dai L, Tian HW, Cheng L, Zhang XM, Li C, Zhang JF, Xu F, Shi G, Chen XL, Du T, Li YM, Wei YQ, Deng HX (2013) Regulation of epidermal growth factor receptor signaling by plasmid-based microRNA-7 inhibits human malignant gliomas growth and metastasis in vivo. Neoplasma 60(3):274–283. doi:10.4149/neo_2013_036
Papagiannakopoulos T, Friedmann-Morvinski D, Neveu P, Dugas JC, Gill RM, Huillard E, Liu C, Zong H, Rowitch DH, Barres BA, Verma IM, Kosik KS (2012) Pro-neural miR-128 is a glioma tumor suppressor that targets mitogenic kinases. Oncogene 31(15):1884–1895. doi:10.1038/onc.2011.380
Sengupta S, den Boon JA, Chen IH, Newton MA, Stanhope SA, Cheng YJ, Chen CJ, Hildesheim A, Sugden B, Ahlquist P (2008) MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci USA 105(15):5874–5878. doi:10.1073/pnas.0801130105
Liu N, Tang LL, Sun Y, Cui RX, Wang HY, Huang BJ, He QM, Jiang W, Ma J (2013) MiR-29c suppresses invasion and metastasis by targeting TIAM1 in nasopharyngeal carcinoma. Cancer Lett 329(2):181–188. doi:10.1016/j.canlet.2012.10.032
Friedman JM, Liang G, Liu CC, Wolff EM, Tsai YC, Ye W, Zhou X, Jones PA (2009) The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res 69(6):2623–2629. doi:10.1158/0008-5472.CAN-08-3114
Guo Y, Chen Z, Zhang L, Zhou F, Shi S, Feng X, Li B, Meng X, Ma X, Luo M, Shao K, Li N, Qiu B, Mitchelson K, Cheng J, He J (2008) Distinctive microRNA profiles relating to patient survival in esophageal squamous cell carcinoma. Cancer Res 68(1):26–33. doi:10.1158/0008-5472.CAN-06-4418
Stamatopoulos B, Meuleman N, Haibe-Kains B, Saussoy P, Van Den Neste E, Michaux L, Heimann P, Martiat P, Bron D, Lagneaux L (2009) microRNA-29c and microRNA-223 down-regulation has in vivo significance in chronic lymphocytic leukemia and improves disease risk stratification. Blood 113(21):5237–5245. doi:10.1182/blood-2008-11-189407
Matsuo M, Nakada C, Tsukamoto Y, Noguchi T, Uchida T, Hijiya N, Matsuura K, Moriyama M (2013) MiR-29c is downregulated in gastric carcinomas and regulates cell proliferation by targeting RCC2. Mol Cancer 12(1):15. doi:10.1186/1476-4598-12-15
Li Y, Wang F, Xu J, Ye F, Shen Y, Zhou J, Lu W, Wan X, Ma D, Xie X (2011) Progressive miRNA expression profiles in cervical carcinogenesis and identification of HPV-related target genes for miR-29. J Pathol 224(4):484–495. doi:10.1002/path.2873
Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y, Jia WH, Zhuang SM (2010) Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 51(3):836–845. doi:10.1002/hep.23380
Nguyen T, Kuo C, Nicholl MB, Sim MS, Turner RR, Morton DL, Hoon DS (2011) Downregulation of microRNA-29c is associated with hypermethylation of tumor-related genes and disease outcome in cutaneous melanoma. Epigenetics 6(3):388–394
Tanaka H, Sasayama T, Tanaka K, Nakamizo S, Nishihara M, Mizukawa K, Kohta M, Koyama J, Miyake S, Taniguchi M, Hosoda K, Kohmura E (2013) MicroRNA-183 upregulates HIF-1alpha by targeting isocitrate dehydrogenase 2 (IDH2) in glioma cells. J Neurooncol 111(3):273–283. doi:10.1007/s11060-012-1027-9
Bai J, Mei PJ, Liu H, Li C, Li W, Wu YP, Yu ZQ, Zheng JN (2012) BRG1 expression is increased in human glioma and controls glioma cell proliferation, migration and invasion in vitro. J Cancer Res Clin Oncol 138(6):991–998. doi:10.1007/s00432-012-1172-8
Mei PJ, Bai J, Liu H, Li C, Wu YP, Yu ZQ, Zheng JN (2011) RUNX3 expression is lost in glioma and its restoration causes drastic suppression of tumor invasion and migration. J Cancer Res Clin Oncol 137(12):1823–1830. doi:10.1007/s00432-011-1063-4
Armeanu-Ebinger S, Herrmann D, Bonin M, Leuschner I, Warmann SW, Fuchs J, Seitz G (2012) Differential expression of miRNAs in rhabdomyosarcoma and malignant rhabdoid tumor. Exp Cell Res 318(20):2567–2577. doi:10.1016/j.yexcr.2012.07.015
Chan JA, Krichevsky AM, Kosik KS (2005) MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65(14):6029–6033. doi:10.1158/0008-5472.CAN-05-0137
Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello JF, Bergers G, Weiss WA, Alvarez-Buylla A, Hodgson JG (2008) miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med 6:14. doi:10.1186/1741-7015-6-14
Gal H, Pandi G, Kanner AA, Ram Z, Lithwick-Yanai G, Amariglio N, Rechavi G, Givol D (2008) MIR-451 and imatinib mesylate inhibit tumor growth of glioblastoma stem cells. Biochem Biophys Res Commun 376(1):86–90. doi:10.1016/j.bbrc.2008.08.107
Zhang Y, Chao T, Li R, Liu W, Chen Y, Yan X, Gong Y, Yin B, Qiang B, Zhao J, Yuan J, Peng X (2009) MicroRNA-128 inhibits glioma cells proliferation by targeting transcription factor E2F3a. J Mol Med (Berl) 87(1):43–51. doi:10.1007/s00109-008-0403-6
Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z, You Y (2008) hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res 1236:185–193. doi:10.1016/j.brainres.2008.07.085
Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, Mendell JT (2008) Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 40(1):43–50. doi:10.1038/ng.2007.30
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Hochegger H, Takeda S, Hunt T (2008) Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat Rev Mol Cell Biol 9(11):910–916. doi:10.1038/nrm2510
Lee MH, Yang HY (2003) Regulators of G1 cyclin-dependent kinases and cancers. Cancer Metastasis Rev 22(4):435–449
Ding DP, Chen ZL, Zhao XH, Wang JW, Sun J, Wang Z, Tan FW, Tan XG, Li BZ, Zhou F, Shao K, Li N, Qiu B, He J (2011) miR-29c induces cell cycle arrest in esophageal squamous cell carcinoma by modulating cyclin E expression. Carcinogenesis 32(7):1025–1032. doi:10.1093/carcin/bgr078
Pham K, Chauviere A, Hatzikirou H, Li X, Byrne HM, Cristini V, Lowengrub J (2012) Density-dependent quiescence in glioma invasion: instability in a simple reaction-diffusion model for the migration/proliferation dichotomy. J Biol Dyn 6(Suppl 1):54–71. doi:10.1080/17513758.2011.590610
Groblewska M, Siewko M, Mroczko B, Szmitkowski M (2012) The role of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in the development of esophageal cancer. Folia Histochem Cytobiol 50(1):12–19. doi:10.2478/18691
Tapia A, Salamonsen LA, Manuelpillai U, Dimitriadis E (2008) Leukemia inhibitory factor promotes human first trimester extravillous trophoblast adhesion to extracellular matrix and secretion of tissue inhibitor of metalloproteinases-1 and -2. Hum Reprod 23(8):1724–1732. doi:10.1093/humrep/den121
Sun ZF, Wang L, Gu F, Fu L, Li WL, Ma YJ (2012) Expression of Notch1, MMP-2 and MMP-9 and their significance in glioma patients. Zhonghua Zhong Liu Za Zhi 34(1):26–30
Dhup S, Dadhich RK, Porporato PE, Sonveaux P (2012) Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des 18(10):1319–1330
Yancopoulos GD (2010) Clinical application of therapies targeting VEGF. Cell 143(1):13–16. doi:10.1016/j.cell.2010.09.028
Acknowledgments
This project is supported by Grants from the Health Department Foundation of Jiangsu province (No. H201019).
Conflict of interest
We declare that we have no conflict of interest.
Ethics statement
This study was performed under a protocol approved by the Institutional Review Boards of The Affiliated Hospital of Xuzhou Medical College and all examinations were performed after obtaining written informed consents.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yue-chao Fan and Peng-jin Mei contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2013_1223_MOESM1_ESM.tif
Fig. S1 Analysis of miR-29a expression in glioma cell lines and tissues. a Real-time analysis of miR-29a expression in normal human astrocytes NHA and glioma cell lines, including U251, U87, T98G, A172, SHG44. The average miR-29a expression was normalized to U6 expression. b The expression of miR-29a was examined in paired primary glioma tissues (T) and glioma adjacent non-tumor tissues (ANT) from ten individual patients. The average miR-29a expression was normalized to U6 expression. Each bar represents the mean of three independent experiments. *P < 0.05. Supplementary material 1 (TIFF 188 kb)
11060_2013_1223_MOESM2_ESM.tif
Fig. S2. Analysis of miR-29b expression in glioma cell lines and tissues. a Real-time analysis of miR-29b expression in normal human astrocytes NHA and glioma cell lines, including U251, U87, T98G, A172, SHG44. The average miR-29b expression was normalized to U6 expression. b The expression of miR-29b was examined in paired primary glioma tissues (T) and glioma adjacent non-tumor tissues (ANT) from ten individual patients. The average miR-29b expression was normalized to U6 expression. Each bar represents the mean of three independent experiments. *P < 0.05. Supplementary material 2 (TIFF 188 kb)
11060_2013_1223_MOESM3_ESM.tif
Fig. S3. a, b Western blot analysis of the relative protein level of Pro-caspase-3, Cleaved-caspase-3, Pro-caspase-9, Cleaved-caspase-9, Bax, Bcl-2 and Actin in miR-29c overexpressed and control group for both U251 and U87 cell lines. Supplementary material 3 (TIFF 930 kb)
Rights and permissions
About this article
Cite this article
Fan, Yc., Mei, Pj., Chen, C. et al. MiR-29c inhibits glioma cell proliferation, migration, invasion and angiogenesis. J Neurooncol 115, 179–188 (2013). https://doi.org/10.1007/s11060-013-1223-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1223-2