Abstract
Elderly or frail patients with high-grade gliomas (HGG) can be effectively treated with an abbreviated course of radiation therapy (RT) consisting of 40 Gy in 15 fractions. Concurrent temozolomide (TMZ) improves survival in non-elderly patients with glioblastoma treated with standard schedule of 60 Gy in 30 fractions. We describe our institutional experience of combining abbreviated RT with concurrent TMZ for treatment of HGG. Between 1/1/2004 and 2/5/2010 31 patients were treated. Survival was estimated with the Kaplan–Meier method. Toxicity was scored according to CTCAE 3.0. Median age was 66 years (range 32–90), and 17 patients had Karnofsky performance score <70. At the time of analysis, 30 patients (98 %) had died, with a followup of 14 months in the surviving patient. Median survival was 11 months (range 1–20), and 41 % of patients were alive at 12 months. Thirty patients (97 %) had a decreased corticosteroid requirement after completion of therapy. Only one new hospitalization for worsening neurologic status was required during therapy. Grade 3–4 hematologic toxicity occurred in 11 patients. Abbreviated RT with concurrent TMZ provides a clinical benefit, is safe and tolerable in patients of advanced age or poor functional status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant gliomas are the most common primary brain tumor with highest incidence among the elderly. They are universally fatal and are generally associated with a rapidly progressive course, especially in the elderly or patients with low performance status [1, 2]. Radiation therapy (RT) improves survival in patients with malignant gliomas as compared to supportive care alone [3, 4]. Given the short expected survival of patients in the poor prognosis group, efforts have been made to shorten the RT treatment time [5–8]. Based on a randomized trial, an abbreviated schedule of 40 Gy/15 fractions is comparable to the standard radiation schedule of 60 Gy/30 fractions with regard to survival and post-treatment quality of life in patients with glioblastoma multiforme (GBM) age 60 or over [9].
Temozolomide (TMZ), an oral alkylating agent with good CNS penetrance has been shown to improve survival with minimal hematologic toxicity when given concurrently with conventionally fractionated radiation (60 Gy) in patients under 70 and with good functional status [10]. The efficacy of TMZ together with its favorable toxicity profile and ease of administration, has led our institution to offer it to patients in the poor prognosis group, due to advanced age or poor functional status, undergoing abbreviated RT. Here we review our experience in order to describe the safety and tolerability of this approach.
Methods and materials
After obtaining IRB approval, our institutional database was searched for patients with high-grade gliomas (HGG) who received 15 or fewer fractions of radiation between 1/1/2004 and 2/5/2010. Thirty-one consecutive patients scheduled for abbreviated RT (37.5–41.4 Gy in 14–15 fractions) with concurrent TMZ were identified and their records reviewed.
Pre-radiotherapy variables recorded included patient’s age, gender, date and type of surgery, histology of the tumor, daily steroid requirement, Karnofsky performance score (KPS) and baseline hematology profile. All the hematologic laboratory data for the weeks on treatment were queried. Post-treatment variables included use of adjuvant chemotherapy, daily steroid requirement within 2 weeks, KPS within 3 months, number and length of hospitalizations lasting over 24 h within 2 months of completing concurrent therapy. Pre- and post-RT KPS values were available in the medical record for 25 patients. For the remaining five patients KPS was estimated as < or ≥70 to characterize the cohort only (Table 1); these data were not used for pre- and post-RT KPS comparison. Median survival from the date of surgery (biopsy or resection) was estimated with the Kaplan–Meier method.
Results
Patient and tumor characteristics
Among the 31 consecutive patients scheduled to receive abbreviated RT with concurrent TMZ, all were offered this fractionation scheme because of advanced age or poor KPS. The median age was 66 years (range 32–90 years), and 19 patients (61 %) were 60 or older (Table 1). Seventeen patients had KPS <70, and all those younger than 60 were in this group. Twenty-six patients had a histopathologic diagnosis of WHO grade 4 glioma, including 25 glioblastomas and one gliosarcoma. Two patients had anaplastic astrocytomas, one patient had a HGG with oligodendromal and astrocytic features, and two patients had gliomatosis cerebri. Promoter methylation status of the O6-methylguanine-DNA methyltransferase gene was unknown in the majority of cases (n = 28).
Treatment characteristics
Debulking surgery was performed in 17 patients (55 %) and biopsy was the only surgery in 14 patients (45 %). RT was delivered using conventional 2D technique (n = 9), 3D conformal technique (n = 2), IMRT (n = 18) and a combination of techniques (n = 2). Computer tomography-based simulation was performed with patients immobilized in the supine position using a thermoplastic mask. 3D and IMRT treatment plans were generated using our in-house treatment planning system. Twenty-seven patients received 40 Gy in 15 fractions, two patients received 37.5 Gy and 41.4 Gy in 15 fractions, each. One patient with prior history of receiving 14 Gy in seven fractions 7 months previously (before discontinuing to pursue alternative therapy), received 37.4 Gy in 14 fractions at the time of his second presentation to our institution with disease progression. One patient was prescribed 40 Gy in 15 fractions, but after five fractions she required a treatment break due to unrelated intercurrent medical issues. She eventually completed an additional five fractions of 350 cGy for a total dose of 30.85 Gy. Thus, a full course of radiotherapy was completed as initially prescribed in 30 of 31 cases, and it was modified and completed as a more hypofractionated course by one patient.
Concurrent TMZ was prescribed at a dose of 75 mg/m2 per day, 7 days per week from the first to the last day of radiotherapy for a median of 20 days (range 4–26 days). Twenty-two patients received adjuvant TMZ starting within 1 month of completing RT for a median of 4 months using the following dosing schedules: 150–200 mg/m2 during days 1–5 of a 28 day cycle (20 patients), 150 mg/m2 on days 1–7 and 15–21 of a 28 day cycle (one patient), and 50 mg/m2 daily (one patient). One patient received concurrent and adjuvant bevacizumab at a dose of 10 mg/kg every 2 weeks for 4.5 months.
Survival and quality of life
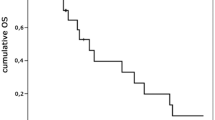
At the time of the final analysis, 30 patients (98 %) had died, with follow up of 14 months in the remaining patient. Median survival was 11 months (range 1–20 months) (Fig. 1). Survival was the same when 25 patients with GBMs were considered separately (data not shown). The patient who received bevacizumab survived 9 months. 41 % of the patients were alive at 12 months.
After completion of abbreviated RT with concurrent TMZ, 25 patients (81 %) had decreased and five patients (16 %) had stable daily corticosteroid requirements (Tables 2, 3). The median KPS after diagnosis-defining surgery and before initiation of RT was 70 (Interquartile range, IQR 60–70) (Tables 1, 2). In keeping with well-established trends [9], the median KPS declined to 60 (IQR 55–67.5) within 1–3 months after completion of RT. KPS was stable or improved in 16 of the 25 evaluable patients (64 %) (Table 3). The patient who received bevacizumab was tapered off corticosteroids within 2 weeks of finishing RT, and had a stable KPS of 80 at 2 months.
Treatment tolerability
Treatment interruptions and new hospitalizations were assessed to evaluate treatment tolerability. Fifteen patients were hospitalized during initiation of RT, but only 11 of them remained hospitalized for the duration of treatment, 5 patients resided in subacute rehab facilities and 10 lived at home. Of ten subjects with outpatient status at the start of RT, five new hospitalizations were recorded for neurologic decline (one patient), dapsone-associated acute renal failure and hemolysis (one patient), urosepsis with absolute neutrophil count of 1,300 cells/mcL (one patient), spontaneous retroperitoneal hemorrhage in the absence of thrombocytopenia (one patient), and manic episode (one patient) (Table 4). Two patients (6.5 %), including the patient with dapsone toxicity and patient with retroperitoneal hemorrhage, required radiotherapy interruptions for management of acute medical problems, lasting 16 and 10 days, respectively. Twenty-nine patients completed a full course of concurrent TMZ ranging from 15 to 26 days. TMZ was discontinued after four doses in the patient with dapsone-associated hemolysis. One patient had a 7 day interruption in concurrent TMZ in the setting of non-neutropenic urosepsis.
Hematologic toxicity
Among 28 patients, who had three or more complete blood counts available while receiving RT, 12 Grade 3 or 4 hematologic events in 11 patients were recorded. Grade 3 anemia and grade 4 lymphocytopenia occurred in one patient with underlying T cell lymphoma. Additional ten patients developed grade 3 lymphocytopenia. All patients received Bactrim (25 patients) or another form of PCP prophylaxis (six patients). There were no grade 5 events noted.
Discussion
As a tertiary referral center, our institution treats many patients with HGG. Many of these patients are elderly or frail, and thus not eligible for standard post-operative therapy consisting of 60 Gy and concurrent TMZ. With the goal of extending meaningful life without excessive toxicity, we offer these patients an abbreviated course of RT (40 Gy in 15 fractions) together with concurrent TMZ. This retrospective analysis of our experience shows this approach to be safe and tolerable.
RT is associated with improved survival and quality of life in patients with HGG and is an integral part of HGG treatment [4]. Yet the treatment can be especially taxing and burdensome for the elderly who have a poor expected survival and therefore, will spend a larger proportion of their remaining life undergoing treatment. A randomized trial comparing 60 Gy versus best supportive care in patients 70 or older, showed a significant survival benefit to RT, which prolonged life by more than twice the amount of time spent on treatment (2.6 months) [4]. Furthermore, an abbreviated schedule of 40 Gy in 15 fractions, was shown in a randomized trial to be non inferior to the standard course in a group of patients 60 or older, in terms of survival and several quality of life measures, while significantly reducing treatment time [9].
In patients with GBMs under 70 and with good performance status, TMZ has been shown to significantly improve survival over radiation alone [10]. TMZ is easy to administer and has a favorable toxicity profile. It is been well tolerated by GBM patients over 70 when administered alone [11, 12] or after radiation [13]. In fact, adjuvant TMZ was shown in a prospective cohort study to improve survival in patients with GBM 65 or older receiving standard RT (60 Gy) from 11.2 to 14.9 months [13].
However, there is some suggestion that the relative benefit of the concurrent RT/TMZ may be reduced with age or RPA class [2, 14]. Therefore the therapeutic ratio of concurrent RT and TMZ in the elderly has been a subject of some debate. Several single arm studies evaluating the standard course of RT (60 Gy) with concurrent TMZ with or without adjuvant TMZ in the elderly showed OS times of 10.6–13.7 months (Table 5) [15–18]. This significantly exceeds the OS seen in the studies of the elderly treated with radiation alone, however it is of the same magnitude as OS seen in the sequential setting when 60 Gy was followed by adjuvant TMZ [13]. In an effort to reduce the treatment time for the poor-prognosis group of patients, several institutions have been evaluating the use of abbreviated RT with concurrent TMZ. These single institution retrospective series have shown OS times ranging from 6.9 to 12.4 months [19–21]. To confound the issue, comparisons to same institution control groups receiving RT alone have yielded conflicting results as to the benefit of concurrent TMZ [20, 21]. Such analyses are complicated by unbalanced and small comparison groups, and should, therefore, be interpreted cautiously.
Here we present a retrospective analysis of 31 elderly or frail patients treated at our center with abbreviated RT (40 Gy/15 fractions) with concurrent TMZ. Although comparisons among different studies is difficult, especially when relatively small and heterogeneous patient populations are compared, the OS in our group (median age 66, median KPS 70) exceeds that of a group of older patients (median age 71, median KPS 70) treated with the same schedule of RT alone [9], and is comparable to the cohorts of a similar age and KPS treated with abbreviated RT or standard schedule with concurrent and adjuvant TMZ [15, 16, 19–22] (Table 5).
Concurrent therapy was well tolerated with one case of grade 3 anemia, and no grade 3 or higher neutropenia or thrombocytopenia. Two patients had interruptions in the radiotherapy, but 30 of 31 completed a course as planned, and one patient completed a more hypofractionated course after a modification made while on treatment.
Poor-prognosis HGG patients are quite debilitated and frequent hospital visits and hospitalizations are required. Half of the patients in our study were hospitalized before initiation of chemoradiation. Although it is possible that in some cases hospitalization was necessitated by daily radiation treatments, it also attests to the poor performance status of these patients and helps to define the burden of the disease on the individual. Of five new hospitalizations recorded during the concurrent phase of therapy, only one may be directly related to therapy in a patient who had a temporary worsening of neurologic function requiring a short inpatient stay, but not discontinuation of therapy.
Unfortunately, the KPS continues to decline within months among the elderly with GBM despite most effective treatment [9]. Therefore, quality of the remaining life is an important factor in choosing the most appropriate therapy for these patients. Using daily corticosteroid requirements and KPS as broad surrogate measures of neurologic function we noted a favorable response to therapy with 97 % of patients having decreased or stable corticosteroid requirement and 64 % of patients having improved or stable KPS. Moreover, only four of 31 patients required an intensive level of medical care offered by an inpatient unit within two months after undergoing concurrent treatment, while most patients could be treated in the outpatient setting. Meaningful time with their loved ones in a home or residential setting is an important treatment goal in this patient population. Combining an abbreviated radiotherapy course with concurrent TMZ helps patients achieve this goal by allowing them to finish their treatment faster with reasonable outcomes. Some concerns have been raised regarding the detrimental effect of concurrent therapy on neurocognitive decline in the elderly or frail [16]. If true, this may be further exacerbated by increase in fraction size associated with abbreviated therapy warranting future prospective evaluation of neurocognitive function.
A currently open phase III EORTC 26062–22061 trial is aimed at assessing the efficacy of concurrent TMZ with abbreviated RT verses abbreviated RT alone in the elderly in a definitive manner. In addition, TMZ alone has recently emerged as a potential alternative to RT in this population [23, 24], suggesting further avenues for future investigations to define the most appropriate treatment strategy for poor-prognosis patients. In the meantime, we believe that the concurrent approach described here represents a reasonable option for frail and/or elderly patients with HGG.
References
Curran WJ Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE et al (1993) Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 85:704–710
Mirimanoff RO, Gorlia T, Mason W, Van den Bent MJ, Kortmann RD, Fisher B, Reni M, Brandes AA, Curschmann J, Villa S, Cairncross G, Allgeier A, Lacombe D, Stupp R (2006) Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol 24:2563–2569
Walker MD, Green SB, Byar DP, Alexander E Jr, Batzdorf U, Brooks WH, Hunt WE, MacCarty CS, Mahaley MS Jr, Mealey J Jr, Owens G, Ransohoff J II, Robertson JT, Shapiro WR, Smith KR Jr, Wilson CB, Strike TA (1980) Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 303:1323–1329
Keime-Guibert F, Chinot O, Taillandier L, Cartalat-Carel S, Frenay M, Kantor G, Guillamo JS, Jadaud E, Colin P, Bondiau PY, Menei P, Loiseau H, Bernier V, Honnorat J, Barrie M, Mokhtari K, Mazeron JJ, Bissery A, Delattre JY (2007) Radiotherapy for glioblastoma in the elderly. N Engl J Med 356:1527–1535
Slotman BJ, Kralendonk JH, van Alphen HA, Kamphorst W, Karim AB (1996) Hypofractionated radiation therapy in patients with glioblastoma multiforme: results of treatment and impact of prognostic factors. Int J Radiat Oncol Biol Phys 34:895–898
Jeremic B, Shibamoto Y, Grujicic D, Milicic B, Stojanovic M, Nikolic N, Dagovic A, Aleksandrovic J (1999) Short-course radiotherapy in elderly and frail patients with glioblastoma multiforme. A phase II study. J Neurooncol 44:85–90
Idbaih A, Taillibert S, Simon JM, Psimaras D, Schneble HM, Lopez S, Lang P, Toubiana T, Feuvret L, Delattre JY, Mazeron JJ (2008) Short course of radiation therapy in elderly patients with glioblastoma multiforme. Cancer Radiother 12:788–792
Bauman GS, Gaspar LE, Fisher BJ, Halperin EC, Macdonald DR, Cairncross JG (1994) A prospective study of short-course radiotherapy in poor prognosis glioblastoma multiforme. Int J Radiat Oncol Biol Phys 29:835–839
Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, Chan A, Fisher B, Fulton D, Gulavita S, Hao C, Husain S, Murtha A, Petruk K, Stewart D, Tai P, Urtasun R, Cairncross JG, Forsyth P (2004) Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol 22:1583–1588
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Chinot OL, Barrie M, Frauger E, Dufour H, Figarella-Branger D, Palmari J, Braguer D, Hoang-Xuan K, Moktari K, Peragut JC, Martin PM, Grisoli F (2004) Phase II study of temozolomide without radiotherapy in newly diagnosed glioblastoma multiforme in an elderly populations. Cancer 100:2208–2214
Glantz M, Chamberlain M, Liu Q, Litofsky NS, Recht LD (2003) Temozolomide as an alternative to irradiation for elderly patients with newly diagnosed malignant gliomas. Cancer 97:2262–2266
Brandes AA, Vastola F, Basso U, Berti F, Pinna G, Rotilio A, Gardiman M, Scienza R, Monfardini S, Ermani M (2003) A prospective study on glioblastoma in the elderly. Cancer 97:657–662
Laperriere N OCC, Ding K (2008) Rationale and design for a phase III randomized controlled trial in elderly patients with glioblastoma multiforme. NCIC CTG CE 6 13th Biannual Canadian Neuro-Oncology Meeting
Combs SE, Wagner J, Bischof M, Welzel T, Wagner F, Debus J, Schulz-Ertner D (2008) Postoperative treatment of primary glioblastoma multiforme with radiation and concomitant temozolomide in elderly patients. Int J Radiat Oncol Biol Phys 70:987–992
Brandes AA, Franceschi E, Tosoni A, Benevento F, Scopece L, Mazzocchi V, Bacci A, Agati R, Calbucci F, Ermani M (2009) Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer 115:3512–3518
Minniti G, De Sanctis V, Muni R, Filippone F, Bozzao A, Valeriani M, Osti MF, De Paula U, Lanzetta G, Tombolini V, Maurizi Enrici R (2008) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol 88:97–103
Gerstein J, Franz K, Steinbach JP, Seifert V, Fraunholz I, Weiss C, Rodel C (2010) Postoperative radiotherapy and concomitant temozolomide for elderly patients with glioblastoma. Radiother Oncol 97:382–386
Minniti G, Lanzetta G, Scaringi C, Caporello P, Salvati M, Arcella A, De Sanctis V, Giangaspero F, Enrici RM (2012) Phase II study of short-course radiotherapy plus concomitant and adjuvant temozolomide in elderly patients with glioblastoma. Int J Radiat Oncol Biol Phys 83:93–99
Cao JQ, Fisher BJ, Bauman GS, Megyesi JF, Watling CJ, Macdonald DR (2012) Hypofractionated radiotherapy with or without concurrent temozolomide in elderly patients with glioblastoma multiforme: a review of ten-year single institutional experience. J Neurooncol 107(2):395–405
Sijben AE, McIntyre JB, Roldan GB, Easaw JC, Yan E, Forsyth PA, Parney IF, Magliocco AM, Bernsen H, Cairncross JG (2008) Toxicity from chemoradiotherapy in older patients with glioblastoma multiforme. J Neurooncol 89:97–103
Fiorica F, Berretta M, Colosimo C, Stefanelli A, Ursino S, Zanet E, Palmucci T, Maugeri D, Malaguarnera M, Palmucci S, Grasso M, Tirelli U, Cartei F (2010) Glioblastoma in elderly patients: safety and efficacy of adjuvant radiotherapy with concomitant temozolomide. Arch Gerontol Geriatr 51: 31–35
Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, Papsdorf K, Steinbach JP, Sabel M, Combs SE, Vesper J, Braun C, Meixensberger J, Ketter R, Mayer-Steinacker R, Reifenberger G, Weller M. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 13: 707–715
Malmstrom A, Gronberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, Abacioglu U, Tavelin B, Lhermitte B, Hegi ME, Rosell J, Henriksson R (2012) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 13:916–926
Acknowledgments
We would like to thank Eve Ferdman for her help with manuscript preparation.
Conflicts of interest
Marsha Reyngold: none; Andrew B. Lassman: Consultant/Advisory role for Bristol-Myers, Squibb, Eisai, Enzon, Genentech, Imclone, Schering-Plough, Sigma Tau, Honoraria-Genentech, Schering-Plough, Research funding-AstraZeneca, Genentech, Keryx, Schering- Plough, Sigma Tau; Timothy A. Chan: none; Yoshiya Yamada: Consultant/Advisory role for American Brachytherapy Society, Varian Medical Systems; Honoraria: Institute of Medical Education; Philip Gutin: none; Kathryn Beal: none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reyngold, M., Lassman, A.B., Chan, T.A. et al. Abbreviated course of radiation therapy with concurrent temozolomide for high-grade glioma in patients of advanced age or poor functional status. J Neurooncol 110, 369–374 (2012). https://doi.org/10.1007/s11060-012-0972-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-0972-7