Abstract
Purpose
As the efficacy of all pediatric high-grade glioma (HGG) treatments is similar and still disappointing, it is essential to also investigate the toxicity of available treatments.
Methods
Prospectively recorded hematologic and nonhematologic toxicities of children treated with radiochemotherapy in the HIT GBM-C/D and HIT-HGG-2007 trials were compared. Children aged 3–18 years with histologically proven HGG (WHO grade III and IV tumors) or unequivocal radiologic diagnosis of diffuse intrinsic pontine glioma (DIPG) were included in these trials. The HIT-HGG-2007 protocol comprised concomitant radiochemotherapy with temozolomide, while cisplatinum/etoposide (PE) and PE plus ifosfamide (PEI) in combination with weekly vincristine injections were applied during radiochemotherapy in the HIT GBM-C/D protocol.
Results
Regular blood counts and information about cellular nadirs were available from 304 patients (leukocytes) and 306 patients (thrombocytes), respectively. Grade 3–4 leukopenia was much more frequent in the HIT GBM-C/D cohort (n = 88, 52%) vs. HIT-HGG-2007 (n = 13, 10%; P <0.001). Grade 3–4 thrombopenia was also more likely in the HIT GBM-C/D cohort (n = 21, 12% vs. n = 3,2%; P <0.001). Grade 3–4 leukopenia appeared more often in children aged 3–7 years (n = 38/85, 45%) than in children aged 8–12 years (n = 39/120, 33%) and 13–18 years (24/100, 24%; P =0.034). In addition, sickness was more frequent in the HIT GBM-C/D cohort (grade 1–2: 44%, grade 3–4: 6% vs. grade 1–2: 28%, grade 3–4: 1%; P <0.001).
Conclusion
Radiochemotherapy involving cisplatinum-based polychemotherapy is more toxic than radiotherapy in combination with temozolomide. Without evidence of differences in therapeutic efficacy, the treatment with lower toxicity, i. e., radiotherapy with temozolomide should be used.
Zusammenfassung
Zielsetzung
Die Wirksamkeit verschiedener Protokolle zur Radiochemotherapie bei Kindern mit hochmalignen Gliomen („high-grade glioma“, HGG) ist ähnlich und leider noch enttäuschend. Es erscheint vordringlich, auch die Toxizität verschiedener Therapieprotokolle zu untersuchen.
Methoden
Es wurden prospektiv erhobene hämatologische und nichthämatologische Toxizitäten von Kindern verglichen, die in der HIT-GBM-C/D- bzw. HIT-HGG-2007-Studie mit einer Radiochemotherapie behandelt wurden. In diesen Studien wurden Kinder im Alter von 3–18 Jahren mit histologisch gesichertem HGG (WHO-Grad-III-und -Grad-IV-Tumore) oder eindeutiger radiologischer Diagnose eines diffusen intrinsischen Ponsglioms (DIPG) eingeschlossen. Das HIT-HGG-2007-Protokoll umfasste eine konkomitante Radiochemotherapie mit Temozolomid; das HIT-GBM-C/D-Protokoll bestand aus Cisplatin/Etoposid (PE) und PE plus Ifosfamid (PEI) in Kombination mit wöchentlichen Vincristin-Injektionen während der Radiochemotherapie.
Ergebnisse
Regelmäßige Blutbilder waren von je 304 (Leukozyten) und 306 Patienten (Thrombozyten) verfügbar. Eine Grad-3/4-Leukopenie trat deutlich häufiger in der HIT-GBM-C/D- (n = 88, 52 %) als in der HIT-HGG-2007-Kohorte (n = 13, 10 %; P <0,001) auf. Ebenso war eine Grad-3/4-Thrombopenie in der HIT-GBM-C/D-Kohorte deutlich wahrscheinlicher (n = 21, 12 % vs. n = 3, 2 %; P <0,001). Eine Grad-3/4-Leukopenie trat bei Kindern zwischen 3–7 Jahren (38/85, 45 %) häufiger auf als bei Kindern zwischen 8–12 (39/120, 33 %) bzw. 13–18 Jahren (24/100, 24 %; P =0,034). Auch nichthämatologische Toxizität, z. B. Übelkeit, war in der HIT-GBM-C/D-Kohorte häufiger (Grad 1–2: 44 %, Grad 3–4: 6 % vs. Grad 1–2: 28 %, Grad 3–4: 1 %; P <0,001).
Schlussfolgerung
Eine Cisplatin-basierte Polychemotherapie ist toxischer als die Radiochemotherapie mit Temozolomid. Bei fehlender Evidenz für therapeutische Überlegenheit sollte die Behandlung mit geringerer Toxizität, d. h. Radiochemotherapie mit Temozolomid, verwendet werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Radiotherapy combined with temozolomide is standard treatment in adults with glioblastoma [1, 2] and its value in anaplastic astrocytoma is under evaluation [3]. In children, an evidence-based standard treatment for high-grade glioma (HGG) does not exist. The current treatment for pediatric patients and adolescents with HGG is defined by a multimodal approach with best possible tumor resection and radiotherapy as well as chemotherapy and/or other drug treatment strategies. Radiochemotherapy is generally used and widely accepted for the treatment of pediatric HGG since the phase III clinical trial CCG-943 by the North American Children’s Cancer Group (CCG) showed that radiotherapy plus chemotherapy significantly improved event-free survival (EFS) in children with HGG compared to patients treated with radiotherapy alone [4].
However, it still appears unclear which adjuvant chemotherapy or drug treatment is associated with the most favorable feasibility, toxicity, and outcome. From the experience in adults, combination treatment with temozolomide seems rational and promising; however, combination of radiotherapy and adjuvant temozolomide did not prove to be superior in children with HGG [5,6,7,8].
Facing this situation, choosing best evidence-based radiochemotherapy in pediatric HGG appears to be challenging. As there is obviously no clear evidence for an advantage of a specific treatment protocol with regard to survival of pediatric HGG, a favorable tolerability and toxicity profile—influencing the quality of life of these patients—seems to be essential and underreported thus far. Especially the question of the toxicity profile of radiotherapy and concurrent chemotherapy has never been addressed although this treatment element may account very strongly for the overall toxicity and tolerability of a treatment protocol for pediatric HGG.
Thus, we report in the present study on the acute toxicity and feasibility of radiotherapy with two different concurrent chemotherapy regimens in pediatric HGG: a mostly inpatient-based intravenous chemotherapy regimen with cisplatin and other agents (HIT-GBM C and D trials) [9, 10] versus oral, outpatients-based temozolomide (HIT-HGG-2007).
Methods and materials
Patients’ characteristics and inclusion criteria
Patient data were obtained from the HIT-HGG database of the Society of Pediatric Oncology and Hematology (Gesellschaft für Paediatrische Onkologie und Haematologie, GPOH) in Germany, Austria, and (German-speaking treatment centers of) Switzerland. The HIT-HGG database contains clinical data of patients enrolled in the various HIT GBM trials and the ongoing HIT-HGG-2007 trial. For the present study, the following inclusion criteria were defined:
-
Patients enrolled in the following trials or observational studies: HIT GBM-C [7], HIT GBM-D pilot [8], HIT-GBM-D (NCT 00278278), and HIT-HGG-2007 (EudraCT 2007-000128-42; ISRCTN19852453). Furthermore, pediatric patients who were treated as observational patients with temozolomide radiochemotherapy were also included.
-
Central neuropathological re-review (TP, GHG, German Brain Tumor Reference Center of the DGNN, Department of Neuropathology, Bonn, Germany) of a histopathological diagnosis of a pediatric HGG as defined by the 2007 WHO classification of central nervous system tumors [11]. Pediatric HGG (pedHGG) include the following diagnoses: glioblastoma (GBM, WHO IV), anaplastic astrocytoma (WHO III), anaplastic oligodendroglioma (WHO III), anaplastic oligoastrocytoma (WHO III), pilocytic astrocytoma with anaplasia (WHO III), anaplastic ganglioglioma (WHO III), pleomorphic xanthoastrocytoma with anaplasia (WHO III), giant cell glioblastoma (WHO IV), and gliosarcoma (WHO IV).
-
Central neuroradiological review (MW-M, BB, Department of Neuroradiology, Wuerzburg, Germany) of tumors affecting the pons or displaying the neuroradiological characteristics of gliomatosis cerebri. A diffuse intrinsic pontine glioma (DIPG) was defined by tumor infiltration centered in the pons covering more than 50% of the total diameter in a patient with “classical” brainstem symptoms (e. g., cranial nerve deficit or long tract signs, or ataxia, or combination of any two) [12]. A gliomatosis cerebri was defined diffuse tumorous infiltration of more than two lobes in the presence of astrocytic histology which had to be WHO grade II or higher.
-
Patient 3–18 years of age at time of pedHGG diagnosis.
-
Initiated treatment with chemotherapy and radiation therapy (intention to treat population).
-
Regular documentation of toxicities according to Common Terminology Criteria for Adverse Events (CTCAE) grades v3.0 during and at the end of radiochemotherapy. For evaluation of hematological toxicity, weekly control of blood cell counts during radiochemotherapy. Patients treated with craniospinal irradiation have not been included in this analysis.
All patients and/or their parents had given informed consent for data storage and statistical analyses at the time of enrollment in the various trials in accordance with the Declaration of Helsinki.
Treatment protocols
In each of the mentioned trials, best feasible tumor resection was recommended before starting radiochemotherapy.
Radiotherapy (HIT GBM-C/D, HIT-HGG-2007 including observational patients)
Initiation of radiation was recommended within 14 days after diagnosis with 5 × 1.8 Gy fractions per week, up to a total dose of 54 Gy for patients 6 years and younger, as well as to brainstem locations and up to 59.4 Gy for older patients with tumors in other locations. Treatment planning was based on postoperative magnetic resonance imaging (MRI) and fusion with planning computer tomography images (CT). Clinical target volumes (CTV) comprised resection cavities or gadolinium-enhancing tumor on axial scans with a safety margin of 2 cm (HIT GBM-C/D) and 1.5 cm (HIT-HGG-2007) including edema, respecting anatomical borders. The planning target volume (PTV) comprised an additional 0.5–1 cm. Three-dimensional (3D) treatment planning was performed; the target dose was prescribed on the basis of the ICRU 50/62 report. Organs at risk of radiation damage were delineated; organ-specific prescribed doses were calculated and documented.
Chemotherapy in HIT GBM-C and HIT GBM-D trials
In HIT-GBM-C, two chemotherapy cycles, consisting of cisplatinum/etoposide (PE), and PE plus ifosfamide (PEI) added by weekly vincristine injections were given during radiotherapy. Maintenance chemotherapy consisted of cisplatin, etoposide, ifosfamide, and vincristine [9]. In HIT GBM-D, radiochemotherapy was performed as in HIT GBM-C, whereas maintenance therapy consisted of prednisolone, vincristine, and lomustine (CCNU). An additional induction with two courses high-dose methotrexate before radiotherapy was studied for feasibility in a pilot study (HIT-GBM pilot D) [10] and was further evaluated as a randomized question in HIT GBM-D.
Chemotherapy in HIT-HGG-2007
Concomitant chemotherapy consisted of temozolomide at a dose of 75 mg/m2/day, given 7 days per week from the first day of radiotherapy until the last day of radiotherapy, but for no longer than 49 consecutive days. After a 4-week break, patients received 12 cycles of adjuvant temozolomide according to the standard 5‑day schedule every 28 days [1]. The dose was 150 mg/m2/day for the first cycle and was increased to 200 mg/m2/day beginning with the second cycle, as long as there were no significant toxic side effects.
Surveillance during radiochemotherapy and follow-up (HIT GBM-C/D and HIT-HGG-2007)
During concurrent radiochemotherapy, patients were clinically seen and full blood cell counts were taken at least once a week. Toxic effects were graded according to the National Cancer Institute Common Toxicity Criteria, version 3.0, with a score of 1 indicating mild adverse effects, a score of 2 moderate adverse effects, a score of 3 severe adverse effects, and a score of 4 life-threatening adverse effects.
Statistics
Descriptive statistics were performed to describe the frequency of toxicities observed during concurrent radiochemotherapy of the HIT GBM C/D and HIT-HGG-2007 trials. For comparison of toxicity grades, χ2 test was used (α < 0.05). Statistical analysis was performed with SPSS V22.0 (IBM).
Results
General information
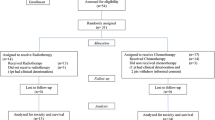
A total of 306 patients fulfilled all inclusion criteria of the present study: 171 patients in HIT GBM-C/D and 135 patients in HIT-HGG-2007.
Within HIT GBM-C/D 98 of patients (57.3%) were male and 73 (42.7%) female; in HIT-HGG 2007, 74 patients were male (54.8%) and 61 female (45.2%). Median age was 10.6 years (HIT GBM-C/D) and 11.4 years (HIT-HGG 2007).
Between the patient cohorts, DIPG was more frequent in the HIT-HGG-2007 cohort, whereas other grade IV tumors were more frequent in HIT GBM-C/D (HIT GBM-C/D: 47 DIPG [27.5%], 64 grade III tumors [37.4%] and 60 grade IV tumors [35.1%]; HIT-HGG-2007: 44 DIPG [32.6], 49 grade III tumors [36.2%] and 40 grade IV tumors [29.6%]). All patient characteristics are summarized in Table 1.
Hematologic toxicity
Hematologic toxicity is listed in Table 2. Regular blood counts and information about cellular nadirs were available from 304 patients (leukocytes) and 306 (thrombocytes), respectively.
Among these, grade 3–4 leukopenia was much more frequent in the HIT GBM-C/D cohort (n = 88, 51.8%) than in HIT-HGG-2007/temozolomide patients (n = 13, 9.7%; P <0.001). Grade 3–4 thrombopenia occurred in both cohorts quite rarely but was observed more often in patients treated in the HIT GBM-C/D trials than in patients treated with HIT-HGG-2007 protocol (n = 21, 12.3% vs. 3 patients, 2.2%; P <0.001).
Erythrocyte transfusions due to anemia ≥ grade 3 were reported more often in HIT GBM-C/D (n = 45, 28.3%) than in HIT-HGG-2007/temozolomide patients (n = 1, 0.7% of patients; P <0.001).
The same applied to platelet transfusions that became necessary in 29 (18.6%) of HIT GBM-C/D patients compared to 7 (5.1%) HIT-HGG-2007/temozolomide patients (P <0.001).
Hematologic toxicity in regards to age and gender
There were no differences observed regarding the frequency of leukopenia (P =0.475) and thrombopenia (P =0.376) between males and females.
The hematologic toxicity with regard to age and gender are listed in Tables 3 and 4. Grade 3–4 leukopenia appeared more often in children aged 3–7 years (n = 38/84, 45.2%) than in older children aged 8–12 years (n = 39/120, 32.5%) and children aged 13–18 years (24/100, 24%; P =0.034).
In HIT GBM-C/D, children aged 3–7 years showed grade 3–4 leukopenia in 31 of 50 (62%) cases compared to 36 of 67 (53.7%) children aged 8–12 years and 21 of 53 (31.6%) children aged 13–18 years (P =0.099). In HIT-HGG-2007/temozolomide treated patients, children aged 3–7 years had grade 3–4 leukopenia in 7 of 34 (20.6%) cases compared to 3 of 53 (5.7%) children aged 8–12 years or 3 of 47 (6.4%) children aged 13–18 years (P =0.361). Boxplot analyses of leukocyte nadirs for all age groups are displayed in Fig. 1, 2 and 3.
Other toxicities
The most frequent other toxicities (Table 5) were skin reactions/alopecia (67% grade I–II, 0% grade III–IV), sickness (36% grade I–II, 4% grade III–IV), and headache (32% grade I–II, 6% grade III–IV). Infections (8% grade I–II, 3% grade III–IV), mucositis (7% grade I–II, 2% grade III–IV), and seizures (7% grade I–II, 1% grade III–IV) were less common.
Sickness was more frequent in the HIT GBM-C/D cohort (grade 1–2: 44%, grade 3–4: 6%) than in the HIT-HGG-2007/temozolomide cohort (grade 1–2: 28%, grade 3–4: 1%; P < 0.001). The frequency of infections, mucositis, seizures, headache, and skin reactions was not different between the two treatment regimens.
Treatment interruptions and early terminations of treatment
Treatment interruptions occurred in 29% of patients (n = 88; Table 6). Most interruptions occurred for technical reasons or due to patients/parents wish. Toxicity was the reason in 20% of cases (n = 19). There was a trend for more toxicity-related interruptions in the HIT GBM C/D cohort: 30% (n = 15) vs. 10% (n = 4) than in the HIT-HGG-2007/temozolomide group (P = 0.08). Early terminations of treatment occurred rarely in 3% (n = 8) of patients; HIT GBM-C/D: 6 patients (3%), HIT-HGG-2007/temozolomide group: 2 patients (1%). In only 2 patients—one in each cohort—was toxicity stated as the reason.
Discussion
To date, there is still no standard of care for pediatric HGG [13]. Since the prognosis of pediatric HGG is often still poor regardless of the underlying treatment approach, it is of outmost relevance to investigate not only the efficacy of a novel treatment but also toxicity and feasibility. Today’s treatment protocols for pediatric HGG very often consist of best possible tumor resection, concurrent radiotherapy with chemotherapy, and finally maintenance chemotherapy. The present study tried to evaluate the toxicity profile of radiotherapy and concurrent chemotherapy. Two large cohorts of pediatric patients treated with two different chemotherapeutic regimens parallel to radiotherapy were studied: one cohort was treated with an intensive, mostly inpatient-based intravenous chemotherapy regimen with cisplatinum and other agents and another cohort was treated with oral, outpatient-based temozolomide. Both regimens are in use for treatment of pediatric HGG worldwide.
Thus, we report on clinically highly relevant findings indicating that radiochemotherapy involving concurrent cisplatinum, ifosfamide, and etoposide (ICE regimen; synonym: PEI regimen) is much more toxic than radiotherapy in combination with oral temozolomide. Severe hematologic toxicity—as the major toxicity—was five times more frequent in treatment with ICE. In addition, ICE-induced toxicity was associated with more interruptions of treatment. Of note, ICE is normally administered during in-patient care, resulting in considerable reduction of the quality of life of children and adolescents, i.e., spending less time at home with their friends and families.
Our patient cohort receiving concomitant treatment with temozolomide represents one of the largest series of pediatric patients with a HGG involving radiochemotherapy with temozolomide. Thus, the present study might have some impact on confirming the favorable toxicity profile of combined radiotherapy with temozolomide: 9.7% of patients encountered grade 3–4 leukopenia and only 2.2% of patients grade 3–4 thrombopenia.
As a limitation of this analysis, a small effect of intercohort PTV differences on leukopenia or thrombopenia cannot be fully excluded. However, a major systematic difference between the two groups appears very unlikely. With the same fraction and total irradiation dose and some minor (“random”) differences in target delineation between the many participating centers, no significant systematic difference should exist between the two cohorts.
The rates of hematologic toxicities appear similar to that in large randomized trials in adults involving radiotherapy in combination with temozolomide in the treatment—or in control arms. Grade 3–4 leukopenia was reported in 6–9% of patients [1, 14, 15], while grade 3–4 thrombopenia was reported in 4–18% of patients [1, 14,15,16].
In single arm trials in children, the rates of severe leukopenia vary between 13–33% [5,6,7,8]. The rates of severe thrombopenia ranged between 8 and 29% [5,6,7,8]. Our results appear to be comparatively moderate and favorable compared to these prior series. This might be partially explained by the use of higher doses of temozolomide in other trials (90 mg/m2) [7] or more intense prior therapies (irinotecan) [4], >50% craniospinal irradiation in medulloblastoma patients [6]. In comparison to other regimens, e. g., CCNU/prednisolone with severe hematologic toxicities of 9–22% or an eight drug chemotherapy regimen with severe hematologic toxicity of 38–57% [17], our results appear indeed favorable.
Interestingly, there appeared to be age dependency with regards to hematologic toxicity. Grade 3–4 leukopenia appeared more often in young children with the age of 3–7 years compared to older children. In a recent analysis, Pixberg et al. [18] did not observe an age dependency of hematologic toxicity; however, their patient cohort was much more heterogeneous and included various tumor types and treatments.
In adults with malignant glioma, an age effect is known: hematologic toxicity can be more severe in elderly patients after concomitant radiochemotherapy with temozolomide [19,20,21] and after monotherapy with temozolomide [22]. It is thought that elderly cancer patients are prone to treatment-related myelotoxicity due to reduction of functional reserves [23]. However, mechanisms of hematologic deprivation are likely to be totally different in young children. Dividing hematologic stem cells in early childhood could be more vulnerable to alkylating chemotherapy than that of older children/adolescents [24]. As an alternative explanation, the body-surface-based calculation of dose appears to be detrimental to the younger population [25].
Conclusion
It appears valuable to retrieve valid, age-stratified, comparable data on short- and long-term toxicity of concurrent radiochemotherapy from large prospective trials in pediatric HGG. Since there is currently no hint that any other concurrent chemotherapy approach generates a superior therapeutic effect, the treatment with the lowest toxicity, i. e., radiochemotherapy with temozolomide, should be used. A temozolomide-based initial radiochemotherapy was also used in the Children’s Oncology Group ACNS0423 trial where the addition of lomustine only in the maintenance element generated a superior survival to maintenance temozolomide alone [26]. This might be a blueprint for future trials: adding less toxic drugs to temozolomide in the initial radiotherapy element or supposedly more toxic drug only in the subsequent maintenance element.
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Nachbichler SB, Schupp G, Ballhausen H et al (2017) Temozolomide during radiotherapy of glioblastoma multiforme daily administration improves survival. Strahlenther Onkol. https://doi.org/10.1007/s00066-017-1110-4
Mayer A, Schwanbeck C, Sommer C et al (2015) Adjuvant temozolomide-based chemoradiotherapy versus radiotherapy alone in patients with WHO III astrocytoma: The Mainz experience. Strahlenther Onkol 191(8):665–671
Sposto R, Ertel IJ, Jenkin RD et al (1989) The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: Results of a randomized trial. A report from the Childrens Cancer Study Group. J Neurooncol 7:165–177
Broniscer A, Chintagumpala M, Fouladi M et al (2006) Temozolomide after radiotherapy for newly diagnosed high-grade glioma and unfavorable low-grade glioma in children. J Neurooncol 76:313–319
Nicholson HS, Kretschmar CS, Krailo M et al (2007) Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: A report from the Children’s Oncology Group. Cancer 110:1542–1550
Cohen KJ, Pollack IF, Zhou T et al (2011) Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro-Oncology 13:317–323
Cohen KJ, Heideman RL, Zhou T et al (2011) Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: A report from the Children’s Oncology Group. Neuro-Oncol 13:410–416
Wolff JEA, Driever PH, Erdlenbruch B et al (2010) Intensive chemotherapy improves survival in pediatric high-grade glioma after gross total resection: Results of the HIT-GBM-C protocol. Cancer 116:705–712
Wolff JE, Kortmann R‑D, Wolff B et al (2011) High dose methotrexate for pediatric high grade glioma: Results of the HIT-GBM-D pilot study. J Neurooncol 102:433–442
Louis et al (2007) WHO classification of tumors of the central nervous sytem. IARC Press, Lyon
Warmuth-Metz M, Bison B, Leykamm S (2009) Neuroradiologic review in pediatric brain tumor studies. Klin Neuroradiol 19:263–273
Fangusaro J, Warren KE (2013) Unclear standard of care for pediatric high grade glioma patients. J Neurooncol 113(2):341–342
Stupp R, Hegi ME, Gorlia T et al (2014) Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 15:1100–1108
Gilbert MR, Dignam JJ, Armstrong TS et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699–708
Chinot OL, Wick W, Mason W et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370:709–722
Finlay JL, Boyett JM, Yates AJ et al (1995) Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Childrens Cancer Group. J Clin Oncol 13:112–123
Pixberg C, Koch R, Eich HT et al (2016) Acute toxicity grade 3 and 4 after irradiation in children and adolescents: Results from the IPPARCA collaboration. Int J Radiat Oncol Biol Phys 94(4):792–799
Minniti G, De Sanctis V, Muni R et al (2008) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol 88:97–103
Sijben AE, McIntyre JB, Roldán GB et al (2008) Toxicity from chemoradiotherapy in older patients with glioblastoma multiforme. J Neurooncol 89:97–103
Brandes AA, Franceschi E, Tosoni A et al (2009) Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: Correlation with MGMT promoter methylation status. Cancer 115:3512–3518
Wick W, Platten M, Meisner C et al (2012) Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: The NOA-08 randomised, phase 3 trial. Lancet Oncol 13:707–715
Balducci L, Colloca G, Cesari M et al (2010) Assessment and treatment of elderly patients with cancer. Surg Oncol 19:117–123
Gupta AA, Anderson JR, Pappo AS et al (2012) Patterns of chemotherapy-induced toxicities in younger children and adolescents with Rhabdomyosarcoma: A report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. Cancer 118(4):1130–1137
Elias GP, Antoniali C, Mariano RC et al (2005) Comparative study of rules employed for calculation of pediatric drug dosage. J Appl Oral Sci 13(2):114–119
Jackacki RI, Cohen KJ, Buxton A et al (2016) Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: A report of the Children’s Oncology Group ACNS0423 study. Neuro Oncol 18(10):1442–1450
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. M. Kramm reports grants from Deutsche Kinderkrebsstiftung, Bonn, Germany, during the conduct of the study; grants from Merck, KGaA Darmstadt, Germany, outside the submitted work. B. Bison reports personal fees and nonfinancial support from Deutsche Kinderkrebsstiftung, during the conduct of the study; personal fees and nonfinancial support from Deutsche Kinderkrebsstiftung, outside the submitted work. C. Seidel, A.O. von Bueren, S. Bojko, M. Hoffmann, T. Pietsch, G.H. Gielen, M. Warmuth-Metz and R.‑D. Kortmann declare that they have no competing interests.
Additional information
Clemens Seidel and André O. von Bueren contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Seidel, C., von Bueren, A.O., Bojko, S. et al. Concurrent radiotherapy with temozolomide vs. concurrent radiotherapy with a cisplatinum-based polychemotherapy regimen. Strahlenther Onkol 194, 215–224 (2018). https://doi.org/10.1007/s00066-017-1218-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-017-1218-6