Abstract
Glioblastoma (GBM) is a rare primary brain tumor in children, and extracranial metastases of pediatric GBM are particularly uncommon. We present the case of a 10-year-old girl with pediatric GBM who developed multiple extracranial metastases, including cervical lymph nodes, spine, and lung. We discuss the rarity of extracranial metastases in GBM and explore possible mechanisms of dissemination. The patient underwent surgical resections, radiotherapy, and chemotherapy, but the metastatic disease progressed despite treatment. We emphasize the need to consider extracranial metastases in pediatric GBM patients and adopt multimodal treatment approaches for managing this rare clinical entity. As the survival rates of pediatric GBM patients are improving, awareness of extracranial metastases is crucial for optimizing treatment outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM) is a primary brain tumor that is rare in children, accounting for only 3–15% of pediatric central nervous system tumors [1]. Despite its rarity, pediatric GBM is a devastating disease with a median survival duration ranging from 13 to 73 months and a 5-year survival rate of less than 20% [2,3,4]. Extracranial metastases were reported in 0.4–0.5% of all glioblastomas, and cases of extracranial metastases in pediatric GBM are especially rare [2,3,4]. In the literature, leptomeningeal spreading or extension via surgical path explains most metastases [5, 6]; however, more than 10% of extracranial metastases occur in patients without prior operation [7]. To the best of our knowledge, the patient described herein is the third reported case of pediatric GBM with multiple extracranial metastases and the second case of pediatric GBM with metastasis to cervical lymph node [8]. The literature is reviewed, and the possible pathophysiology is discussed.
Case report
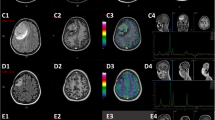
A 10-year-old girl presented with a history of headaches and dizziness for 2 months. Magnetic resonance imaging (MRI) of the brain revealed a cystic tumor in the left parieto-occipital region (Fig. 1). The patient underwent craniotomy for tumor removal, and pathological examination confirmed the diagnosis of glioblastoma. As there were no standards for adjuvant chemotherapy in pediatric high-grade glioma patients, the treatment decisions were made by our pediatric neuro-oncology team based on limited literature [9, 10].
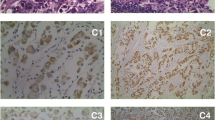
The patient received adjuvant concurrent chemoradiotherapy (CCRT) with radiotherapy, 6.9 Gy in 30 fractions, temozolomide for 42 days, followed by monotherapy with temozolomide. Her dizziness improved initially, but she developed low back pain 4 months after the first surgery. MRI of the spine showed a compression fracture with an enhanced lesion at the L1 level (Fig. 2), which was confirmed to be an osteolytic bone metastasis of glioblastoma by whole body bone scan with single photon emission computed tomography/computed tomography (SPECT/CT) (Fig. 3). The patient underwent percutaneous transpedicular biopsy, and pathological examination disclosed glioblastoma with immunohistochemistry (IHC) stains with S-100 ( +), epithelial membrane antigen, EMA (focal +), glial fibrillary acidic protein, GFAP ( +), cytokeratin AE1/AE3 ( −), and INI-1 (understaining).
Whole-body bone scan with Tc-99 m methylene diphosphonate (Tc-99 m MDP) and SPECT/CT revealing increased MDP uptake in the lateral aspect of left 6th rib (a), decreased MDP uptake in the vertebral body of L1 level, and mildly increased MDP uptake at the bilateral superior articular processes of L1 level (b)
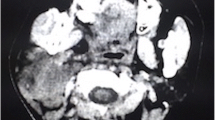
Five months after the first craniotomy, the child experienced vertigo and aphasia. MRI of the brain showed recurrence of the cystic tumor. She underwent craniectomy with tumor removal and received bevacizumab (Avastin) in combination with temozolomide, as well as additional radiotherapy for spinal metastases and recurrent brain tumor bed. However, her low back pain worsened, and MRI of the brain demonstrated an enlarged neck lymph node (Fig. 4). The presence of glioblastoma in neck lymph nodes was pathologically confirmed through IHC stains, showing positive expression of GFAP (focal +) and INI-1 ( +).
However, the patient developed chest pain 8 months after the first surgery, and CT revealed lung metastasis. Ten months after the first craniotomy, she experienced generalized tonic clonic seizure, and brain CT showed contralateral cerebral hemisphere involvement by the tumor. Finally, she received hospice care and succumbed to pneumonia 11 months after the first brain surgery.
Discussion
Extracranial dissemination of glioblastoma to lung, chest wall, and soft tissue of an arm was first reported in 1928 [11]. With advances in treatment, more cases of extracranial metastases in pediatric GBM have been reported. Our case adds to the literature on the metastatic potential of pediatric GBM. Understanding the mechanism of extracranial metastases is essential for improving treatment outcomes.
Several hypotheses have been put forward to explain why extracranial metastases of glioblastoma are so rare. Lun et al. described several biological factors that may prevent tumor cells from infiltrating the neural environment, including the lack of a lymphatic system in the brain and spinal cord, the dense dura surrounding intracranial veins, and the absence of adequate nurturing stroma in other organs to support the proliferation of glioblastoma cells [2].
In the literature, leptomeningeal spreading or extension via surgical path explains most metastasis [5, 6]. Reports have identified cases of leptomeningeal dissemination, essentially diagnosed on imaging studies [12]. The seeding of glioblastomas along the track of the ventriculoperitoneal shunt has also been reported, with the potential route being CSF shunting [13]. Hematogenous circulating tumor cells (CTCs) were found in the peripheral blood of 29 out of 141 (20.6%) GBM patients. Immunostaining of enriched mononuclear cells with antibodies targeting glial fibrillary acidic protein (GFAP) was used for detection. This finding suggests that the hematogenous spread of GBM is an inherent characteristic of its biology [14].
Cervical lymph nodes are the second most common locations of extracranial metastases in the glioblastoma according to the literature [8]. This is presumably because they are in the vicinity of the surgical site. Nonetheless, pediatric cases with extracranial metastases are rare. The lymphatic fluid channels along the cranial nerves and vascular structures were found to be directly connected to the cervical lymph nodes by non-invasive MR imaging in a recent report, which may explain the occurrence of cervical metastases in pediatric GBM [15]. In summary, the possible routes of extracranial metastases of pediatric GBM include leptomeningeal, hematogenous, and lymphatic dissemination, as well as CSF shunting.
There is no current standardized protocol for the management of extracranial metastases of gliomas. No significant difference in clinical efficacy has been found between radiotherapy and/or chemotherapy in most patients [16, 17]. Our patient underwent two brain surgeries and open spine surgery, and received radiotherapy for both the brain tumor bed and spinal metastases. Our surgery debulked her tumor volumes, improved her vertigo and aphasia, stabilized her spine structures, and improved her neurological functions in walking; radiotherapy relieved her low back pain and benefited her walking; however, the ability of chemotherapy to slow down the progression of multiple metastases in our patient was limited.
In conclusion, we describe the case of a female pediatric patient who developed multiple extracranial metastases involving lymph nodes, spine, and lung. Based on our literature review, the patient described herein is the third case of pediatric GBM with multiple extracranial metastases and the second case of pediatric GBM with metastasis to cervical lymph node. With the increasing survival rates of pediatric GBM patients, it is anticipated that the occurrence of this rare clinical entity will also increase. It is important to keep extracranial metastases in mind if a patient has lesions at different sites and to adopt multimodal approaches in the management of such patients.
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Perkins SM, Rubin JB, Leonard JR, Smyth MD, El Naqa I, Michalski JM, Simpson JR, Limbrick DL, Park TS, Mansur DB (2011) Glioblastoma in children: a single-institution experience. Int J Radiat Oncol Biol Phys 80:1117–1121. https://doi.org/10.1016/j.ijrobp.2010.03.013
Lun M, Lok E, Gautam S, Wu E, Wong ET (2011) The natural history of extracranial metastasis from glioblastoma multiforme. J Neurooncol 105:261–273. https://doi.org/10.1007/s11060-011-0575-8. (Epub 2011 Apr 22)
Smith DR, Hardman JM, Earle KM (1969) Metastasizing neuroectodermal tumors of the central nervous system. J Neurosurg 31:50–58. https://doi.org/10.3171/jns.1969.31.1.0050
Pasquier B, Pasquier D, N’Golet A, Panh MH, Couderc P (1980) Extraneural metastases of astrocytomas and glioblastomas: clinicopathological study of two cases and review of literature. Cancer 45:112–125. https://doi.org/10.1002/1097-0142(19800101)45:1%3c112::aid-cncr2820450121%3e3.0.co;2-9
Amitendu S, Mak SK, Ling JM, Ng WH (2012) A single institution experience of the incidence of extracranial metastasis in glioma. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 19:1511–1515. https://doi.org/10.1016/j.jocn.2011.08.040
Kraft M, Lang F, Braunschweig R, Janzer RC (2008) Parotid gland metastasis from glioblastoma multiforme: a case report and review of the literature. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery 265:709–711. https://doi.org/10.1007/s00405-007-0499-2
Anzil AP (1970) Glioblastoma multiforme with extracranial metastases in the absence of previous craniotomy. Case report. J Neurosurg 33:88–94. https://doi.org/10.3171/jns.1970.33.1.0088
Oktay K, Yildirim DC, Acikalin A, Ozsoy KM, Cetinalp NE, Erman T (2021) Extensive extraneural metastases of cerebral glioblastoma in a pediatric patient: an extreme case report and comprehensive review of the literature. Pediatr Neurosurg 56:300–305. https://doi.org/10.1159/000515348
Braunstein S, Raleigh D, Bindra R, Mueller S, Haas-Kogan D (2017) Pediatric high-grade glioma: current molecular landscape and therapeutic approaches. J Neurooncol 134:541–549. https://doi.org/10.1007/s11060-017-2393-0
MacDonald TJ, Aguilera D, Kramm CM (2011) Treatment of high-grade glioma in children and adolescents. Neuro Oncol 13:1049–1058. https://doi.org/10.1093/neuonc/nor092
Davis L (1928) Spongioblastoma multiforme of the brain. Ann Surg 87:8–14
Sethi R, Allen J, Donahue B, Karajannis M, Gardner S, Wisoff J, Kunnakkat S, Mathew J, Zagzag D, Newman K, Narayana A (2011) Prospective neuraxis MRI surveillance reveals a high risk of leptomeningeal dissemination in diffuse intrinsic pontine glioma. J Neurooncol 102:121–127. https://doi.org/10.1007/s11060-010-0301-y
Gelder CL, Hawkins C, Zapotocky M, Dirks P, Bartels U, Bouffet E (2019) Diffuse intrinsic pontine glioma ventricular peritoneal shunt metastasis: a case report and literature review. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 35:861–864. https://doi.org/10.1007/s00381-019-04069-4
Müller C, Holtschmidt J, Auer M, Heitzer E, Lamszus K, Schulte A, Matschke J, Langer-Freitag S, Gasch C, Stoupiec M, Mauermann O, Peine S, Glatzel M, Speicher MR, Geigl JB, Westphal M, Pantel K, Riethdorf S (2014) Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med 6:247ra101. https://doi.org/10.1126/scitranslmed.3009095
Albayram MS, Smith G, Tufan F, Tuna IS, Bostancıklıoğlu M, Zile M, Albayram O (2022) Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat Commun 13:203. https://doi.org/10.1038/s41467-021-27887-0
Sun Q, Xu R, Xu H, Wang G, Shen X, Jiang H (2017) Extracranial metastases of high-grade glioma: the clinical characteristics and mechanism. World journal of surgical oncology 15:181. https://doi.org/10.1186/s12957-017-1249-6
Piccirilli M, Brunetto GM, Rocchi G, Giangaspero F, Salvati M (2008) Extra central nervous system metastases from cerebral glioblastoma multiforme in elderly patients. Clinico-pathological remarks on our series of seven cases and critical review of the literature. Tumori 94:40–51. https://doi.org/10.1177/030089160809400109
Author information
Authors and Affiliations
Contributions
Wei-Zhi Huang, Hung-Chieh Chen, and Yu-Cheng Chou conceptualized and drafted the manuscript. Hung-Chieh Chen also edited the manuscript. Yu-Cheng Chou revised the manuscript and supervised the research work. Te-Kau Chang reviewed the literature and performed chemotherapy. Weir Chiang You reviewed the literature and performed radiotherapy. Yee-Jee Jan made the histopathologic diagnosis and reviewed the literature.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board (IRB) of Taichung Veterans General Hospital (IRB: CE19140B).
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, WZ., Chen, HC., Chang, TK. et al. Extracranial metastasis of pediatric glioblastoma: case report and literature review. Childs Nerv Syst 40, 933–937 (2024). https://doi.org/10.1007/s00381-023-06229-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06229-z