Abstract
Gefitinib, a selective epidermal growth factor receptor tyrosine kinase inhibitor, is under clinical testing and use in cancer patients, including glioma. However, the molecular mechanisms involved in gefitinib-mediated anticancer effects against glioma remain largely uncharacterized. Gefitinib inhibits cell growth and induces apoptosis in human glioma cells. Gefitinib also induces death of H4 cells with characteristics of the intrinsic apoptotic pathway, including Bax mitochondrial translocation, mitochondrial outer membrane permeabilization, cytochrome c cytosolic release, and caspase-9/caspase-3 activation. The importance of Bax in mediating gefitinib-induced apoptosis was confirmed by the attenuation of apoptosis by Bax siRNA and Bax channel blocker. Gefitinib caused Bad dephosphorylation, particularly in serine-112, and increased its binding preference to Bcl-2 and Bcl-xL. The dephosphorylation of Bad in gefitinib-treated cells was accompanied by reduced intracellular cyclic AMP content and protein kinase A (PKA) activity. Adenylyl cyclase activator forskolin attenuated, but PKA inhibitor H89 augmented, gefitinib-induced Bad dephosphorylation, Bax mitochondrial translocation, caspase-9/caspase-3 activation, and viability loss. Intriguingly, a nonselective protein phosphatase inhibitor okadaic acid alleviated gefitinib-induced alterations, except Bad dephosphorylation. In parallel with the higher basal PKA activity, response of U87 cells to gefitinib treatment was delayed and relatively resistant compared with that of H4 and T98G cells. Inactivation of PKA sensitized H4, T98G, and U87 cells to gefitinib cytotoxicity, Bad dephosphorylation in serine-112, and caspase-9/caspase-3 activation. Our findings suggest the involvement of the Bad/Bax signaling pathway in gefitinib-induced glioma apoptosis. Furthermore, the inactivation of PKA was shown to play a role in triggering the proapoptotic function of Bad.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas are among the most lethal types of human adult cancer. Malignant gliomas generally prove refractory to treatment by surgery, irradiation, and conventional chemotherapy. They have several genetic and signaling abnormalities that lead to uncontrolled growth, invasiveness, and angiogenesis, and ultimately facilitate cell proliferation and survival [1, 2]. These dysregulated pathways provide the basis for designing molecular-targeted therapy for treatment of gliomas.
The intracellular tyrosine kinase of the epidermal growth factor receptor (EGFR) activates signaling cascades leading to cell proliferation, angiogenesis, and inhibition of apoptosis [3]. Therefore, the EGFR is an attractive target for cancer therapy. Gefitinib (ZD1839, Iressa; AstraZeneca) is a novel, oral, low-molecular weight tyrosine kinase inhibitor that reversibly inhibits tyrosine kinase activity associated with EGFR [4]. Gefitinib has been clinically approved for treatment of non-small cell lung cancer in some countries [5]. Somatic mutations in the EGFR tyrosine kinase domain are important predictors of an individual’s response to gefitinib [6]. However, some patients in whom EGFR tyrosine kinase domain mutations are absent also respond to gefitinib [7]. In addition to non-small cell lung cancer, gefitinib also has antitumor activity against variety of human cancer cell lines and xenograft models [8].
EGFR gene amplification is one of the most frequent alterations, occurring in 30–40% of malignant gliomas. In gliomas with this gene amplification, ~40% also express a constitutively autophosphorylated variant of the EGFR that lacks the extracellular ligand-binding domain known as EGFR variant III (EGFRvIII). EGFR amplification in gliomas has been associated with tumor invasiveness, angiogenesis, poor survival, and resistance to radiation therapy [9, 10]. Given the presence of EGFR amplification and EGFRvIII in malignant gliomas, inhibition of EGFR seems to be a promising antiglioma therapy. A cell study has shown that gefitinib can induce apoptosis in several human glioma cells but requires high doses [11]. Clinically, monotherapy with gefitinib is of marginal benefit in recurrent malignant gliomas [12]. There is ample evidence that the family of Bcl-2 related proteins is important in glioma cell resistance. Silencing Bcl-2/Bcl-xL protein expression renders gliomas sensitive to apoptotic execution [13]. Exogenous administration of Bad protein induces glioma cell apoptosis [14]. The expression of Bax protein is correlated with increased survival of glioma patients and its silenced expression has marked resistance to multiple cell death inducers [15, 16]. Therefore, better understanding of the involvement of signatures of gefitinib resistance in Bcl-2-mediated cell survival and death signaling may be useful in the development of new therapeutic strategies which can overcome gefitinib resistance and improve patient outcomes.
Bax is of central importance in regulating cell apoptosis [17]. The role of Bax and upstream regulatory mechanisms involved in gefitinib-mediated apoptosis remain largely uncharacterized in human glioma cells. In this study, the cellular and molecular mechanisms of gefitinib action against human glioma cells were examined.
Materials and methods
Cell cultures
Human neuroglioma (H4) and glioblastoma (U87 and T98G) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). During experiments, cells were maintained in DMEM containing 2% FBS.
Viability assessment
For cell proliferation, cells were seeded into six-well plates (6.6 × 104 cells/well). The number of viable cells was determined by the Trypan blue dye exclusion method. MTS (Promega, Madison, WI, USA) assay was performed to measure cell viability in a 96-well plate (4 × 103 cells/well), in accordance with the manufacturer’s instructions. Cell damage was assessed by measuring the activity of lactate dehydrogenase (LDH) in the culture media by using an LDH diagnostic kit (Promega) in a 96-well plate (4 × 103 cells/well). The cytotoxicity index was indicated by the ratio of released LDH to total LDH activity.
Small interfering RNA (siRNA) transfection
The siRNAs against human Bax (SignalSilence Bax siRNA I) and Bad (SignalSilence Bad siRNA I) and control siRNA (SignalSilence Control siRNA) were purchased from Cell Signaling Technology (Beverly, MA, USA). H4 cells were transfected with siRNAs by use of INTERFERin siRNA transfection reagent (Polyplus-transfection, New York, NY, USA) in accordance with the manufacturer’s instructions.
Caspase activity assay
After treatment, cells were homogenized on ice in a lysis buffer containing 20 mM HEPES, pH 7.4, 4 mM EDTA, 1 mM EGTA, 5 mM MgCl2, and 1 mM DTT. The supernatant (50 μl) was incubated with an equal volume of the reaction buffer containing 20 mM HEPES, pH 7.4, 4 mM EDTA, 0.2% CHAPS, 10 mM DTT, and caspase-specific fluorogenic peptide substrates (BioVision, Mountain View, CA, USA). Enzymatic release of free AMC was measured at an excitation wavelength of 380 nm and an emission wavelength of 460 nm. The arbitrary activity was expressed as the fluorescence change per amount of protein.
Western blot
Obtained cell extracts were separated by SDS-PAGE and electrophoretically transferred to poly(vinylidene difluoride) membranes. After blocking, the membranes were incubated with the indicated antibodies against: PARP-1, FAK, Bax, cytochrome c, cytochrome oxidase IV (COX IV), Bad, phospho-Bad (Ser-112), phospho-Bad (Ser-136), phospho-Bad (Ser-155), Bcl-2, Bcl-xL, EGFR, phospho-EGFR (Tyr-845 and Tyr-1068), Akt, phospho-Akt (Ser-473) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), 14-3-3 (Zymed, San Francisco, CA, USA), and β-tubulin (Sigma–Aldrich, St Louis, MO, USA). The membranes were then incubated with horseradish peroxidase-labeled IgG. The blots were developed using enhanced chemiluminescence (ECL) Western blotting reagents. The intensity of each signal was determined by use of a computer image-analysis system (IS1000; Alpha Innotech).
Mitochondria membrane potential measurement
The change of mitochondria membrane potential was measured by use of the fluorescent probe JC-1 (Molecular Probes, Eugene, OR, USA). After treatment, cells were incubated in medium containing 5 μg/ml JC-1 for 30 min. The cells were then washed twice with phosphate-buffered saline (PBS). When excited at 485 nm, the fluorescence emission of JC-1 was measured at wavelengths corresponding to its monomer (538 nm) and J aggregate (590 nm) forms. Fluorescence was measured in a fluorescent plate reader. A decrease of the Em590-to-Em538 ratio indicates a drop in mitochondria membrane potential.
Subcellular fractionation
Cells were resuspended in buffer containing 75 mM NaCl, 8 mM Na2PO4, 1 mM NaH2PO4 (pH 7.4), 250 mM sucrose, 1 mM EDTA, 0.4 mM PMSF, 20 μM leupeptin, 0.005 U/ml aprotinin, and 2 μg/ml soybean trypsin inhibitor on ice for 20 min. Cells were disrupted by passage through 26-gauge needles 20 times. Cell lysates were centrifuged at 750 × g for 10 min at 4°C to eliminate unlysed cells and nuclei. Supernatant was were centrifuged at 10,000 × g for 20 min at 4°C. The pellet served as the mitochondrial fraction and the supernatant as the cytosolic fraction.
Immunoprecipitation
Cells were washed twice with PBS and harvested in RIPA buffer [18]. Protein A-agarose beads were washed with RIPA buffer and then incubated with antibodies for 1 h at room temperature. After removal of the unbound antibodies, protein extracts (200 μg) were added with gentle shaking and incubated for an additional 4 h at room temperature. Immunoprecipitates were washed with RIPA buffer and then eluted for further analysis.
Cyclic AMP (cAMP) measurement
The amount of cellular cAMP was measured by use of a commercially available cyclic AMP EIA kit (Cayman Chemical Company, Ann Arbor, MI, USA) in accordance with the manufacturer’s instructions.
Protein kinase A (PKA) activity measurement
PKA activity was measured by use of a commercially available PKA kinase activity assay kit (Assay Designs, Ann Arbor, MI, USA) in accordance with the manufacturer’s instructions.
Statistical analysis
Data are expressed as mean values ± standard deviation. Statistical analysis was carried out using one-way analysis of variance, followed by Dunnett’s test to assess the statistical significance of differences between treated and untreated groups. A level of P < 0.05 was considered statistically significant.
Results
Gefitinib had cytostatic and cytotoxic effects on human glioma cells
To determine the effect of gefitinib on human glioma cell proliferation, H4 cells were treated with different concentrations of gefitinib and viable cells were measured daily by the Trypan blue dye exclusion method. As shown in Fig. 1a, at concentrations lower than 25 μM gefitinib inhibited the growth of H4 cells in a concentration-dependent manner. After incubation for three days the cell number of 1, 5, and 10 μM gefitinib-treated cultures was reduced by approximately 6, 35, and 55%, respectively. The growth of H4 cells was almost completely suppressed in the presence of 20 μM gefitinib for three days. The cell numbers were similar to those of initial seeding. When treated with 25 μM gefitinib, there was no increase of cell number and an apparent decrease of cell number was found after incubation for 48 h (reducing to 80% of initial cell number) and 72 h (reducing to 66% of initial cell number). In contrast, treatment with 40 and 50 μM gefitinib caused a dramatic reduction in cell number after incubation for 24 h, and this continued to decrease. These two concentrations of gefitinib caused indistinguishable cytotoxicity. The cell numbers were maintained at approximately 55, 30, and 5% of those observed at the initial seeding after incubation for 24, 48, and 72-h, respectively. These results suggest that gefitinib has both cytostatic and cytotoxic effects against human glioma H4 cells.
Gefitinib caused glioma cell death. H4 cells were treated with different concentrations of gefitinib for different times. The number of viable cells was determined by the Trypan blue dye exclusion method (a). H4 cells were treated with different concentrations of gefitinib for 24 h. Representative phase contrast images were obtained (b). Scale bar 50 μm. Cell viability was determined by MTS reduction assay (c) and cell damage was measured by LDH efflux assay (d). *P < 0.05 and **P < 0.01 vs. each control (0 μM group) and # P < 0.05 and ## P < 0.01 vs. the corresponding control (0 h group), n = 4
Gefitinib induced apoptosis in human glioma cells
To further characterize gefitinib-induced cytotoxicity, cell morphology was observed with a light microscope after treatment with different concentrations of gefitinib for 24 h. There was no remarkable morphological change in 10 and 20 μM gefitinib-treated cells. Slight morphological change was observed in H4 cells after treatment with 25 μM gefitinib. Distinctive morphological changes, including cellular rounding, shrinkage, membrane blebbing, and separation from neighboring cells were observed for H4 cells treated with 40 μM gefitinib (Fig. 1b). In parallel with these morphological changes, gefitinib reduced cell viability (Fig. 1c) and increased cell damage (Fig. 1d) in a concentration-dependent manner. To investigate whether the cytotoxic effect of gefitinib was because of induction of apoptosis, proteolytic activity of caspase family proteases was measured. Elevated caspase-8, caspase-9, and caspase-3 activity was detected in gefitinib-treated cells, and maximum activity was seen 3 and 6 h after incubation. However, in the same treated cells, change in caspase-12 activity was not found (Fig. 2a). To determine whether particular caspases are crucially involved in gefitinib-induced apoptosis, specific caspase inhibitors were investigated. As depicted in Fig. 2b, non-selective caspase and specific caspase-8, caspase-9, and caspase-3, but not caspase-12 inhibitors protected the cells from death because of gefitinib treatment. These observations suggest that caspase-8, caspase-9, and caspase-3 activation are of crucial importance in the gefitinib-triggered apoptotic process in H4 cells.
Gefitinib induced glioma cell apoptosis. H4 cells were treated with gefitinib (40 μM) for different times. Protein extracts were isolated and subjected to fluorogenic protease assay. *P < 0.05 and **P < 0.01 vs. each control, n = 4 (a). H4 cells were treated with medium or gefitinib (40 μM) or gefitinib (40 μM) in combination with Z-VAD-FMK (broad-spectrum caspase inhibitor, Pan-C, 10 μM), Z-DEVD-FMK (caspase-3 inhibitor, C-3, 10 μM), Z-IETD-FMK (caspase-8 inhibitor, C-8, 10 μM), Z-LEHD-FMK (caspase-9 inhibitor, C-9, 10 μM), or Z-ATAD-FMK (caspase-12 inhibitor, C-12, 10 μM) for 24 h. Cell viability was determined by MTS reduction assay. **P < 0.01 vs. control (without gefitinib) and # P < 0.05 and ## P < 0.01 vs. gefitinib control, n = 3 (b)
Bax involvement in gefitinib-induced apoptosis
The activation of caspase-9 implies that the mitochondria-mediated intrinsic apoptotic pathway is involved in gefitinib-induced glioma cell apoptosis. The integrity of the outer mitochondrial membrane and formation of mitochondrial permeability transition pores are critical determinants in regulating the release of cytochrome c and other proapoptotic factors which control the initiation of mitochondria-mediated apoptosis [19]. To determine the involvement of mitochondrial events in apoptosis in H4 cells after gefitinib treatment, the change in mitochondrial membrane permeability was measured. Mitochondrial damage is often associated with loss of mitochondrial membrane potential, which is measurable by use of JC-1 staining [20]. A high JC-1 ratio (Em590 nm/Em538 nm) was detected in control H4 cells. Treatment with gefitinib caused a drop in JC-1 ratio, indicating a collapse of mitochondrial membrane potential (Fig. 3a). Treatment with mitochondrial permeability transition pore inhibitor, cyclosporin A [21], attenuated the gefitinib-induced collapse in mitochondrial membrane potential (Fig. 3a). The restoration of mitochondrial membrane potential by cyclosporin A also alleviated gefitinib-induced caspase-9 and caspase-3 activation (Fig. 3b). These results show that mitochondrial membrane permeability is a crucial target in determining gefitinib-triggered apoptosis. Bax is a critical gateguard in the regulation of mitochondrial membrane permeability [17]. Western blotting revealed that gefitinib caused PARP-1 and FAK protein cleavage, indicating the execution of apoptosis. Expression of Bax was unchanged (Fig. 3c). However, the collapse of mitochondrial membrane potential caused by gefitinib was associated with elevated mitochondrial distribution of Bax and mitochondrial release of cytochrome c into the cytosol (Fig. 3d). Silencing Bax expression by siRNA (Fig. 3e) and inhibition of Bax channel activity by Bax-channel blocker all caused reduction in gefitinib-induced caspase-9 and caspase-3 activity (Fig. 3f, g). These results suggest that the Bax membrane permeabilization pathway might be involved in gefitinib-induced apoptosis.
Gefitinib activated Bax. H4 cells were treated with medium, gefitinib (40 μM), or cyclosporine A (5 μM), alone or in combination for 3 h. The level of mitochondrial membrane potential was determined by JC-1 staining. The Em590/Em538 ratio was determined (n = 4) (a). Proteins were isolated and subjected to fluorogenic protease assay. n = 4 (b). H4 cells were treated with medium or gefitinib (40 μM) for 3 h. Proteins were isolated and subjected to Western blot with antibodies against PARP-1, FAK, Bax, and β-tubulin (c). Proteins obtained from the cytosolic and mitochondrial fractions were subjected to Western blot with antibodies against Bax, cytochrome c, β-tubulin, and COX IV (d). H4 cells were transfected with mock, control siRNA (1 nM), or Bax siRNA (1 nM) for 48 h. Proteins were isolated and subjected to Western blot with antibodies against Bax and β-tubulin. **P < 0.01 vs. mock, n = 3 (e). The resulting transfected cells were treated with medium or gefitinib (40 μM) for 3 h. Proteins were isolated and subjected to fluorogenic protease assay. n = 4 (f). H4 cells were treated with medium, gefitinib (40 μM), Bax channel blocker (1 μM), alone or in combination for 3 h. Proteins were isolated and subjected to fluorogenic protease assay. n = 3 (g). One of four independent experiments is shown (c, d, and e). *P < 0.05 and **P < 0.01
Gefitinib decreased Bad phosphorylation in human glioma cells leading to apoptosis
Mitochondrial membrane permeability is regulated by Bcl-2 family proteins. Among the BH3-only Bcl-2 family proteins, Bad promotes mitochondrial membrane permeabilization and apoptosis and its function can be neutralized by phosphorylation [17, 22]. A change in total Bad protein expression was not found in gefitinib-treated H4 cells. Western blot analysis for phospho-Bad revealed that gefitinib treatment reduced the phosphorylation of serine-112, serine-136, and serine-155 residues. Dephosphorylation of serine-112 occurred before that of serine-136 and serine-155 (Fig. 4a). To directly address the possible ivolvement of Bad in gefitinib-induced apoptosis, expression of endogenous Bad was down-regulated by siRNA. Silencing of Bad expression (Fig. 4b) alleviated gefitinib-induced caspase-9 and caspase-3 activity (Fig. 4c). In most theories, protein–protein interaction is important in proapoptotic activation or inactivation of preexisting Bad. Phosphorylation of Bad changes its interactive binding partners between 14-3-3 and antiapoptotic Bcl-2 family proteins and the consequences of apoptosis [17, 19]. Gefitinib had a negligible effect on total protein expression in Bad, 14-3-3, Bcl-2, and Bcl-xL (Fig. 4d). In controlled H4 cells, Bad formed protein complexes with 14-3-3, Bcl-2, and Bcl-xL (Fig. 4e). Immunoprecipitation/Western blot analysis showed that Bad had tendency to bind with 14-3-3 rather than Bcl-2 and Bcl-xL in controlled H4 cells. The binding preference of Bad for Bcl-2 and Bcl-xL was increased in gefitinib-treated cells (Fig. 4f). These results show that Bad is a crucial molecule in mediating gefitinib-induced apoptosis by dephosphorylation and consequent sequestration of Bcl-2 and Bcl-xL.
Gefitinib caused Bad dephosphorylation. H4 cells were treated with medium or gefitinib (40 μM) for 1 or 3 h. Proteins were isolated and subjected to Western blot with antibodies against β-tubulin, Bad, and phospho-Bad (Ser112, Ser136, and Ser155) (a). H4 cells were transfected with mock, control siRNA (1 nM), or Bad siRNA (1 nM) for 48 h. Proteins were isolated and subjected to Western blot with antibodies against Bad and β-tubulin. **P < 0.01 vs. mock, n = 3 (b). The resulting transfected cells were treated with medium or gefitinib (40 μM) for 3 h. Proteins were isolated and subjected to fluorogenic protease assay. n = 4 (c). H4 cells were treated with medium or gefitinib (40 μM) for 3 h. Proteins were isolated and subjected to Western blot with antibodies against Bcl-2, Bcl-xL, Bad, 14-3-3, and β-tubulin (d). Proteins obtained from medium-controlled H4 cells were immunoprecipitated (IP) by IgG or anti-Bad antibody, and the immunoprecipitates were subjected to Western blot with antibodies against Bad, 14-3-3, Bcl-2, and Bcl-xL (e). Proteins obtained from both groups were immunoprecipitated by anti-Bad antibody, and the immunoprecipitates were subjected to Western blot with antibodies against Bad, 14-3-3, Bcl-2, and Bcl-xL (f). One of three independent experiments is shown (a, b, d, e, and f). *P < 0.05 and **P < 0.01
Gefitinib reduced PKA activity
Depending on the cellular environment, multiple protein kinases and phosphatases have been implicated in dynamic regulation of Bad phosphorylation. Evidence shows that PKA is one upstream kinase for the phosphorylation of Bad [22]. Gefitinib treatment reduced intracellular cAMP content (Fig. 5a) and PKA activity (Fig. 5b). The results show that gefitinib has a negative effect on PKA activity.
PKA and protein phosphatases were involved in gefitinib-induced apoptosis
Because gefitinib modulates PKA activity, we conducted experiments to elucidate the effects of pharmacological agents of PKA and protein phosphatases on gefitinib-induced Bad dephosphorylation and apoptosis. Western blot analysis revealed that adenylyl cyclase activator forskolin attenuated and PKA inhibitor H89 augmented gefitinib-induced Bad dephosphorylation in serine-112. Surprisingly, nonselective protein phosphatase inhibitor okadaic acid did not prevent gefitinib-induced Bad dephosphorylation in serine-112 (Fig. 6a). Subcellular fractionation and biochemical assay further showed that forskolin alleviated gefitinib-induced Bax mitochondrial translocation (Fig. 6b), caspase-9 and caspase-3 activation (Fig. 6c), and viability loss (Fig. 6d). These gefitinib-induced alterations were potentiated by H89 (Fig. 6b–d). In contrast with its effect on Bad dephosphorylation, okadaic acid attenuated gefitinib-induced Bax mitochondrial translocation (Fig. 6b), caspase-9 and caspase-3 activation (Fig. 6c), and viability loss (Fig. 6d). The results show that activation of PKA or inactivation of protein phosphatase has a protective effect against gefitinib-induced apoptosis in H4 cells.
Forskolin, H89, and okadaic acid differently affected gefitinib-induced alterations. H4 cells were treated with medium or gefitinib (40 μM) alone or in combination with forskolin (5 μM), H89 (5 μM), or okadaic acid (5 nM) for 3 h. Proteins were isolated and subjected to Western blot with antibodies against Bad, phospho-Bad (Ser112), and β-tubulin. The intensity of phospho-Bad in the gefitinib-untreated control is defined as 100%. *P < 0.05 and **P < 0.01 vs. gefitinib-untreated control and # P < 0.05 vs. gefitinib-treated control (a). Proteins obtained from the cytosolic and mitochondrial fractions were subjected to Western blot with antibody against Bax. The relative amount of mitochondrial distribution of Bax in the gefitinib-untreated group is defined as 100%. **P < 0.01 vs. gefitinib-untreated control and # P < 0.05 vs. gefitinib-treated control (b). One of four independent experiments is shown (a and b). Proteins were isolated and subjected to fluorogenic protease assay. **P < 0.01 vs. gefitinib-untreated control and # P < 0.05 and ## P < 0.01 vs. gefitinib-treated control, n = 4 (c). H4 cells were treated with medium or gefitinib (40 μM) alone or in combination with forskolin (5 μM), H89 (5 μM), or okadaic acid (5 nM) for 24 h. Cell viability was determined by MTS assay. **P < 0.01 vs. gefitinib-untreated control and # P < 0.05 and ## P < 0.01 vs. gefitinib-treated control, n = 4 (d)
Gefitinib induced apoptosis in U87 and T98G glioblastoma cells
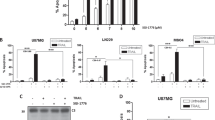
To determine whether PKA inactivation is a general event contributing to gefitinib-induced apoptosis, U87 and T98G cells were also included for investigation. There was greater basal PKA activity in U87 than H4 and T98G cells (Fig. 7a). Gefitinib concentration-dependently reduced cell viability in U87 and T98G cells (Fig. 7b). Similar to that in H4 cells, gefitinib also caused both cytostatic and cytotoxic effects on T98G (Fig. 7c) and U87 (Fig. 7d) cells. However, U87 cells were more resistant to gefitinib-induced cell death than H4 and T98G cells. Despite the presence of different basal PKA activity, reduced cAMP content (Fig. 8a), PKA activity (Fig. 8b), and Bad phosphorylation in serine-112 (Fig. 8c), and elevated caspase-9 (Fig. 8d) and caspase-3 (Fig. 8e) activity were also observed in gefitinib-treated U87 and T98G cells. These alterations were detected 3 h after treatment of T98G and H4 cells. Intriguingly, changes were not apparent until 8 h after treatment for U87 cells. Forskolin attenuated and H89 augmented gefitinib-induced alteration in U87 and T98G cells (Fig. 8c–e). To further investigate the crucial importance of PKA activity in cell viability, intracellular PKA activity was inhibited by H89. As shown in Fig. 8f, H89 had different cytotoxicity against H4, T98G, and U87 cells, in a concentration-dependent manner. These results show that gefitinib cytotoxicity involves PKA inactivation.
Gefitinib caused different cytotoxicity in U87 and T98G cells. Proteins (50 μg) were isolated from subconfluent H4, T98G, and U87 cells and subjected to PKA analysis, n = 4 (a). U87 and T98G cells were treated with different concentrations of gefitinib for 24 h. Cell viability was determined by MTS assay. *P < 0.05 and **P < 0.01 vs. vehicle control, n = 4 (b). T98G (c) and U87 (d) cells were treated with different concentrations of gefitinib for different times. The number of viable cells was determined by the Trypan blue dye exclusion method. **P < 0.01 vs. each control (0 μM group) and ## P < 0.01 vs. the corresponding control (0 h group), n = 4
Gefitinib caused apoptosis in U87 and T98G cells. U87 and T98G cells were treated with medium or gefitinib (40 μM) for 8 and 3 h, respectively. The amount of cAMP was measured (a). The whole-cell lysates were isolated and subjected to enzymatic assay for measurement of PKA (b). *P < 0.05, n = 4. U87 and T98G cells were treated with medium or gefitinib (40 μM) alone or in combination with forskolin (5 μM) or H89 (5 μM) for 8 and 3 h, respectively. Proteins were isolated and subjected to Western blot with antibodies against Bad and phospho-Bad (Ser112). The intensity of phospho-Bad in gefitinib-untreated control is defined as 100%. *P < 0.05 and **P < 0.01 vs. gefitinib-untreated control and # P < 0.05 and ## P < 0.01 vs. gefitinib-treated control, n = 3 (c). Proteins were isolated and subjected to enzymatic assay for caspase-9 (d) and caspase-3 (e). **P < 0.01 vs. gefitinib-untreated control and # P < 0.05 and ## P < 0.01 vs. gefitinib-treated control, n = 4. H4, U87, and T98G cells were treated with different concentrations of H89 for 24 h. Cell viability was determined by MTS assay. *P < 0.05 and **P < 0.01 vs. vehicle control, n = 4 (f)
H89 increased cytotoxic sensitivity to gefitinib
The abovementioned results suggested the importance of PKA in the viability of H4, T98G, and U87 cells. To investigate the potential involvement of PKA in determining cytotoxic sensitivity to gefitinib, a sub-toxic concentration of H89 was used. H89 (5 μM) increased cell sensitivity to gefitinib treatment and potentiated gefitinib-induced cytotoxicity. In the presence of a sub-toxic concentration of H89, the concentration of gefitinib required for induction of cytotoxicity was higher in U87 cells (20 μM) than in H4 (10 μM) and T98G (10 μM) cells (Fig. 9a). The combination of non-toxic concentrations of H89 and gefitinib elevated caspase-9 and caspase-3 activity (Fig. 9b) and reduced Bad phosphorylation in serine-112 (Fig. 9c). These results show that H89 might increase cell sensitivity to gefitinib cytotoxicity and trigger Bad-related apoptotic signaling.
H89 increased cell sensitivity to gefitinib cytotoxicity. H4, T98G, and U87 cells were treated with different concentrations of gefitinib in the absence (0 μM) or presence (5 μM) of H89 for 24 h. Cell viability was determined by MTS assay (a). *P < 0.05 and **P < 0.01 vs. vehicle control, n = 4. H4, T98G, and U87 cells were treated with medium, H89, or gefitinib alone or in combination for 8 h. Proteins were isolated and subjected to enzymatic assay for caspase-9 and caspase-3 (b). **P < 0.01 vs. gefitinib/H89-untreated control, n = 4. Proteins were isolated and subjected to Western blot with antibodies against Bad and phospho-Bad (Ser112) (c). **P < 0.01 vs. untreated control. One of three independent experiments is shown
Gefitinib blocked EGF/EGFR signaling
To demonstrate the inhibitory effect of gefitinib on EGF/EGFR signaling in glioma cells, the activation of EGFR and its downstream effector Akt were analyzed by measuring their phosphorylation levels (Fig. 10). H4 and U87 cells expressed similar levels of EGFR protein. However, T98G cells expressed a lower level of EGFR protein (~70% of that of H4 or U87 cells). Intriguingly, gefitinib treatment reduced EGFR protein (reduction by 20–35% in H4, T98G, and U87 cells) and the reduction was blocked by EGF. Although gefitinib itself slightly reduced basal level of EGFR protein, a low concentration of gefitinib (10 μM) had profound inhibitory effects on basal and EGF-stimulated EGFR phosphorylation and Akt phosphorylation. Despite the distinct expression levels of EGFR protein, these results suggest that H4, T98G, and U87 cells all expressed functional EGFR and their signaling could be blocked by a low concentration of gefitinib.
Gefitinib inhibited EGF/EGFR signaling. H4, T98G, and U87 cells were treated with medium, EGF (10 ng/ml), or gefitinib (10 μM) alone or in combination for 1 h. Proteins were isolated and subjected to Western blot with antibodies against EGFR, phospho-EGFR (P-EGFR1068), phospho-EGFR (P-EGFR845), Akt, phospho-Akt (P-Akt473), and β-tubulin. One of three independent experiments is shown
Discussion
Apoptosis has been described as multiple pathways converging from numerous different initiating events and insults. Accumulating evidence shows that efficacy of antitumor agents is related to the intrinsic propensity of the target tumors cells to respond to these agents by apoptosis. In addition to cytostatic effect, gefitinib induced cytotoxicity in glioma cells involving apoptotic mechanism. Several pathways have been reported to be involved in the induction of apoptosis by gefitinib. The Fas/Fas-ligand death-signaling pathway and subsequent activation of the caspase-8/caspase-3 cascade have been reported to relay the apoptotic signaling in cells exposed to gefitinib [23–26]. Apoptotic cascades other than the death receptor pathway are also of major importance in gefitinib-induced apoptotic cell death. Inactivation of antiapoptotic Bcl-2 and/or activation of proapoptotic Bim, Puma, Bad, or Bax has been observed in gefitinib-treated cells, implying the mitochondrial apoptotic signaling pathway is involved in gefitinib-triggered apoptosis [24, 27–29]. An increase in caspase-8 and caspase-9 activity was observed after gefitinib treatment, and gefitinib-induced cytotoxicity could be attenuated by caspase-8 or caspase-9 inhibitor. By measurement of mitochondrial membrane potential and biochemical assays, we found that mitochondrial outer membrane permeabilization was associated with gefitinib-induced cytochrome c cytosolic release and caspase-9 and caspase-3 activation. Inhibition of mitochondrial outer membrane permeabilization alleviated gefitinib-induced mitochondrial membrane potential loss and caspase-9/caspase-3 activation. Evidence suggests that Bax is important in the apoptotic response of cells and is a crucial mediator in gefitinib-induced apoptosis [27]. There was no change in the level of Bax protein expression; instead, its mitochondrial distribution was promoted by gefitinib. The importance of Bax in gefitinib-induced glioma cell apoptosis was further supported by the findings that inactivation of Bax either by siRNA or Bax channel blocker reduced gefitinib-induced caspase-9/caspase-3 activation and apoptosis. These observations suggest that gefitinib induces apoptosis in glioma cells through both the death receptor and mitochondria signaling pathways.
Much independent evidence indicates that Bcl-2 family proteins are important in the control of mitochondrial integrity. The Bax or Bak proteins are proapoptotic and perturb mitochondrial membrane permeability when activated. To prevent mitochondrial membrane permeabilization and cell apoptosis, Bax and Bak are bound and inhibited by the antiapoptotic members of the Bcl-2 family (for example Mcl-1, Bcl-2, and Bcl-xL). The BH3-only proteins, for example Bid, Puma, Noxa, Bim, and Bad, promote mitochondrial membrane permeabilization and initiate apoptosis signaling by binding and antagonizing the prosurvival Bcl-2 family members, thereby causing activation of Bax and Bak [17, 19]. Bad, a ligand of the prosurvival protein 14-3-3, is a switch protein for the mitochondria-induced apoptotic pathway. When dissociated from 14-3-3, Bad may interact with Bcl-2/Bcl-xL and release Bax, revealing its apoptotic activity. Binding of Bad to Bcl2/Bcl-xL has also been reported to free activator BH3-only molecules, for example Bim, Bid, and Puma, to directly activate Bax [17, 19, 22, 30, 31]. We found no change in the level of expression of Bad protein or its binding partners, for example 14-3-3, Bcl-2, and Bcl-xL in gefitinib-treated cells. However, gefitinib slightly changed Bad’s binding preference from 14-3-3 to Bcl-2 and Bcl-xL. Our findings suggest that Bad binds and antagonizes Bcl-2/Bcl-xL activity and serves as an upstream activator of Bax. This hypothesis was further supported by the reduced phosphorylation of Bad by gefitinib, because phosphorylated Bad binds 14-3-3 with preference and hypophosphorylated Bad dissociated from 14-3-3 and targeted Bcl-2/Bcl-xL [17, 19, 22, 30, 31]. We found that gefitinib reduced Bad phosphorylation in serine-112 with greater preference. The dephosphorylation of Bad in serine-112 occurred before the changes in serine-136 and serine-155. Studies have proposed a sequential model of Bad dephosphorylation initiated by serine-112, which may then expose serine-136 and serine-155 residues for dephosphorylation [30, 32]. Therefore, serine-112 dephosphorylation may be the initial dephosphorylation event required for promoting the apoptotic activity of Bad in gefitinib-treated glioma cells.
Protein kinases are of crucial importance in the regulation of Bad phosphorylation. For example, serine-136 is a preferred substrate for Akt [33]. Mitogen-activated protein kinase (MAPK) and PKA phosphorylate Bad on serines 112 and 155 [22, 34]. The event of Bad dephosphorylation in serine-112 is critical to gefitinib-induced Bad inactivation and resulting cell apoptosis. The involvement of MAPKs in Bad dephosphorylation was primarily excluded because their activity is up-regulated by gefitinib (data not shown). The dephosphorylation of Bad was accompanied by reduced intracellular cAMP content and PKA activity. The gefitinib-induced dephosphorylation of Bad in serine-112 was attenuated by forskolin and augmented by H89. Other biochemical assays showed that forskolin alleviated gefitinib-induced Bax mitochondrial translocation, caspase-9/caspase-3 activation, and viability loss, and that H89 exacerbated these gefitinib-induced alterations. It has been shown that combination of PKA antisense and EGFR inhibitors results in cooperative antitumor activity [35]. Although there might be other potential triggering factors, the inactivation of PKA seemed to be a general event contributing to gefitinib-induced apoptosis, because of the occurrence of apoptosis in H4, T98G, and U87 cells. Dramatic PKA inactivation by higher concentrations of H89 (more than 10 μM) caused viability loss in H4, T98G, and U87 cells. In parallel with the higher basal PKA activity, U87 cells had a delayed and relatively resistant response to gefitinib treatment compared with H4 and T98G cells. Despite the different response to gefitinib treatment, inactivation of PKA by a sub-toxic concentration of H89 (5 μM) sensitized H4, T98G, and U87 cells towards gefitinib cytotoxicity, Bad dephosphorylation in serine-112, and caspase-9/caspase-3 activation. Taken together, our findings provide evidence which supports the postulate that PKA inactivation contributes to gefitinib-mediated Bad dephosphorylation and subsequent mitochondrial apoptotic signaling.
In addition to protein kinase inhibition, activation of protein phosphatase also leads to protein dephosphorylation. However, okadaic acid, a classic protein phosphatase inhibitor, failed to reverse gefitinib-induced Bad dephosphorylation in serine-112. This raised uncertainty about whether protein phosphatases are involved in gefitinib-induced Bad dephosphorylation. In contrast with its insensitivity to Bad phosphorylation in serine-112, okadaic acid alleviated gefitinib-induced Bax mitochondrial translocation, caspase-9/caspase-3 activation, and viability loss. There is evidence that okadaic acid can protect against apoptosis by modulating Bax phosphorylation [36, 37]. The Bax conformational change and relocation to mitochondria is also controlled by phosphorylation [38]. Therefore, the beneficial effect of okadaic acid against gefitinib-induced apoptosis might be independent of Bad phosphorylation and may exert its effect via other mechanisms. The event of Bax phosphorylation might be a potential target.
The coexpression of the EGFRvIII oncogene and the PTEN tumor suppressor protein is strongly associated with clinical response to EGFR inhibitor therapy [39]. In early clinical trials, 10–20% of malignant glioma patients seemed to derive benefit from the EGFR inhibitor gefitinib [12]. H4 glioma cells harbor genetic mutations in PTEN but are not EGFRvIII variant. U87 cells are PTEN-deficient and EGFR-wild type. In contrast, T98G cells contain wild-type PTEN and EGFR [40–43]. Although H4, T98G, and U87 cells expressed different basal amounts of EGFR protein, successful EGFR signaling of these cells showed was observed in response to EGF stimulation. Low concentrations of gefitinib were able to inhibit cell proliferation and almost completely inhibit basal and EGF-stimulated EGFR activity. Because EGFR signaling is important in cell proliferation [3], our findings suggest that the cytostatic effect of low concentration of gefitinib on glioma cells was probably mediated by inhibition of EGFR activation. However, the concentrations of gefitinib needed to induce apoptotic cell death were higher than the concentrations needed to inhibit EGFR phosphorylation. Other investigators also observed similar phenomena in different human glioma cell lines [11, 44–46]. Compared with the cytotoxic concentration of gefitinib, the non-apoptotic concentration of gefitinib (10 μM in H4 and T98G cells and 20 μM in U87 cells) had a negligible effect on Bad dephosphorylation in serine-112. Our findings suggest that gefitinib at higher concentrations might act on additional targets other than EGFR, leading to apoptosis in glioma cells. The low response of these three glioma cell lines to apoptosis might be explained by genetic diversity and/or gefitinib resistance. According to the results of this study, cellular PKA activity and PKA/Bad crosstalk might be crucial determinants. Therefore, it seems that gefitinib induces apoptosis not simply by blocking the tyrosine kinase activity of EGFR but by being involved in multiple mechanisms. A combination of gefitinib and various cytotoxic drugs has been found to have an additive or synergistic antitumor effect. Therefore, new therapeutic strategies incorporating logical combinations of molecular targeted agents may be critical to improving patient outcomes. Evidence suggests that a combination of gefitinib with heat shock protein 90 antagonist (17-AAG), mTOR inhibitor (sirolimus), 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor (lovastatin), or radiation might be a potent strategy for treatment of patients with malignant gliomas [11, 46–48]. In considering expression levels of EGFR protein and gefitinib sensitivity of H4, T98G, and U87 cells, the expression level of EGFR protein and functional EGFR signaling seemed not to be crucial in gefitinib-induced glioma apoptosis. These findings contradicted previous reports showing that EGFR-null human head and neck squamous cell carcinoma cells were resistant to EGFR inhibitors [49]. The detailed signatures of glioma towards gefitinib treatment require further investigation via EGFR-null glioma cells.
Despite advances in the development of diagnosis, the limited treatment options for patients with malignant gliomas emphasize the need for investigation of new agents with novel mechanisms of action. Amplification of the EGFR gene is one of the most common oncogenic alterations in malignant gliomas making it a prime target for therapy. Unfortunately, small-molecule inhibitors of the EGFR, including gefitinib, have little activity in clinical trials for malignant gliomas. Recently, a clinical trial showed that an extremely high gefitinib concentration (~40 μM) is occasionally reached in some human glioblastoma tumors and produces an off-target effect to regulate EGFR downstream effectors irrespective of EGFR inhibition [50]. Our in-vitro study suggests that high concentrations of gefitinib were able to cause glioma cell apoptosis, presumably in an off-target effect involving PKA inactivation. Currently, the translational implication of this study is limited and requires further investigation because of the potential difficulties of achieving such tumor concentrations of gefitinib while avoiding adverse effects on normal tissues.
In conclusion, gefitinib is capable of inhibiting growth and inducing apoptosis of human glioma cells. The Bax-mediated mitochondrial outer membrane permeabilization and consequent intrinsic apoptotic signaling pathway is involved in gefitinib-induced apoptosis of glioma cells. The inactivation of PKA by gefitinib is an upstream signal which triggers Bax activation, a process in which the dephosphorylation of Bad is a crucial event. It should be noted that gefitinib-induced apoptosis was alleviated partly by pharmacological agents in this study. Therefore, the anticancer effects of gefitinib in glioma cells may result from multiple mechanisms. Among these, the cytostatic contribution and extrinsic apoptotic mechanisms caused by gefitinib were not addressed in this study.
References
Nagane M, Huang HJ, Cavenee WK (1997) Advances in the molecular genetics of gliomas. Curr Opin Oncol 9:215–222
Soni D, King JA, Kaye AH, Hovens CM (2005) Genetics of glioblastoma multiforme: mitogenic signaling and cell cycle pathway converge. J Clin Neurosci 12:1–5
Lund-Johansen M, Bjerkvig R, Humphrey PA, Bigner SH, Bigner DD, Laerum OD (1990) Effect of epidermal growth factor receptor on glioma cell growth, migration, and invasion in vitro. Cancer Res 50:6039–6044
Culy CR, Faulds D (2002) Gefitinib. Drugs 62:2237–2248
Frampton JE, Easthope SE (2005) Spotlight on gefitinib in non-small cell lung cancer. Am J Pharmacogen 5:133–136
Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500
Chan SK, Gullick WJ, Hill ME (2006) Mutations of the epidermal growth factor receptor in non-small cell lung cancer-search and destroy. Eur J Cancer 42:17–23
Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH (2002) ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res 62:5749–5754
Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, Huang HJ (1994) A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA 91:7727–7731
Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS, Vogelstein B (1992) Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA 89:2965–2969
Premkumar DR, Arnold B, Pollack IF (2006) Cooperative inhibitory effect of ZD1839 (Iressa) in combination with 17-AAG on glioma cell growth. Mol Carcinog 45:288–301
Rich JN, Reardon DA, Peery T, Dowell JM, Quinn JA, Penne KL, Wikstrand CJ, Van Duyn LB, Dancey JE, McLendon RE, Kao JC, Stenzel TT, Ahmed Rasheed BK, Tourt-Uhlig SE, Herndon JE 2nd, Vredenburgh JJ, Sampson JH, Friedman AH, Bigner DD, Friedman HS (2004) Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol 22:133–142
Jiang Z, Zheng X, Rich KM (2003) Down-regulation of Bcl-2 and Bcl-xL expression with bispecific antisense treatment in glioblastoma cell lines induce cell death. J Neurochem 84:273–281
Ichinose M, Liu XH, Hagihara N, Youle RJ (2002) Extracellular Bad fused to toxin transport domains induces apoptosis. Cancer Res 62:1433–1438
Cartron PF, Oliver L, Martin S, Moreau C, LeCabellec MT, Jezequel P, Meflah K, Vallette FM (2002) The expression of a new variant of the pro-apoptotic molecule Bax, Baxpsi, is correlated with an increased survival of glioblastoma multiforme patients. Hum Mol Genet 11:675–687
Busser B, Sancey L, Josserand V, Niang C, Favrot MC, Coll JL, Hurbin A (2010) Amphiregulin promotes BAX inhibition and resistance to gefitinib in non-small-cell lung cancers. Mol Ther 18:528–535
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9:47–59
Raung SL, Chen SY, Liao SL, Chen JH, Chen CJ (2007) Japanese encephalitis virus infection stimulates Src tyrosine kinase in neuron/glia. Neurosci Lett 419:263–268
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87:99–163
Das A, Banik NL, Ray SK (2010) Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer 116:164–176
Oka N, Wang L, Mi W, Zhu W, Honjo O, Caldarone CA (2008) Cyclosporine A prevents apoptosis-related mitochondrial dysfunction after neonatal cardioplegic arrest. J Thorac Cardiovasc Surg 135:123–130
Harada H, Becknell B, Wilm M, Mann M, Huang LJ, Taylor SS, Scott JD, Korsmeyer SJ (1999) Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell 3:413–422
Chang GC, Hsu SL, Tsai JR, Liang FP, Lin SY, Sheu GT, Chen CY (2004) Molecular mechanisms of ZD1839-induced G1-cell cycle arrest and apoptosis in human lung adenocarcinoma A549 cells. Biochem Pharmacol 68:1453–1464
Chang GC, Yu CT, Tsai CH, Tsai JR, Chen JC, Wu CC, Wu WJ, Hsu SL (2008) An epidermal growth factor inhibitor, gefitinib, induces apoptosis through a p53-dependent upregulation of pro-apoptotic molecules and downregulation of anti-apoptotic molecules in human lung adenocarcinoma A549 cells. Eur J Pharmacol 600:37–44
Piechocki MP, Yoo GH, Dibbley SK, Amjad EH, Lonardo F (2006) Iressa induces cytostasis and augments Fas-mediated apoptosis in acinic cell adenocarcinoma overexpressing HER2/neu. Int J Cancer 119:441–454
Rho JK, Choi YJ, Ryoo BY, Na II, Yang SH, Kim CH, Lee JC (2007) p53 enhances gefitinib-induced growth inhibition and apoptosis by regulation of Fas in non-small cell lung cancer. Cancer Res 67:1163–1169
Ariyama H, Qin B, Baba E, Tanaka R, Mitsugi K, Harada M, Nakano S (2006) Gefitinib, a selective EGFR tyrosine kinase inhibitor, induces apoptosis through activation of Bax in human gallbladder adenocarcinoma cells. J Cell Biochem 97:724–734
Cragg MS, Kuroda J, Puthalakath H, Huang DCS, Strasser A (2007) Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med 4:e316
Sun Q, Ming L, Thomas SM, Wang Y, Chen ZG, Ferris RL, Grandis JR, Zhang L, Yu J (2009) PUMA mediates EGFR tyrosine kinase inhibitor-induced apoptosis in head and neck cancer cells. Oncogene 28:2348–2357
Danial NN (2009) BAD: undertaker by night, candyman by day. Oncogene 27:553–570
Zha J, Harada H, Yang E, Jocker J, Korsmeyer SJ (1996) Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 87:619–628
Chiang CW, Kanies C, Kim KW, Fang WB, Parkhurst C, Xie M, Henry T, Yang E (2003) Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Mol Cell Biol 23:6350–6362
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241
Shimamura A, Ballif BA, Richards SA, Blenis J (2000) Rsk1 mediates a MEK-MAP kinase cell survival signal. Curr Biol 10:127–135
Tortora G, Caputo R, Damiano V, Fontanini G, Melisi D, Veneziani BM, Zunino F, Bianco AR, Ciardiello F (2001) Oral administration of a novel taxane, an antisense oligonucleotide targeting protein kinase A, and the epidermal growth factor receptor inhibitor Iressa causes cooperative antitumor and antiangiogenesis activity. Clin Cancer Res 7:4156–4163
Cohen P, Holmes CFB, Tsukitani Y (1990) Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci 15:98–102
Xin M, Deng X (2006) Protein phosphatase 2A enhances the proapoptotic function of Bax through dephosphorylation. J Biol Chem 281:18859–18867
Garibal J, Hollville É, Renouf B, Tétaud C, Wiels J (2010) Caspase-8-mediated cleavage of Bid and protein phosphatase 2A-mediated activation of Bax are necessary for verotoxin-1-induced apoptosis in Burkitt’s lymphoma cells. Cell Signal 22:467–475
Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, Riggs BL, Horvath S, Liau LM, Cavenee WK, Rao PN, Beroukhim R, Peck TC, Lee JC, Sellers WR, Stokoe D, Prados M, Cloughesy TF, Sawyers CL, Mischel PS (2005) Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med 353:2012–2024
Krex D, Mohr B, Hauses M, Ehninger G, Schackert HK, Schackert G (2001) Identification of uncommon chromosomal aberrations in the neuroglioma cell line H4 by spectral karyotyping. J Neurooncol 52:119–128
Rubenstein M, Shaw M, Mirochnik Y, Slobodskoy L, Glick R, Lichtor T, Chou P, Guinan P (1999) In vivo establishment of T98G human glioblastoma. Methods Find Exp Clin Pharmacol 21:391–393
Zhang R, Banik NL, Ray SK (2007) Combination of all-trans retinoic acid and interferon-gamma suppressed PI3K/Akt survival pathway in glioblastoma T98G cells whereas NF-κB survival signaling in glioblastoma U87MG cells for induction of apoptosis. Neurochem Res 32:2194–2202
Lal B, Goodwin CR, Sang Y, Foss CA, Cornet K, Muzamil S, Pomper MG, Kim J, Laterra J (2009) EGFRvIII and c-Met pathway inhibitors synergize against PTEN-null/EGFRvIII+ glioblastoma xenografts. Mol Cancer Ther 8:1751–1760
Tamura S, Hosoi H, Kuwahara Y, Kikuchi K, Otabe O, Izumi M, Tsuchiya K, Iehara T, Gotoh T, Sugimoto T (2007) Induction of apoptosis by an inhibitor of EGFR in neuroblastoma cells. Biochem Biophys Res Commun 358:226–232
Nakagawa K, Tamura T, Negoro S, Kudoh S, Yamamoto N, Yamamoto N, Takeda K, Swaisland H, Nakatani I, Hirose M, Dong RP, Fukuoka M (2003) Phase I pharmacokinetic trial of the inhibitor gefitinib (Iressa, ZD1839) in Japanese patients with solid malignant tumors. Ann Oncol 14:922–930
Cemeus C, Zhao TT, Barrett GM, Lorimer IA, Dimitroulakos J (2008) Lovastatin enhances gefitinib activity in glioblastoma cells irrespective of EGFRvIII and PTEN status. J Neurooncol 90:9–17
Doherty L, Gigas DC, Kesari S, Drappatz J, Kim R, Zimmerman J, Ostrowsky L, Wen PY (2006) Pilot study of the combination of EGFR and mTOR inhibitors in recurrent malignant gliomas. Neurology 67:156–158
Geoerger B, Gaspar N, Opolon P, Morizet J, Devanz P, Lecluse Y, Valent A, Lacroix L, Grill J, Vassal G (2008) EGFR tyrosine kinase inhibition radiosensitizes and induces apoptosis in malignant glioma and childhood ependymoma xenografts. Int J Cancer 123:209–216
Gustafson DL, Frederick B, Merz AL, Raben D (2008) Dose scheduling of the dual VEGFR and EGFR tyrosine kinase inhibitor vandetanib (ZD6474, Zactima) in combination with radiotherapy in EGFR-positive and EGFR-null human head and neck tumor xenografts. Cancer Chemother Pharmacol 61:179–188
Hegi ME, Diserens AC, Bady P, Kamoshima Y, Kouwenhoven MCM, Delorenzi M, Lambiv WL, Hamou MF, Matter MS, Koch A, Heppner FL, Yonekawa Y, Merlo A, Frei K, Mariani L, Hofer S (2010) Pathway analysis of glioblastoma tissue after preoperative treatment with the EGFR tyrosine kinase inhibitor gefitinib—a phase II trial. Mol Cancer Ther. 10(6):1102–1112. doi:10.1158/1535-7163.MCT-11-0048
Acknowledgments
This study was supported by grants from Central Region Hospital Alliance, Department of Health, Taiwan, ROC (9909) and the Yen Tjing Ling Medical Foundation (CI-97-11). The authors thank AstraZeneca (Cheshire, UK) for the donation of gefitinib.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chang, CY., Shen, CC., Su, HL. et al. Gefitinib induces apoptosis in human glioma cells by targeting Bad phosphorylation. J Neurooncol 105, 507–522 (2011). https://doi.org/10.1007/s11060-011-0632-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0632-3