Abstract
Introduction
Diffuse intrinsic pontine glioma (DIPG) is the most common form of brainstem glioma. The present study was performed to assess if hypofractionated radiotherapy completed in < 3 weeks with temozolomide improves survival in DIPG.
Material and methods
The present study is a phase II open label randomized trial. The study included newly diagnosed patients with DIPG. Patients in arm A received conventional fractionated RT of 60 Gy in 30 fractions over 6 weeks while patients in arm B received hypo-fractionated radiotherapy of 39 Gy in 13 fractions over 2.6 weeks along with concurrent Temozolomide (TMZ) 75 mg/m2 from day 1 to day 17 followed by adjuvant TMZ for six cycles. The survival analysis was performed with modified intention to treat analysis.
Results
A total of 35 patients were randomized. 33 patients were evaluable. 93% (n = 14) of patients in the conventional arm completed treatment while only 17% (n = 3) of the children could complete planned course of treatment in the experimental arm. The median overall survival (OS) was 11 months (95% CI − 7.5 to 14.5 months) in the conventional arm and 12 months (95% CI − 10.5 to 13.5 months) in the experimental arm (p = 0.208). 28% (n = 5) patients in the experimental arm developed grade 3 or 4 hematological toxicity.
Conclusion
The above study shows that hypofractionated radiotherapy with concurrent and adjuvant temozolomide does not improve OS and has higher hematological toxicity. Conventional radiotherapy remains the standard of care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse intrinsic pontine glioma (DIPG) is the most common form of brainstem glioma and accounts for 70% to 80% of all brainstem tumors [1]. Definitive radiotherapy to a dose of 54 to 60 Gy delivered in 30 daily fractions over 6 weeks is the current standard of care [2]. Even after definitive radiation most patients progress within 6 months and have a median survival of < 1 year [3, 4]. Due to this poor outcome, multiple trials have been conducted over the years by changing the fractionation of radiation therapy and by using certain chemotherapeutic agents [3,4,5,6,7,8,9,10]. Most of the trials have failed to improve survival in DIPG. The present study was performed to assess if hypofractionated radiotherapy completed in < 3 weeks with temozolomide improves survival in DIPG. The effect of treatment on toxicity and quality of life was also documented. We report the survival and toxicity analysis in this paper and plan to present our quality of life data later.
Patients and methods
The present study is a phase II open label randomized trial. Randomization was done by a simple computer-generated randomization chart into Arm A (Conventional radiotherapy) and Arm B (Experimental Arm). The study included newly diagnosed patients with DIPG. Patients of either sex from the age of 3 years to 40 years with no prior history of chemoradiotherapy, Karnofsky performance score of greater than 50, normal complete blood count, liver and kidney function tests and a signed informed consent from the patient guardian or the patient itself were included. The patients were included if they had a contrast enhanced MRI (CEMRI) showing features suggestive of diffuse intrinsic pontine glioma with involvement of more than half of pons and encasement of basilar artery. All patients underwent a comprehensive clinical examination including complete neurological examinations. Baseline quality of life assessment was done as per PedsQLTM (Version 4). Blood investigations including complete blood counts, liver function test, and kidney function test were done.
Radiation therapy
For radiotherapy planning, gross tumor volume (GTV) was delineated as the T1 post-contrast abnormality visible in the CEMRI. The Clinical Target Volume (CTV) included T2 FLAIR abnormality with 2 cm isotropic, anatomically constrained expansion. An isotropic expansion of 5 mm was added on CTV to generate the Planning target volume (PTV). Patients in arm A received conventional fractionated RT of 60 Gy in 30 fractions over 6 weeks while patients in arm B received hypo-fractionated radiotherapy of 39 Gy in 13 fractions over 2.6 weeks along with concurrent Temozolomide (TMZ) 75 mg/m2 from day 1 to day 17.
Concurrent and adjuvant TMZ
Concurrent TMZ was given to patients in Arm B at 75 mg/m2 from day 1 to day 17. Four weeks after completion of radiotherapy these patients were started on adjuvant TMZ. The First cycle was prescribed at 150 mg/m2 D1 through D5 and then the dose was increased to 200 mg/m2 D1 through D5 repeated every 4 weeks for a total of 6 cycles based on tolerance.
Dose modification protocol
Toxicity was monitored using CTCAE version 4.03. Toxicity was monitored weekly during chemoradiotherapy and monthly during adjuvant chemotherapy. Any decrease in absolute neutrophil count (ANC) to less than 1000 and platelets to less than 100,000 would result in discontinuation of TMZ with the remaining fractions of radiotherapy. During adjuvant TMZ, a dose reduction by 50 mg/m2 was done when ANC was reduced to less than 1000 or platelet less than 75,000. If toxicity persisted, a further reduction of 50 mg/m2 was done till a minimum of 100 mg/m2. TMZ was discontinued if toxicity beyond this.
Follow up protocol
First follow up was done after 4 weeks of completion of treatment with subsequent follow up at 4 weekly intervals for 6 months. CEMRI Brain was done at first follow up followed by 3 monthly MRI or when patient became symptomatic. Progression was defined as per the RANO criteria and was taken from date of MRI showing unequivocal progression.
Statistical analysis
For sample size calculation, the 1 year OS for the control arm was defined as 35%. The assumption for experimental arm was improvement of 1 year OS to 60%. For a power of 80% with alpha error of 0.05, the sample size was calculated to 63 patients in each arm (126 patients in total). Keeping in mind the rarity of tumour and logistics we decided on an accrual goal of 35 patients over 2 years so as create data to support or refute conduct of a bigger trial. All baseline characteristics were tabulated in an excel sheet and chi square test performed to identify any significant differences between the arms. Categorical variables were summarized by frequency and percentage and quantitative variables by the median and range. The survival analysis was performed with modified intention to treat analysis i.e. patients who were randomized but did not start any form of treatment were excluded but all patients who started treatment were included. This was done as DIPG is highly aggressive and some patients expire even before treatment is started. Inclusion of such patients may skew the results away from an effective treatment. Progression free survival (PFS) and Overall survival (OS) were reported in months and were compared using the log rank test. A p value < 0.05 was considered significant.
Results
Patient characteristics
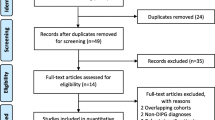
During the study period between July 2016 and May 2018, a total of 35 patients were recruited for this study; 17 patients were randomized to the conventional treatment arm (Arm A) and 18 patients to the experimental arm (Arm B) (Consort diagram). Of this, 20 were females and 15 were males. The median age of patients in this study was 7 years (Range 4–35 years) with median age in Arm-A being 7 years and that of Arm-B 9 years. Fifteen children had presented for treatment within one month of onset of symptoms while twenty children presented with more than one month of symptoms. The most common symptoms were limb weakness followed by visual disturbance in the form of diplopia or squint, ataxia and difficulty in swallowing. All patients had cranial nerve deficits. 4 children required a MPVP shunt (Midline Posterior Ventriculo-Peritoneal Shunt) before start of treatment for management of raised intracranial tension. The patient characteristics are summarized in Table 1.
Treatment characteristics
A total of 33 children were evaluable of which 16 were from conventional treatment arm and 17 from the experimental arm. Two children, one in each arm developed features of raised intracranial pressure and succumbed to the disease even before the treatment could be started. Two children were lost to follow up after the treatment started, one from each arm. One patient in the conventional arm was not able to afford accommodation near the hospital and went back to their native place after 7 fractions of radiotherapy. The second patient chose alternative medicine after 13 fractions of radiotherapy with concurrent temozolomide. 93% (n = 14) of patients in the conventional arm completed treatment while only 17% (n = 3) of the children could complete the planned course of treatment in the experimental arm although all except one (n = 17) completed the course of hypofractionated radiotherapy. Other patients (n = 14) progressed during adjuvant TMZ and therefore could not complete the planned course of treatment. No patient had to discontinue treatment due to toxicity of adjuvant TMZ.
Survival analysis
The median overall survival (OS) was 11 months (95% CI − 7.5 to 14.5 months) in the conventional arm and 12 months (95% CI − 10.5 to 13.5 months) in the experimental arm. This was not statistically significant with a p value of 0.208 (Fig. 1). The median progression free survival (PFS) was 7 months (95% CI − 3.6 to 10.3 months) in the conventional arm and 8 months (95% CI − 6.7 to 9.3 months) in the experimental arm. This was not statistically significant with a p value of 0.198 (Fig. 1). The estimated 18 month OS in arm A was 11% while for arm B it was 27%. At last follow up, 3 (18.7%) patients in arm A and 8 (47.1%) patients in arm B were alive.
Toxicity
28% (n = 5) of patients in the experimental arm developed grade 3 or 4 hematological toxicity during adjuvant temozolomide of whom all 5 developed grade 3 or 4 thrombocytopenia, 2 children developed grade 3 or 4 leukopenia and neutropenia and one developed grade 3 anemia. None of the children had any grade 3 or grade 4 toxicity associated with concurrent temozolomide. In the conventional treatment arm, 7% (n = 1) developed grade3 or 4 toxicity, in the form of a subdural hemorrhage while on radiation after a dose of 48 Gy (24 fractions). He was subsequently diagnosed to have intra-tumoral bleeding and was admitted under the department of neurosurgery and managed conservatively for the same. The child was not given any further radiation and was kept on monthly follow up. There was no documented radiation necrosis on MRI in any of the patients in both arms. There was also no documented grade 2 or higher fatigue during course of radiation in both arms. The toxicities are summarized in Table 2.
Discussion
The management of DIPG is one of the biggest challenges in pediatric oncology. Andrea Chassot et al. [5] evaluated the efficacy and toxicity of radiotherapy to a dose of 54 Gy in 30 fractions along with concurrent TMZ (75 mg/m2/day) followed by adjuvant TMZ (200 mg/m2/day, days 1–5) in 21 children with median age of 6.4 years with DIPG. Four weeks after completing the initial RT–TMZ schedule, adjuvant TMZ was given every 28 days up to six cycles. They showed a median survival of 11.7 months. The 1-year PFS and the 1-year OS were 33 and 50%, respectively. A similar study was conducted by Jalali et al. [7] in 20 children with DIPG who were treated with focal RT to a dose of 54 Gy in 30 fractions along with concurrent daily TMZ (75 mg/m2, Days 1–42). Four weeks after completing the initial RT–TMZ schedule, adjuvant TMZ (200 mg/m2, Days 1–5) was given every 28 days to a maximum of 12 cycles. They showed a median overall survival and progression-free survival were 9.15 months and 6.9 months, respectively. Both the above studies concluded that there was no improvement in survival with the addition of Temozolomide to conventional radiotherapy along with the added toxicity of Temozolomide. The same was shown in a phase 2 trial by Cohen et al. [3]. It should be noted that the above studies were a single arm, non-randomized trial, and the conclusion of the same should be taken with caution. A randomized controlled trial by Zaghoul et al. [6] compared hypofractionated radiotherapy with 39 Gy in 13 fractions over 2.6 weeks to conventional radiotherapy of 54 Gy in 30 fractions over 6 weeks in the treatment of 71 children with DIPG. The 18-month OS difference was 2.2% in that study with the conventional arm doing better. In view of single arm design of some of the studies using TMZ and negative results by using only hypofractionation we decided to test in a randomized manner the effect of hypofractionation with TMZ in comparison to standard of care. The present randomized trial reports a slightly better OS in the experimental arm but at the cost of increased incidence of hematologic toxicity. The median OS was 11 months in the conventional arm and 12 months in the experimental arm with a p value of 0.208. Jalali et al. [7] reported Grade 3 or 4 toxicity during the concurrent TMZ which included thrombocytopenia in 3 patients, leucopenia in 2, and vomiting in 7. During the adjuvant TMZ phase, they reported Grade 3 and 4 leucopenia, which developed in 2 patients and Grade IV thrombocytopenia in 1 patient which accounted for 35% of the patients in their cohort. This was similar to the toxicity in our study with 7% and 28% patients in the conventional and hypofractionated arm developing grade 3 or 4 toxicity respectively. Unlike described in the above trial, our study did not show any toxicity during the phase of concurrent temozolomide. This could be because concurrent TMZ was given for a lesser duration (3 weeks) in our study.
This study does come with the limitations of a small sample size and no blinding in the study. The strength of this study is that this is the only randomized study comparing conventional treatment with a new experimental treatment which includes hypofractionated radiotherapy along with concurrent and adjuvant temozolomide.
Conclusion
The above study shows that hypofractionated radiotherapy with concurrent and adjuvant temozolomide shows no significant improvement in OS as compared with present standard of care and has higher hematological toxicity. Conventional radiotherapy remains the standard of care.
References:s
Walker DA, Punt JA, Sokal M (1999) Clinical management of brain stem glioma. Arch Dis Child 80(6):558–564
Warren KE (2012) Diffuse intrinsic pontine glioma: poised for progress. Front Oncol 2:205
Cohen KJ, Heideman RL, Zhou T, Holmes EJ, Lavey RS, Bouffet E et al (2011) Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group. Neuro Oncol 13(4):410–416
Mandell LR, Kadota R, Freeman C, Douglass EC, Fontanesi J, Cohen ME et al (1999) There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys 43(5):959–964
Chassot A, Canale S, Varlet P, Puget S, Roujeau T, Negretti L et al (2012) Radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. J Neurooncol 106(2):399–407
Zaghloul MS, Eldebawy E, Ahmed S, Mousa AG, Amin A, Refaat A et al (2014) Hypofractionated conformal radiotherapy for pediatric diffuse intrinsic pontine glioma (DIPG): a randomized controlled trial. Radiother Oncol 111(1):35–40
Jalali R, Raut N, Arora B, Gupta T, Dutta D, Munshi A et al (2010) Prospective evaluation of radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys 77(1):113–118
Frappaz D, Schell M, Thiesse P, Marec-Bérard P, Mottolese C, Perol D et al (2008) Preradiation chemotherapy may improve survival in pediatric diffuse intrinsic brainstem gliomas: final results of BSG 98 prospective trial. Neuro-oncology 10(4):599–607
Korones DN, Fisher PG, Kretschmar C, Zhou T, Chen Z, Kepner J et al (2008) Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children's Oncology Group phase II study. Pediatr Blood Cancer 50(2):227–230
Bernier-Chastagner V, Grill J, Doz F, Bracard S, Gentet JC, Marie-Cardine A et al (2005) Topotecan as a radiosensitizer in the treatment of children with malignant diffuse brainstem gliomas: results of a French Society of Paediatric Oncology Phase II Study. Cancer 104(12):2792–2797
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any potential conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee.
Informed consent
It was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Izzuddeen, Y., Gupta, S., Haresh, K.P. et al. Hypofractionated radiotherapy with temozolomide in diffuse intrinsic pontine gliomas: a randomized controlled trial. J Neurooncol 146, 91–95 (2020). https://doi.org/10.1007/s11060-019-03340-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03340-7