Abstract
To review the safety and efficacy of linear accelerator-based stereotactic radiosurgery (SRS) for brainstem metastases. We reviewed all patients with brain metastases treated with SRS at DF/BWCC from 2001 to 2009 to identify patients who had SRS to a single brainstem metastasis. Overall survival and freedom-from-local failure rates were calculated from the date of SRS using the Kaplan–Meier method. Prognostic factors were evaluated using the log-rank test and Cox proportional hazards model. A total of 24 consecutive patients with brainstem metastases had SRS. At the time of SRS, 21/24 had metastatic lesions elsewhere within the brain. 23/24 had undergone prior WBRT. Primary diagnoses included eight NSCLC, eight breast cancer, three melanoma, three renal cell carcinoma and two others. Median dose was 13 Gy (range, 8–16). One patient had fractionated SRS 5 Gy ×5. Median target volume was 0.2 cc (range, 0.02–2.39). The median age was 57 years (range, 42–92). Follow-up information was available in 22/24 cases. At the time of analysis, 18/22 patients (82%) had died. The median overall survival time was 5.3 months (range, 0.8–21.1 months). The only prognostic factor that trended toward statistical significance for overall survival was the absence of synchronous brain metastasis at the time of SRS; 1-year overall survival was 31% with versus 67% without synchronous brain metastasis (log rank P = 0.11). Non-significant factors included primary tumor histology and status of extracranial disease (progressing vs. stable/absent). Local failure occurred in 4/22 cases (18%). Actuarial freedom from local failure for all cases was 78.6% at 1 year. RTOG grade 3 toxicities were recorded in two patients (ataxia, confusion). Linac-based SRS for small volume brainstem metastases using a median dose of 13 Gy is associated with acceptable local control and low morbidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases represent the most common intracranial tumor in clinical practice, with an estimated annual incidence of 200,000 cases in the United States. Brainstem metastases are relatively uncommon, however, comprising approximately 5% of brain metastases [1, 2]. Stereotactic radiosurgery is a potentially important treatment modality for brainstem metastases. The management of brainstem metastases presents a unique therapeutic challenge. Surgical resection is not feasible in most instances due to potentially significant morbidity. In the brainstem, both uncontrolled tumor growth and stereotactic radiosurgery have the potential to cause significant neurologic deficits. This potential for morbitiy coupled with a concern regarding possible increased radiosensitivity of brainstem tissue this has led to the exclusion of brainstem metastases from various radiosurgery clinical trials [3–5]. Data on the safety and efficacy of stereotactic radiosurgery in the management of brainstem metastases is lacking therefore, and relies on retrospective reports from single institutions [6–14]. Nine previous reports have been published. All but one utilized the Elekta Gamma Knife radiosurgical delivery system. Here we report the Dana-Farber/Brigham and Women’s experience with stereotactic radiosurgery in the treatment of single brainstem metastases. This is the only the second reported experience of linear accelerator-based radiosurgery for brainstem metastases.
Materials and methods
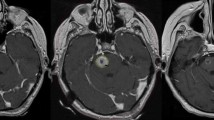
This retrospective study was approved by the Dana-Farber/Harvard Cancer Center institutional review board. All cases of stereotactic radiosurgery performed at Brigham and Women’s Hospital between January 1st 2001 and December 30th 2009 were reviewed to identify patients who received stereotactic radiosurgery for a single brainstem metastasis. In total, 24 consecutive patients were identified. Brainstem stereotactic radiosurgery was offered predominantly as a salvage therapy following previous whole brain radiotherapy (N = 20). However, less frequently it was used as a boost in conjunction with whole brain radiotherapy (N = 3) or as the upfront treatment in patients who refused whole brain radiotherapy (N = 1). The majority of patients (16/24) had brainstem metastases detected on surveillance neuroimaging prior to developing symptoms. Only 4 patients presented with symptoms attributable to brainstem metastases (ataxia, ptosis, cranial nerve III and IV palsies). Four other patients presented with symptomatic brain metastases located outside of the brainstem and had an incidental finding of a brainstem metastasis on MRI.
Stereotactic radiosurgery was performed using the NovalisTM linear accelerator-based radiosurgery platform. Until April 2009 all patients were immobilized using a BRW (Radionics) stereotactic head frame. After April 2009 conventional frame-based radiosurgery at our institution was replaced by frameless delivery using the thermosplastic BrainLAB cranial mask immobilization. A clinical comparison at our institution of patient setup and intra-fraction motion using frame-based radiosurgery versus frameless image-guided radiosurgery for intracranial lesions has previously been reported [15]. The target volume was contoured on fused contrast-enhanced computed tomography and magnetic resonance images in all cases. No margin was added to the contrast-enhancing lesion to define the planning target volume (PTV). Planning was performed using the BrainLAB BrainSCANTM stereotactic treatment planning software. A stereoscopic kilovoltage X-ray system combined with infrared (IR) position tracking (Initially ExacTrac version 3.5 later version 5.0 by BrainLAB) was used for patient localization.
As a balance between efficacy and risk of clinically significant side effects our institutional standard would be to prescribe radiosurgery doses to non-brainstem targets marginally lower than those employed in the Radiation Therapy Oncology Group 95-08 randomized trial. Owing to concern regarding the risk of a potential neurologic deficit following radiosurgery to the brainstem, the prescription dose for radiosurgery was reduced compared to non-brainstem targets. This would constitute a 20–25% reduction on dose employed for non-brainstem targets. Generally plans were normalized to between the 70 and 80% isodoses, but were optimized in individual cases. The institutional practice is to aim for 99% coverage of the target by the prescription doses. Conformity index defined as the ratio of the volume enclosed by the prescription isodose/planning target volume was calculated in all cases. Homogeneity index defined as the ratio of the Dmax/prescribed dose was also calculated in all cases.
Patients underwent a first follow-up MRI at 4–8 weeks following radiosurgery. Many patients had serially MRI scans at 3–4 month intervals. Lack of follow-up imaging in certain cases was due to rapid clinical deterioration or a wish to undergo follow-up at local institutions.
Overall survival and freedom-from local failure were calculated from the date of radiosurgery using the Kaplan–Meier method. Freedom-from local failure was defined as an absence of progression on follow-up MRI studies and would include complete response, partial response and stable disease as defined the Response Evaluation Criteria in Solid Tumors (RECIST) criteria [16]. Patients were censored at the time of last clinical follow-up for estimating overall survival and at the time of last MRI for estimating freedom-from local progression. Prognostic factors for overall survival were evaluated using the log-rank test and Cox proportional hazards model. (Table 1)
Results
A total of 24 patients (10 males, 14 females) underwent stereotactic radiosurgery at Brigham and Women’s Hospital for a single brainstem metastasis during the 9 year period included in this study. With respect to performance status, all but one of the patients was self caring (Karnofsky performance score≥70%) at the time of radiosurgery. The most frequent primary tumor histologies were non-small cell lung carcinoma (n = 8) and breast carcinoma (n = 8). Other primary tumors included melanoma (n = 3), renal cell carcinoma (n = 3) and others (n = 2). Brainstem metastases were located in the midbrain (n = 10), the pons (n = 13) and the medulla (n = 1).
Notably stereotactic radiosurgery was offered as a salvage treatment following previous whole brain radiotherapy in the vast majority of cases (n = 20). The median dose of previously delivered whole brain radiotherapy was 35 Gy (range 30–40). 21/24 cases (87%) had at least 1 synchronous brain metastasis at the time of brainstem radiosurgery.
The median prescription dose was 13 Gy (range 8–16), with a mode of 12 Gy. One patient underwent fractionated stereotactic radiosurgery 5 Gy × 5. The median planning target volume was 0.2 cm3 (range 0.02–2.39). At least 98% of the target was covered by the prescription isodose in all cases. Median normalization was 73% (range 52–90%). The median conformity and homogeneity indices were 1.71 and 1.39, respectively.
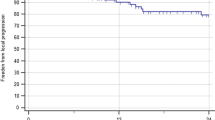
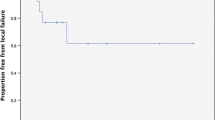
Follow-up imaging was available in 22/24 cases. At the time of analysis 18/22 patients had died. Of those patients who died, 15/18 died of progressive systemic disease. Three deaths were due to progressive CNS disease, including 2 cases of leptomeningeal dissemination. No death was attributable to progressive brainstem disease. The median overall survival following brainstem radiosurgery was 5.3 months (range 0.8–21.1 months). At the time of analysis the four surviving patients had been followed for 5, 8, 14 and 21 months. Actuarial 1-year overall survival was 29%. The only prognostic factor that trended toward statistical significance for overall survival was the absence of synchronous brain metastasis as the time of radiosurgery (1-year overall survival with vs. without synchronous brain metastasis was 31 vs. 67%, log-rank P = 0.11). Non-significant factors included primary tumor histology (P = 0.69) and status of extracranial disease, progressing versus stable/absent (P = 0.41). Local failure occurred as the site of first failure in 4/22 evaluable cases. Isolated distant failure occurred in 13/22 evaluable cases, while simultaneous local and distant failure occurred in 3/22 cases. Actuarial Freedom-From Local Failure was 88.2% at 6 months and 78.6% at 1 year.
Of the patients who presented with symptoms attributable to brainstem metastasis (N = 4), two had symptomatic improvement following SRS (ataxia, ptosis). Two patients had persistent cranial nerve deficits following SRS. However, in follow-up only one patient developed symptoms related to local failure (gaze palsy).
RTOG grade 3 toxicities were recorded in two patients (one patient developed ataxia 10 days post-radiosurgery, which resolved with corticosteroids and a further patient developed acute confusion 48 h following radiosurgery). Both patients progressed at distant sites within the brain on the first follow-up MRI. No late radiation side effects were recorded.
Discussion
Conventional teaching is that the brainstem is more radiosensitive than other regions of the brain [17, 18]. Evidence in support of this is lacking, however. This conclusion is likely based on an early publication by Boden [19], who reported a high incidence of radionecrosis after fractionated radiotherapy for nasopharyngeal or middle ear tumors. It is likely that radiation injury to the brainstem is more likely to result in clinically apparent toxicity than is radiation injury to other locations within the brain given the close aggregation of fiber tracts and cranial nerve nuclei. Furthermore salvage surgery for radionecrosis secondary to stereotactic radiosurgery is not usually a feasible option. Corticosteroids form the mainstay of therapy in the event of radionecrosis, although more recently vascular endothelial growth factor inhibitors, such as bevacizumab, have shown promise in this regard [20, 21]. It is, therefore, the functional consequence of a complication that is the primary concern in delivering radiosurgery to brainstem targets.
Reports on brainstem stereotactic radiosurgery can be divided into treatment of intrinsic lesions, such as metastases or vascular malformations, or extrinsic lesions, such as acoustic neuromas. To date, a total of nine reports of brainstem radiosurgery for metastases have been published [6–14]. All reports reflect retrospective experience at single institutions. All but one used the Gamma Knife radiosurgery delivery system. This is the second reported series of stereotactic radiosurgery to brainstem metastases using linac-based radiosurgery. From a toxicity standpoint, previous reports quote a 0–11% of severe or life-threatening complications following radiosurgery. These results, in line with ours, are comparable to reported rates in the two radiosurgery randomized trials, which both excluded brainstem lesions [4, 5]. However, all brainstem series are limited by retrospective design and the fact that the likelihood of observing late radiation injury in this patient population is limited by poor life expectancy. The recent QUANTEC review of radiation associated brainstem injury addressed studies of single-fraction brainstem radiosurgery for both intrinsic and extrinsic lesions [22]. Most conclusions were drawn from the largest study which reported the incidence of cranial neuropathy following radiosurgery for 149 patients with vestibular schwannoma. Robust recommendations regarding ‘safe’ dose limits are unavailable. Whether radiosurgery to specific locations within the brainstem are more likely to precipitate injury is unknown. In a series of brainstem radiosurgery for arteriovenous malformations, Maruyama et al. [23] reported that the risk of neurologic complications was significantly associated with tectal midbrain location, possibly related to the location of multiple cranial nerve nuclei in this area. In our series both grade 3 toxicities were observed in patients with midbrain lesions, however, neither lesion was located in the tectum. Sharma et al. [24] reported an 18.4% incidence of new imaging findings, defined as adverse radiation imaging effects (ARIEs) after brainstem radiosurgery to median prescription dose of 15 Gy. All patients had non-malignant diagnoses, predominantly vascular anomalies. ARIEs appeared to have a linear relationship with marginal dose escalation from 12 to 25 Gy. Unsurprisingly, the development of adverse radiation effects on MRI correlated with the new neurologic deficits. In contrast, 3 out of a total of 38 patients in this cohort developed new neurologic symptoms in the absence of imaging abnormality, which highlights the limited sensitivity of standard MRI sequences in detecting all clinically significant effects of radiosurgery.
Owing to a balance between toxicity concerns and expected efficacy we utilized lower median dose (13 Gy) than previous reports (median dose range 15–20 Gy). It should be noted that our series included a higher proportion of patients who had undergone previous whole brain radiotherapy (96% vs. 13.6–92.3% in prior reports). Current practice in many radiosurgery centers would be to withhold WBRT in the upfront management of patients with limited numbers of brain metastases, owing to a concern regarding neurocognitive side effects and the lack of a survival benefit to the addition of WBRT to SRS in two randomized trials [5, 25]. No firm recommendations exist regarding prescription dose for patients who have not had prior WBRT, although from the limited retrospective data available, doses in the range of 15–16 Gy for lesions of volumes less than 1 cc appear to be associated with a low risk of symptomatic radionecrosis. However, life expectancy is likely to influence the incidence of radionecrosis, with longer term survivors at higher risk of late radiation side effects. Freedom-from local failure was comparable to previous reports (78.6% vs. 77–100%). With a median target volume of 0.2 cm3, our cohort had smaller target volumes than all previous reports, which may explain why the rate of local control was not reduced by using a lower median dose. The small volume of tumors in our series is likely reflected in the fact that only four patients presented with symptoms attributable to their brainstem metastasis. 2/4 cases improved symptomatically following SRS. Significantly, only 1/4 cases of local failure developed a new symptom related to progressive brainstem disease (gaze palsy).
Our study is limited by a small sample size and retrospective design, however, no prospective study on this subject has been published. We believe our study demonstrates the safety and efficacy of linear accelerator-based radiosurgery as a salvage therapy for small volume brainstem metastases following previous whole brain radiotherapy. However, a single-arm prospective multi-institutional protocol would better address the true incidence of radiation injury.
Conclusion
In patients with good performance status (KPS>70%), linear accelerator-based stereotactic radiosurgery, using a median dose of 13 Gy, as a salvage therapy for small volume single brainstem metastases following previous whole brain radiation therapy is associated with acceptable rates of local control and acute side effects. Absence of late radiation side effects may be a function of poor survival in this population.
References
Chason JL, Walker FB, Landers JW (1963) Metastatic carcinoma in the central nervous system and dorsal root ganglia. A prospective autopsy study. Cancer 16:781–787
Delattre JY, Krol G, Thaler HT et al (1988) Distribution of brain metastases. Arch Neurol 45:741–744
Shaw E, Scott C, Souhami L et al (2000) Single dose radiosurgical treatment of previously irradiated primary brain tumors and brain metastases: final report of RTOG 90–05. Int J Radiat Oncol Biol Phys 47:291–298
Andrews DW, Scott CB, Sperduto PW et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672
Aoyama H, Shirato H, Tago M et al (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491
Koyfman SA, Tendulkar RD, Chao ST et al (2010) Stereotactic radiosurgery for single brainstem metastases: the cleveland clinic experience. Int J Radiat Oncol Biol Phys 78(2):409–414
Lorenzoni JG, Devriendt D, Massager N et al (2009) Brainstem metastases treated with radiosurgery: prognostic factors of survival and life expectancy estimation. Surg Neurol 71:188–195
Kased N, Huang K, Nakamura JL et al (2008) Gamma knife radiosurgery for brainstem metastases: the UCSF experience. J Neuro-oncol 86:195–205
Hussain A, Brown PD, Stafford SL et al (2007) Stereotactic radiosurgery for brainstem metastases: survival, tumor control, and patient outcomes. Int J Radiat Oncol Biol Phys 67:521–524
Fuentes S, Delsanti C, Metellus P et al (2006) Brain stem metastases: management using gamma knife radiosurgery. Neurosurgery 58:37–42
Yen CP, Sheehan J, Patterson G et al (2006) Gamma knife surgery from metastatic brain stem tumors. J Neurosurgery 105:213–219
Shuto T, Fujino H, Asada H et al (2003) Gamma knife radiosurgery for metastatic tumours in the brain stem. Acta Neurochir (Wien) 145:755–760
Huang CF, Kondziolka D, Flickenger JC et al (1999) Stereotactic radiosurgery for brain stem metastases. J Neurosurg 91:563–568
Valery CA, Boskos C, Boisserie G et al (2010) Minimized doses for linear accelerator radiosurgery of brainstem metastasis. Int J Radiat Oncol Biol Phys. doi:10.1016/j.ijrobp.2010.02.028
Ramakrishna N, Rosca F, Friesen S et al (2010) A clinical comparison of patient setup and intra-fraction motion using frame-based radiosurgery versus a frameless image-guided radiosurgery system for intracranial lesions. Radiother Oncol 95:109–115
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European J Cancer Vol 45:228–247
Sheline GE, Wara WM, Smith V (1980) Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys 6:1215–1228
Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE et al (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21:109–122
Boden G (1950) Radiation myelitis of the brain stem. J Fac Radiol 2:79–94
Torcuator R, Zuniga R, Mohan YS, Rock J, Doyle T, Anderson J, Gutierrez J, Ryu S, Jain R, Rosenblum M, Mikkelsen T (2009) Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol 94(1):63–68
Liu AK, Macy ME, Foreman NK (2009) Bevacizumab as therapy for radiation necrosis in four children with pontine gliomas. Int J Radiat Oncol Biol Phys 75(4):1148–1154
Mayo C, Yorke E, Merchant TE (2010) Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys 76(3 Suppl):S36–S41. Review
Maruyama K, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD (2004) Stereotactic radiosurgery for brainstem arteriovenous malformations: factors affecting outcome. J Neurosurg 100(3):407–413
Sharma MS, Kondziolka D, Khan A, Kano H, Niranjan A, Flickinger JC, Lunsford LD (2008) Radiation tolerance limits of the brainstem. Neurosurgery 63(4):728–732 Discussion 732–733
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortman RD, Carrie C, Hassel MB, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP (2010) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. doi:10.1200/JCO.2010.30.1655
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kelly, P.J., Lin, Y.B., Yu, A.Y.C. et al. Linear accelerator-based stereotactic radiosurgery for brainstem metastases: the Dana-Farber/Brigham and Women’s Cancer Center experience. J Neurooncol 104, 553–557 (2011). https://doi.org/10.1007/s11060-010-0514-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0514-0