Abstract
Meningiomas sometimes appear to stop growing after attaining a large size. Commonly used exponential growth models do not reflect this phenomenon. We attempted to find the best curve to simulate their growth. Fifty-two patients with meningioma were followed up for 3.1–21.7 years (mean 7.5 years) with four or more imaging studies each. Thirty-one patients had asymptomatic tumors. The other 21 patients with residual or recurrent tumor were followed up after surgery. Time–volume curves for each tumor were plotted. Nonlinear regression analyses were performed against power, exponential, logistic, and Gompertzian curves. Time–volume curves corresponded to the Gompertzian and logistic growth curves better than to power or exponential curves. When simulating time–volume curves with Gompertzian curves, the majority of benign meningiomas began to slow their growth before patient age of 80 years. Twenty-three of 31 asymptomatic meningiomas had already passed the inflection point before diagnosis. In contrast, this happened less frequently in symptomatic tumors. Especially, all six atypical meningiomas continued to grow quasi-exponentially. Sigmoid curves that approach a plateau were better descriptors of the growth of benign meningiomas than were curves of unlimited growth. However, atypical meningiomas were unlikely to slow their growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Meningiomas usually grow slowly with a long initial asymptomatic phase, and may remain silent until the patient’s death. The incidence of asymptomatic meningioma found at autopsy is 1–2.3% [1–3]. On the other hand, the incidence of symptomatic meningiomas found in population-based studies is 2–4 per 100,000 person-years [4–6]. These results imply that only a small proportion of meningiomas become symptomatic. Actually, only 3–6% of clinically detected asymptomatic meningiomas later become symptomatic [7–9]. This may be due to slow growth of incidentally found asymptomatic meningiomas. Another explanation might be that meningiomas may stop growing at some point, and a key difference between symptomatic and asymptomatic tumors may be their size when they stop growing.
Figures for the proportion of asymptomatic meningiomas which were found to be growing when they were discovered have varied from 11% to 100% [7–15]. This wide range of rate figures is due to differences between measurement methods and the length of follow-up periods. Exact volumetric measurement using image-analysis software usually showed higher rates of tumor growth than calculations using diameter as a growth indicator. Even among studies based on exact volumetry, very few of them set a cutoff point for discriminating between growing and nongrowing tumors in view of measurement error [10, 16].
As the growth rate of a tumor directly affects the patient’s prognosis and therapeutic options, many investigators have attempted to find a model for meningioma growth [9, 12–14, 16–21]. Most of these studies were based on the hypothesis that each meningioma grows at a constant rate. One long-term observation in a limited number of cases revealed that meningiomas showed three growth patterns, namely exponential, linear, and no growth [16]. Other observations including 70 cases for a mean follow-up period of 3.3 years supported that idea [10]. However, both of these studies raise the possibility that these growth patterns may change in the long term; exponentially growing tumors may decelerate their growth and even stop growing. If this is true, it may not be appropriate to use a linear or exponential curve to predict the growth of meningiomas in the long term. Better understanding of the growth kinetics of the tumor is of paramount importance for choice of more successful strategies. We attempted to analyze the growth of meningiomas using various curves to find the best growth model.
Materials and methods
Because growth analysis requires multiple imaging studies over a sufficiently long period, we studied patients with meningiomas that were followed up without radiation therapy, with four or more radiological studies over more than 3 years. We examined the clinical records of patients with meningioma during the period between 1980 and 2004 at the Department of Neurosurgery, Shiga University of Medical Science and during the period between 2001 and 2004 at the Division of Neurosurgery, Shizuoka Cancer Center. Neurofibromatosis cases were excluded. We identified 52 patients, 13 men and 39 women; their median age was 57.6 years at their first imaging study, and the mean follow-up period was 7.7 years (range 3.1–21.7 years). Of those 52, 31 had asymptomatic tumors, and the other 21 were postoperative patients with residual or recurrent tumors; of those 21, 6 had atypical meningioma while the other 15 had benign meningioma. In the 21 patients with residual or recurrent tumors, 5 (that is, 4 atypical and 1 benign) had undergone macroscopically total resection; the volumes of the tumors immediately after macroscopically total resection were defined as equal to 0.1 cm3, as in two previous studies [18, 19]. A further five patients underwent another surgery during the follow-up. Tumor growth in those patients was analyzed both after the first surgery and after the second surgery separately, yielding data for two growth curves each. In each of those five cases the growth curve for the longer follow-up period was used for statistical analysis. The majority of cases had at least one plain computed tomography (CT) scan, although nonenhanced scans were not employed in volume analysis.
Tumors were classified by histology according to the World Health Organization (WHO) classification system [22].
Measurement of tumor volume
Images obtained from CT scanning or magnetic resonance imaging (MRI) were taken into a computer system. Although MRI was used for analysis in most cases, CT scanning was mainly employed in three cases. The tumor area in each slice was measured using NIH image software 1.62, and the tumor volume was calculated by the slice thickness of the image. Each volume was measured three times, and the mean value was calculated. Any change less than 8.2% between the initial and the last volume was considered as no change because the mean standard deviation was 4.1% of the calculated volume using this method [16].
Curve fitting
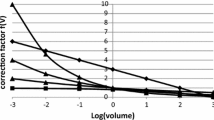
We tested four representative growth curves for analysis of meningioma growth (Fig. 1). Exponential growth is the most commonly used tumor growth model. In this model, the cells divide at a constant rate with constant cell loss, and increase perpetually. The power law is a growth model that shows various shapes of growth curves such as root, linear, and parabolic curves. This group of curves exhibits unlimited growth with various changes in the growth rate. Another class of growth models consists of sigmoid curves such as the logistic and Gompertzian curves. These show quasi-exponential proliferation followed by gradually decelerating growth rate. The point at the beginning of deceleration is called the inflection point, after which the curve approaches a plateau.
A time–volume curve was plotted for each tumor. Nonlinear regression analysis against power, exponential, logistic, and Gompertzian growth curves (Fig. 2, equations shown in the Appendix) were performed using Statistica 4.1 (StatSoft, Tulsa, OK, USA). The correlation coefficients (R) and coefficients of determination (R 2) were calculated. In the Gompertzian and logistic analysis of the time–volume curves, inflection points were calculated from the equation in the Appendix. Patient age at the inflection point was compared with patient age at the first imaging study and at last follow-up, to find whether and when each tumor began to slow its growth. In the previous study, the deceleration of growth speed of benign meningiomas appeared to be related to tumor age [16]. We attempted to analyze patient age when the tumor began to slow its growth (inflection point) using Kaplan–Meier method.
Statistical methods
Statistical analyses were performed using Statistica 4.1 (StatSoft, Tulsa, OK, USA) and Stata version 11.1 (StataCorp LP, College Station, TX, USA). Coefficients of determination were summarized by their quartiles for all cases (N = 44), and separately for benign tumors (n = 38) and atypical tumors (n = 6). Quartiles were estimated by linear interpolation between neighboring sample values as necessary. Comparisons were made between model types (exponential, power, logistic, and Gompertzian) by Wilcoxon’s signed-rank test. Comparisons were made for all tumors, and separately for benign tumors and atypical tumors. Bonferroni’s inequality was used to adjust P-values for multiple comparisons.
We analyzed patient age at the inflection point using a Kaplan–Meier method. Patients who had not passed the inflection point at last follow-up were censored in the analysis.
Results
Of the 52 patients with meningioma, 44 patients’ tumors displayed measurable growth during the observation period covered in this report. Summary statistics for coefficients of determination of curve fits for those 44 tumors are shown in Tables 1 (all tumors), 2 (benign tumors only), and 3 (atypical tumors only). For all tumors (Table 1), as well as for the subset of nonmalignant meningiomas (Table 2), median R 2 was higher for logistic and Gompertzian model types than for exponential and power. R 2 for power model type was higher than that for exponential. In terms of R 2, logistic and Gompertzian models fitted equally well to longitudinal measurements of tumor volume. For the subset of atypical meningiomas (Table 3), a statistically significant difference in R 2 between model types was not detected.
One example of growth of a benign recurrent meningioma, with the longest follow-up in this series, is shown in Fig. 2. The meningioma enlarged about eight times during the first 79 months, and less than twice during the next 89 months. The time–volume curve was fitted by a Gompertzian curve or a logistic curve better than by a power or exponential curve.
Because patient age at the inflection point calculated from Gompertzian curves correlated highly with those from logistic curves (R = 0.98, P < 0.000001) (Fig. 3) and the mean ages were almost equal (60.6 ± 11.2 versus 59.8 ± 11.7 years), we performed the following analysis using the data from Gompertzian curves. Kaplan–Meier analyses of the inflection point in each patient indicated that the majority of benign meningiomas begin to slow their growth before the age of 80 years (Fig. 4). In contrast, atypical meningiomas never decelerated their growth (log-rank test, P = 0.04, Fig. 4).
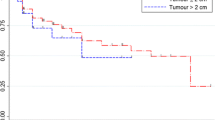
Of tumors with calcification, eight showed no growth, as their mean volume change was measured at 2.8% over the mean 5.7 years. Their growth curves were considered to be in the plateau phase of the Gompertzian or logistic curve. Of the 31 asymptomatic meningiomas, 23 had already passed their inflection point (and 8 of those had stopped growing) before diagnosis, and 3 more passed their inflection point during the mean follow-up period of 5.7 years. Of the 15 symptomatic but benign meningiomas, 6 passed their inflection point before diagnosis, and 5 more did so during the mean follow-up period of 9.9 years. Asymptomatic meningiomas had passed the inflection point before diagnosis more frequently than had symptomatic benign meningiomas (χ 2-test, P = 0.025). However, Kaplan–Meier analyses showed no difference of patient age at inflection point between symptomatic and asymptomatic meningiomas (log-rank test, P = 0.53, Fig. 5). This was partly due to older age at diagnosis in patients with asymptomatic meningiomas (64.5 ± 9.6 years old) than those with symptomatic benign ones (52.2 ± 11.0 years old) (Mann–Whitney U test, P = 0.0008). In contrast, none of the atypical meningiomas passed the inflection point.
Discussion
We showed that the growth curves of meningiomas fitted the sigmoid Gompertzian and logistic curves with high R 2. We previously reported that meningiomas matched three growth patterns, namely exponential, linear, and no growth [16]. Some cases showed changes in apparent growth pattern from exponential to linear and from linear to no growth. In this study, the sigmoid growth model could fit all these changes with one equation, and seems useful for describing the growth of meningiomas. This model is in accordance with the previous observations that meningiomas in the elderly grow more slowly than those in younger people [9, 11–13, 23].
This study demonstrated that growth speed of benign meningiomas might change in the long term. In this context, application of the proper growth model is indispensable for prediction of growth and for decision-making in treatment. Especially, it is very important to know the time when tumors begin to slow their growth (the inflection point). It is expected that a tumor with Gompertzian growth can grow as much as 2.7 times larger after its inflection point. In this study, 26 of 31 incidental meningiomas passed their inflection point before or during the observation period. This explains why most of the incidental meningiomas were found to grow slowly and remain asymptomatic. Although the growth of atypical meningiomas was fitted well by the pre-inflection regions of the Gompertzian and logistic curves, it is unlikely that atypical or anaplastic meningiomas slow their growth spontaneously. The sigmoid curve can be adapted to the growth of tumors that would not have an inflection point, by setting a large value for the inflection point.
Most studies on growth analysis using sigmoid curves utilized cell lines [24–27]. Application of this model to clinical neoplasms has been attempted using historical clinical data [28–30]. In benign tumors, the whole processes are difficult to observe during the follow-up period. In fact, for the mean follow-up period of 7.7 years, we were able to observe both the exponential and decelerating growth phase in only eight tumors. Shorter follow-up may reveal only an apparently exponential, linear, or no-growth phase.
Several biological and biophysical hypotheses have been proposed to explain why the sigmoid growth model is a good descriptor of the biological growth of tumors [24, 25, 31–33]. All of these studies are based on the idea that the tumor volume saturates when the tumor becomes larger. The larger the tumor, the more likely the proliferating cells are to become quiescent, and the less likely the quiescent cells are to re-enter the proliferation cycle. In this context, growth should accelerate again when the volume is reduced by surgery [34]. However, the growth rate of benign meningiomas slowed even when the size became smaller after surgical removal [16]. When comparing patient age at the inflection point among those with asymptomatic and symptomatic meningiomas, they did not differ when the latter experienced surgical removal. Thereafter, it appears that the deceleration of growth of benign meningiomas correlates with tumor or patient age. This means that the mechanism of growth saturation in meningiomas would be different from that of experimental tumors in vitro and in vivo.
This study has several limitations. This was a retrospective study that enrolled selected patients. The design of this study might have included a bias toward selecting tumors which were not growing. However, the incidence of tumor growth among the asymptomatic meningiomas in this series (74.2%) was not lower than in previous reports. Moreover, the finding that even symptomatic tumors with recurrence or regrowth showed growth deceleration in the long run supports the idea of the sigmoid growth model for benign meningiomas. Another limitation of this study arises from the errors of volume measurement. Tumors of small size are apt to pose problems of partial volume effect and slice thickness of the images. If more exact measurement were possible, then a more precise prediction in an early phase might be made. Although we employed Gompertzian and logistic curves as a sigmoid function, there are other forms of sigmoid equations [32]. If more precise measurement becomes possible, it may be determined which is the best descriptor of tumor growth.
Growth may not be self-limiting when benign meningiomas acquire malignant change. However, the reported incidence is low. Schiffer et al. [35] stated that only 2 of 53 recurrent meningiomas progressed from benign to atypical. None in our present series showed malignant change of grade 1 meningioma.
Estimation of growth in the present method has limitations, and a long follow-up period is necessary. Further study may reveal definite radiological or clinical features that distinguish tumors that have passed the inflection point. Calcification of a tumor may be one such candidate. Elucidation of the mechanism of the self-limiting growth model may make it possible to predict the plateau volume and inflection point from a surgical specimen.
Conclusions
We have presented for the first time the applicability of a self-limiting growth model for simulation of benign meningioma growth. Benign meningiomas may pass the inflection point and approach their plateau volume in the long run, while atypical meningiomas are not likely to do so. This difference may be one reason for the lower recurrence rate in benign meningiomas than in higher-grade ones even after incomplete surgery. Further refinement of the methodology and elucidation of the mechanism should enable us to predict growth of meningiomas more precisely and simply.
References
Bellular SN, Chandra V (1981) Meningioma size. Its relationship to other diseases. Arch Neurol 38:458–459
Nakasu S, Hirano A, Shimura T, Llena JF (1987) Incidental meningiomas in autopsy study. Surg Neurol 27:319–322
Rausing A, Ybo W, Stenflo J (1970) Intracranial meningioma—a population study of ten years. Acta Neurol Scand 46:102–110
Elia-Pasquet S, Provost D, Jaffré A, Loiseau H, Vital A, Kantor G, Maire JP, Dautheribes M, Darrouzet V, Dartigues JF, Brochard P, Baldi I, Work Group (2004) Incidence of central nervous system tumors in Gironde, France. Neuroepidemiology 23:110–117
Kuratsu J, Takeshima H, Ushio Y (2001) Trends in the incidence of primary intracranial tumors in Kumamoto, Japan. Int J Oncol 6:183–191
Radhakrishnan K, Mokri B, Parisi JE, O’Fallon WM, Sunku J, Kurland LT (1995) The trends in incidence of primary brain tumors in the population of Rochester, Minnesota. Ann Neurol 37:67–73
Go RS, Taylor BV, Kimmel DW (1998) The natural history of asymptomatic meningiomas in Olmstead County, Minnesota. Neurology 51:1718–1720
Yano S, Kuratsu J, The Kumamoto Brain Tumor Research Group (2006) Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg 105:538–543
Yoneoka Y, Fujii Y, Tanaka R (2000) Growth of incidental meningiomas. Acta Neurochir 142:507–511
Hashiba T, Hashimoto N, Izumoto S, Suzuki T, Kagawa N, Maruno M, Kato A, Yoshimine T (2009) Serial volumetric assessment of the natural history and growth pattern of incidentally discovered meningiomas. J Neurosurg 110:675–684
Herscovici Z, Rappaport Z, Sulkes J, Danaila L, Rubin G (2004) Natural history of conservatively treated meningiomas. Neurology 63:1133–1134
Jung HW, Yoo H, Paek SH, Choi KS (2000) Long-term outcome and growth rate of subtotally resected petroclival meningiomas: experience with 38 cases. Neurosurgery 46:567–574
Nakamura M, Roser F, Michel J, Jacobs C, Samii M (2003) The natural history of incidental meningiomas. Neurosurgery 53:62–70
Olivero WC, Lister JR, Elwood PW (1995) The natural history and growth rate of asymptomatic meningiomas: a review of 60 patients. J Neurosurg 83:224–226
Niiro M, Yatsushiro K, Nakamura K, Kawahara Y, Kuratsu J (2000) Natural history of elderly patients with asymptomatic meningiomas. J Neurol Neurosurg Psychiat 68:25–28
Nakasu S, Fukami T, Nakajima M, Watanabe K, Ichikawa M, Matsuda M (2005) Growth pattern changes of meningiomas: long-term growth analysis. Neurosurgery 56:946–955
Cho KG, Hoshino T, Nagashima T, Murovic JA, Wilson CB (1986) Prediction of tumor doubling time in recurrent meningiomas. Cell kinetics studies with bromodeoxyuridine labeling. J Neurosurg 65:790–794
Jääskeläinen J, Haltia M, Laasonen E, Wahlström T, Valtonen S (1985) The growth rate of intracranial meningiomas and its relation to histology. An analysis of 43 patients. Surg Neurol 24:165–172
Nakaguchi H, Fujimaki T, Matsuno A, Matsuura R, Asai A, Suzuki I, Sasaki T, Kirino T (1999) Postoperative residual tumor growth of meningioma can be predicted by MIB-1 immunohistochemistry. Cancer 85:2249–2254
Van Havenbergh T, Carvalho G, Tatagiba M, Plets C, Samii M (2003) Natural history of petro-clival meningioma. Neurosurgery 52:55–64
Nakasu S, Nakasu Y, Nakajima M, Yokoyama M, Matsuda M, Handa J (1996) Potential doubling time and tumor doubling time in meningiomas and neurinomas. Acta Neurochir 138:763–770
Perry A, Louis DN, Scheithauer BW, Budka H, von Deimling A (2007) Meningiomas. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) WHO classification of tumours of the central nervous system. IARC, Lyon, pp 164–172
Nakamura M, Roser F, Michel J, Jacobs C, Samii M (2005) Volumetric analysis of the growth rate of incompletely resected intracranial meningiomas. Zentralbl Neurochir 66:17–23
Castro MAA, Klamt F, Grieneisen VA, Grivicich I, Moreira JCF (2003) Gompertzian growth pattern correlated with phenotypic organization of colon carcinoma, malignant glioma and non-small cell lung carcinoma cell lines. Cell Prolif 36:65–73
Demicheli R, Foroni R, Ingrosso A, Pratesi G, Soranzo C, Tortoreto M (1989) An experimental Gompertzian description of LoVo cell tumor growth from in vivo and in vitro data. Cancer Res 49:6543–6546
Norton L (1988) A Gompertzian model of human breast cancer growth. Cancer Res 48:7067–7071
Steel GG, Lametron LF (1966) The growth of human tumors. Br J Cancer 20:74–86
Hart D, Shochat E, Agur Z (1998) The growth law of primary breast cancer as inferred from mammography screening trial data. Br J Cancer 78:382–387
Norton L, Simon R, Brereton H, Bodgen AE (1976) Predicting the course of Gompertzian growth. Nature 264:542–545
Speer JF, Petrosky VE, Retsky MW, Wardwell RH (1984) A stochastic numerical model of breast cancer growth that simulates clinical data. Cancer Res 44:4124–4130
Gyllenberg M, Webb GF (1989) Quiescence as an explanation of Gompertzian tumor growth. Growth Develop Aging 53:25–33
Kozusko F, Bourdeau M (2007) A unified model of sigmoid tumour growth based on cell proliferation and quiescence. Cell Prolif 40:824–834
Kozusko F, Bajzer Z (2003) Combining Gompertzian growth and cell population dynamics. Math Biosci 185:153–167
Lazareff JA, Suwinski R, De Rosa R, Olmstead CE (1999) Tumor volume and growth kinetics in hypothalamic-chiasmatic pediatric low grade gliomas. Pediatr Neurosurg 30:312–319
Schiffer D, Ghimenti C, Fiano V (2005) Absence of histological signs of tumor progression in recurrences of completely resected meningiomas. J Neuro-oncol 73:125–130
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Growth curve equations used in this article are shown below, where α, β, and γ are constants, and V(t) is the volume at time t.
Power curve [28] : \(V(t)\, = \,\left( {\alpha t\left( {1 - \beta } \right) + \gamma } \right)^{1/1 - \beta } \)
The power curves show rapid increase of volume when 0 < β < 1, linear growth when β = 0, and gradual deceleration when β < 0.
Ti was described as days after the initial observation time. A negative Ti value indicates that the inflection point was before the initial imaging study. However, if Ti is beyond the observation period, the tumor was still growing quasi-exponentially.
Rights and permissions
About this article
Cite this article
Nakasu, S., Nakasu, Y., Fukami, T. et al. Growth curve analysis of asymptomatic and symptomatic meningiomas. J Neurooncol 102, 303–310 (2011). https://doi.org/10.1007/s11060-010-0319-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0319-1