Abstract
Introduction Treating high grade gliomas in the elderly is a challenge for multidisciplinary teams. Most studies on this topic exclude patients aged >65 and a Karnofsky Performance Status (KPS) score of <70, a group most likely to have a poor outcome. We undertook this study to analyze the outcomes in a cohort of patients which included such patients. Methods Ours was a retrospective cohort study. About 71 consecutive patients with high grade gliomas, who were seen in the neurooncology clinic in 2004, were included. The case records of these patients were scrutinized for the demographic, clinical data, follow-up and survival. The cohort was divided into two groups; Age ≥65 and age <65 for analysis. The factors influencing survival were analyzed using the Cox’s proportional hazards model in each group. Results In the age group ≥65 years, patients treated with a radical resection ± adjuvant therapy had a lower risk of death (hazard ratio 0.14, 95%CI 0.04–0.51, P = 0.003) when compared to patients undergoing a biopsy ± adjuvant therapy and palliative treatment. In the group <65 years, the greater the age, greater was the risk of death (hazard ratio 2.05, 95%CI 1.13–3.73, P = 0.01). The median survival was 12 months in the group <65 years and 5 months in age ≥65 years (P = 0.001). In the group ≥65 years, those patients who had radical resection ± adjuvant treatment had a median survival of 7 months as compared to 3 months in the patients who had biopsy ± adjuvant treatment (P = 0.003). KPS, presence of co-morbidities, duration of symptoms, location of the lesion and sex were not found to be significant independent predictors of survival in our study. Conclusions Age is an important predictor of survival in younger patients, however in the elderly treatment matters most. Elderly patients undergoing radical surgery ± adjuvant treatment had a longer median survival as compared to the elderly patients undergoing a biopsy ± adjuvant treatment. KPS was not found to be a significant independent predictor of survival probably because of underrepresentation of patients with poor KPS. Radical treatment should not be denied to elderly patients who are deemed fit as the outcome is significantly better.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treating malignant brain tumours in an elderly population is a challenge that is unique to the current times [1–10]. Most cancer registries across the world have reported increasing trends in the incidence of brain tumours, especially in the elderly [5, 11–13]. A recent report suggests that the incidence of brain cancer increased until 1985 in the elderly but the incidence rates were stable thereafter [14]. Survival rates for patients with glioblastoma multiforme (GBM) have not shown any improvement in the last two decades [14]. The generally accepted median survival rates for patients with high grade gliomas is 6 months to a year [14, 15]. Since the life expectancy has increased over the years, the elderly form a predominant part of the patient load of any neurooncology service and therefore treating high grade gliomas in the elderly is becoming increasingly more relevant in present times [5, 6, 14].
Age is perhaps the most important prognostic factor when treating high grade gliomas [6, 16, 17] and becomes an important issue in determining treatment. It is recognized that elderly patients with these tumors may present late to the hospital, present with different symptoms, may be diagnosed late and may receive suboptimal care due to poor prognosis [5–7, 16, 17]. Even in published literature, this particular group seems to be underrepresented or omitted from analysis due to a poor prognosis [6, 18]. Recent studies have questioned the ageist policy in treating malignant gliomas in the elderly [7]. We undertook this retrospective cohort study to analyze our results in treating high grade gliomas. The objective was to study the prognostic factors for survival in the patients aged 65 years and over (‘elderly’) and in a similar subset of patients aged less than 65 years (‘young’).
Our study is unique as we did not exclude any patients on the basis of their age or the Karnofsky Performance Status (KPS) score from the analysis and for the first time have shown by a comparative analysis a difference between factors that influence survival in patients <65 and patients ≥65.
Methods
Ours was a retrospective cohort study. A group of 71 consecutive patients with diagnosed and treated high grade gliomas (primary Grade III or IV gliomas [19] according to the World Health Organization classification) that attended the neurooncology follow-up clinic during 2004 were identified and included in the study. There were no exclusions. A detailed review of their case notes was undertaken for the demographic and treatment related information. The data recorded included the age at diagnosis, presenting symptoms, duration of symptoms, location of the tumor (eloquent or non-eloquent as per the recognized grading after Sawaya et al. [20, 21]), treatment (biopsy ± adjuvant treatment, radical resection ± adjuvant treatment, palliative care), duration of hospital stay, pre treatment KPS score, duration of follow-up, Glasgow Outcome Score (GOS) at last follow-up and duration of survival. All surgeries were performed by or closely supervised by one surgeon (PJK) which was either a biopsy or a radical resection. The extent of gross total tumor resection at surgery was over 95% (surgeon’s subjective opinion) and this was confirmed on post-operative scans. The scans were performed primarily to check for any residual tumor volume, and no detailed attempt was to calculate the percentage resection. Adjuvant treatment was either radiotherapy or chemotherapy or both for the purposes of this study. Importantly, Temozolomide was not a part of the chemotherapeutic regimen as it was not available on the National Health Service (NHS).
These patients were subsequently followed up in the neurooncology clinics. There was also a regular telephone follow-up conducted by the neurooncology specialist nurse (GH). Telephonic GOS was recorded for those still alive. A death and its probable cause were recorded as and when reported. The data was entered onto a Microsoft Excel™ spreadsheet and analysed using SPSS™ Version 13.0 (SPSS Inc. Chicago, IL).
Statistical analysis
The patients were divided into two groups with age ≥65 (‘elderly’) and age <65(‘young’) for the purposes of analysis and comparison. Continuous variables were checked for normality using the one sample Kolmogorov-Smirnov test. Demographic characteristics were compared between the two groups using Chi-square test for categorical variables and independent sample t-test for continuous variables. A Cox’s proportional hazards model was used to analyze the factors affecting the survival of patients with high grade gliomas within both the groups (age <65 and age ≥65). The variables used in this multivariable analysis were age, duration of symptoms (continuous variables) and sex, co-morbidity, location, treatment and pre treatment KPS (categorical variables). All analysis was done using SPSS version 13.0 (SPSS Inc. Chicago, IL).
Results
A histogram showing the age distribution in the whole sample along with the normal curve is shown in Fig. 1. The demographic details of the patients are presented in Table 1. The main predictors of survival in the two groups, age ≥65 (n = 34) and age <65 (n = 37), as per the Cox’s proportional hazards analysis, are summarized in Table 2.
Demography
The median age of diagnosis in the group age <65 was 59 years (range 25–64 years) and the median age of diagnosis in the group age ≥65 was 72 years (range 65–88 years). At the time of analysis, none of the 34 patients in the age group age >65 were alive while 15 of the 37 patients in the younger age group were still alive. The duration of the presenting symptoms (P = 0.05) was the only variable that was significantly different in the two groups, being longer in the younger age group.
The number of patients with co-morbidities was seven in the ‘young’ group and 10 in the ‘elderly’ group. Of the seven patients with co-morbidities in the age group <65 years, two patients suffered from only asthma and chronic obstructive pulmonary disease, two suffered from Type II diabetes mellitus and hypertension, while the remaining three had Type II diabetes mellitus with ischemic heart disease and hypertension. One of these last three patients one also had renal failure. In the age group ≥65 years, 10 out of the 34 patients had co-morbidities. One of them had a combination of Type II diabetes mellitus, ischemic heart disease and congestive cardiac failure. Three had asthma and chronic obstructive pulmonary disease in addition to Type II diabetes mellitus. The remaining six patients had varying degrees of ischemic heart disease, arrhythmias, hypertension and Type II diabetes mellitus and two of them had significant diabetic nephropathy. Although the objective details of the severity of the disease in these patients were not obtained accurately from the retrospective case-note review, it seemed that the elderly patients had a combination of more than one disease and greater number of co-morbidities than the younger.
Eleven of the 37 patients in the age group <65 had tumors in eloquent locations. Seven were located in the left parietoccipital region, three in the left temporal lobe and one in the thalamus. Of the remaining 26 patients in this age group with tumors in non-eloquent locations, 17 were right frontal, 6 were right parietoccipital and 3 were left frontal. Six of the 34 in the age group ≥65 years had tumors in eloquent locations, four in the left parietoccipital region and one each in the brainstem and thalamus. Of the 28 tumors in this age group located in non-eloquent regions 16 were right frontal, 10 were left frontal and 2 in the right parietoccipital region.
Post operative stay in the hospital was short in our sample, with the younger patients having a significantly shorter length of stay (P = 0.04). There were four complications in the patients aged <65 (two urinary tract infections, one chest infection and one seizure) while there were seven post operative complications in the patients aged ≥65 (three urinary tract infections, two chest infections and one deep venous thrombosis of the calf veins).
Factors influencing survival
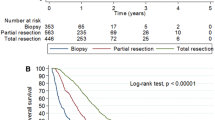
When the median survival was studied within the two age groups, it was found that in the ‘elderly’ group, patients who had a radical resection ± adjuvant treatment had the maximum median survival (7 months) and this was significantly (Log Rank test, P = 0.003) greater than patients treated with just a biopsy ± adjuvant treatment who had a median survival of 3 months. The median survival in the age group <65 was maximum in the group that underwent a radical resection ± adjuvant treatment (14 months) although this was not significantly different from the patients who had a biopsy ± adjuvant treatment (Log rank test, P = 0.33). The Kaplan Meier graphs illustrating the above two results are shown in Figs. 2 and 3 respectively. A multivariable Cox Proportional Hazards analysis was conducted, and the results can be seen in Table 2. This shows that for patients <65 the only significant predictor of survival was the patient’s age, with older patients having a higher risk of death (hazard ratio 2.05, 95%CI 1.13–3.73, P = 0.01). For those 65 and over the risk of death in patients undergoing a radical resection ± adjuvant therapy was significantly lower (hazard ratio 0.14, 95%CI 0.04–0.51, P = 0.003) than those patients undergoing a biopsy ± adjuvant therapy or palliative treatment. The duration of symptoms, KPS, sex, location of the lesion and co-morbidities did not significantly influence survival in our study (Table 2).
Discussion
Salient features and comparison to other studies
The results of our simple study are significant in the fact that we have shown that contrary to popular belief, age is not the most important factor in determining survival when treating patients aged 65 years or more. In this age group the risk of death in patients undergoing a radical resection ± adjuvant therapy was significantly lower (hazard ratio 0.14, 95%CI 0.04–0.51, P = 0.003) than those patients undergoing a biopsy ± adjuvant therapy or palliative treatment. There was a definite survival advantage in patients undergoing radical surgery ± adjuvant therapy (median survival 7 months) when compared to patients undergoing a biopsy ± adjuvant therapy (median survival 3 months) (Log Rank test, P = 0.003). This implies that elderly patients with a good performance status if treated aggressively, survived a significantly longer time when compared to those who did not undergo a more radical treatment. We observed that the mean duration of symptoms prior to diagnosis was significantly longer in the patients with age <65 contrary to what has been reported in literature [5]. The multivariable analysis for those aged <65 showed that the only significant predictor of survival was the patients age, with older patients having a higher risk of death (hazard ratio 2.05, 95%CI 1.13–3.73, P = 0.01). We have clearly demonstrated a difference in factors that significantly influence survival in patients with high grade gliomas in age <65 and age ≥65 years.
Our findings were comparable to the studies previously published in terms of median survival and ultimate outcome [1, 2, 5–7, 16, 17, 22, 23]. Patwardhan et al. [8] in 2003 reported a similar series with a median survival of 3.2 months in the group treated with biopsy only, 5.5 months for patients treated with resective surgery and radiotherapy and up to 13.6 months in patients who underwent resective surgery with radiotherapy and chemotherapy. Like us Patwardhan et al. [8] did not observe and significant influence of KPS on the results, primarily because their mean KPS was uniform over the analysis groups. Pierga et al. [9] in their series in 1999 reported a median survival of 36 weeks and found KPS to be the most important predictor of survival unlike Patwardhan et al. [8] and the present study. In the most recent report on this subject, Mangiola et al. [7] report a median survival of 10.5 months in series of 34 patients treated for high grade gliomas. There are significant differences between Mangiola et al’s [7] series and the others which may explain the better results reported by them. Mangiola et al. [7] did not have any patients treated with only a biopsy, a group expected to have a bad outcome. They also had patients who had repeat debulking in their series [7] which was not the case in previously reported series [8, 9] indicating a very aggressive surgical approach. Their series used Temozolomide as part of the chemotherapeutic regimen and radioimmunotherapy both of which could have significantly added to the survival advantage [7]. In keeping with the findings of Lowry et al. [5] we also found that delayed diagnosis was not an issue with gliomas in the elderly and in fact the mean duration of symptoms was significantly less in the elderly (Table 1).
Factors influencing survival
High grade glioma in the elderly population is a problem that is yet unconquered [1–3]. It is a problem unique to the ageing population in today’s world and one which any multidisciplinary team with an interest in their treatment should be well equipped to handle [1–9, 24]. Treating brain tumors in the elderly is more challenging because most of the tumors in this age groups are highly malignant, disease progression is rapid, there is a reluctance to treat primary brain tumors aggressively in the elderly and the outcome is bleak. Cancer registries in most developing countries have reported a rise in the incidence rates of this condition [11–14, 25, 26]. The mortality rate from malignant high grade gliomas in the elderly has not changed substantially in the last 2–3 decades despite rapid advances in treatment [14, 27, 28]. The factors that determine outcome or survival in such patients are well documented in literature. Age stands out as the single most important factor [16, 17, 22, 29]. KPS score [1, 2, 16, 17, 30], treatment offered [1, 2, 8, 16, 17, 24, 31], location of the tumour [16, 17], the grade of the tumor [16, 17], the extent of necrosis [16, 17], presence of ring enhancement on CT scans [32], proliferation index [32], midline shift and involvement of the corpus callosum [32] have all been documented to have prognostic significance.
One of the notable exceptions in most studies has been the relative avoidance of the elderly population and poor representation of patients with low KPS scores [6, 18]. The reasons for this are understandable; surgeons are reluctant to operate on patients with poor KPS scores [17, 33, 34] and oncologists are reluctant to offer chemo/radiotherapy due to the high risk of complications and poor survival [1, 2]. Lowry et al. [5] have reported an actual increase in the number of patients undergoing no treatment from their data spanning two decades. The policies of different surgeons and oncologists that dictate the aggressiveness of management decisions are not explicit and in most cases seem to be guided by age although other factors such as location of the tumour, resectability, fitness for a general anaesthetic and multifocal disease play an important part in the decision making [17, 34]. This practice has been questioned in recent literature [1, 2, 7, 23] and there is a school of thought developing which would advocate a rather more aggressive management approach for older patients with high grade gliomas with a good performance status irrespective of age [1, 2, 7, 23]. It is only appropriate that when dealing with an ageing population, the treating multidisciplinary team should try to give the best chance to the fittest of the group. Our study reiterates this point.
Limitations
Our simplistic approach has certain drawbacks. The patient group was pre-selected and small. The intention was to review our practice in treating high grade gliomas hence the choice of the sample. The sample was not large enough to adequately represent all age groups and possible variations of pathology, location and co-morbidity and sensitively distinguish between the contributions of minor variations of individual factors on patient survival. The patients were ones that had already undergone the treatment, and therefore patients who had come into contact with the neurooncology services but were not offered any active treatment and not followed up in the neurooncology clinic were completely missed out. This fact could have a potential bias; however the small number of such patients would probably count against any significant influence. The lack of follow-up of such patients could have been due to either short duration of survival or the futility of any further treatment because of which these patients were not actively followed up. This subset of patients even though small would be interesting to look at in terms of ultimate survival and also judge their impact on the results of studies such as ours and also others [2, 5]. At our centre, we are currently in the process of establishing a comprehensive database of all patients that come into contact with the neurooncology services which would help us to include even these patients in the analysis.
An attempt to analyze our data most effectively within the acknowledged constraints could be perceived as an oversimplification of a set of extremely complex interactions between factors influencing survival in high grade gliomas; but this was not actually the case. Survival following glioma surgery is influenced by multiple factors. These include age, co-morbidity (nature, number, severity), KPS, functional age, location of tumor, size of lesion, extent of gross total resection, post operative complications, histology and grade of tumor, midline shift, time from symptoms to diagnosis, degree of necrosis and edema and several others. The exact interaction of all these and the role of each in determining survival cannot be determined from a study such as ours which provides a snapshot of our practice rather than a long term follow-up of all treated patients.
We have analysed age in two groups ≥65 and <65 as per a few previous studies [1, 2, 5] which have used a similar age when defining ‘elderly’. This cut-off was used as we were particularly interested in the factors which affected survival in patients aged 65 and over. Analyzing the data in blocks with an age difference of 10 years each would have given us far less numbers in certain groups (Fig. 1). This type of analysis is more suitable for a greater sample size where patients of all ages with high grade gliomas are adequately represented. There have been differences in opinion about clubbing WHO Grade III astrocytomas and WHO Grade IV GBM’s together in the analysis because of the suggestions that WHO Grade III astrocytomas may have a better prognosis [17, 34]. However there have been reports contrary to this as well [19]. We analysed these two groups together primarily because of the ease of analysis and also because the number of patients with Grade III astrocytomas were very few.
KPS has been recognized as an independent factor in several multivariate analyses of the predictors of survival [1, 2, 5, 7, 16, 17, 19, 34] but there is an indication that it may not be a particularly sensitive surrogate for the operative risk. Also KPS may to an extent follow age and co-morbidities. Does this influence the multivariable analysis where both are included as independent variables? This is a fact not touched upon by the previous studies and remains an unanswered question. The impact of KPS on survival in our analysis did not show any statistically significant variations in either age group. One would generally expect a significantly poorer outcome in patients with low KPS scores. This did not stand out in our results. The reasons could be the small number of patients with KPS < 70, in the both age groups which probably rendered the results statistically insignificant. Co-morbidities, their nature and severity would play an important part in survival after any tumor resection. In our study groups the presence of co-morbidities was not significantly different. We did observe that in the elderly age group the number of co-morbidities present together were greater. However with the small number of patients with co-morbidities in our sample, this is unlikely to significantly alter our main result. We did not have detailed data regarding the degree of severity of the co-morbidities. We agree that worse degree of co-morbidities would adversely affect survival; however detailed analysis of these would not have significantly altered our main results primarily because they were uniformly distributed in both groups and secondly because KPS would act a proxy for patients’ medical status. The influence of tumor location on survival has been debated upon in the past and has not been found to be a significant independent predictor of survival in the past [16, 17]. Grouping the tumor locations into eloquent and non-eloquent [20, 21] provides the easiest way of creating a broad classification most likely to influence survival and post-operative results, as the general trend is to be less radical in resecting tumors in eloquent locations, the intention being to leave the patients with minimal post-operative deficits and a good quality of life. We relied on the surgeon’s perception of the extent of gross total resection during surgery and did not make any specific attempts to calculate the extent of resection on post-operative scans. No gross residual tumor volumes were found on any scans performed post-operatively. The surgical technique and resection were similar in all cases as all operations were either performed or closely supervised by one surgeon (PJK).
Another important factor to consider while interpreting our results would be the significant (almost four fold) difference in the time for which the symptoms were present before diagnosis in the two age groups. The fact that younger patients presented late could be due to several reasons. A better KPS and functional status and lesser co-morbidities could probably mean that these patients were not significantly disabled by the symptoms whereas the elderly and possibly frailer patients decompensate earlier and adequate access to health services translates into faster diagnosis. Whether these patients presented at a more advanced stage of the disease due to late presentation and whether this created a bias is difficult to determine. The likelihood is that this delay in diagnosis in younger patients is compensated by a generally fitter functional status and does not significantly influence survival. One could postulate that if screening programs existed and these patients were identified and treated earlier we could identify a subset of patients whose survival could be significantly increased with aggressive surgical treatment.
The future
Our study raises a few further issues. We found that aggressive surgical resection along with adjuvant treatment offers the best chance of survival, but we were unable to determine whether this spans to all tumors (eloquent and non-eloquent locations). This is an important point and leads us to consider whether in this day and age—is any tumor ‘unresectable’ at all? Surely if the survival advantage of radical resection extends to all tumors, there is merit in aggressively advocating this approach. It would have been interesting to look at quality of life after radical resections and see the differences with respect to anatomic location of tumors. Quality of life after a tumor resection remains the most important issue which has not been addressed adequately by most other studies in the past. The question of whether a significant increase in survival (7 months versus 3 months, in the elderly group with radical resection versus biopsy) translates into better quality of life remains unanswered. The retrospective nature of our study made the collection of this data unfeasible and this is one of the planned future studies at our centre. The answer will probably determine a patients approach to accepting surgical treatment. Future studies must be directed at this all important lacuna in knowledge. The factors that make a surgeon or oncologist decide against aggressive management need to be more objective. What determines ‘fit’ for surgery or aggressive management needs to be well standardized and universally agreed. A comprehensive score for the suitability of a patient for a particular treatment could be a topic for future studies. This could potentially be a combination of KPS, co-morbidities and chronological age. Future attempts should be directed towards this.
In conclusion, from our experience in treating high grade malignant gliomas, we have found that treatment and not age is the most significant predictor of survival in the elderly (age ≥65). In this age group patients treated with radical surgery ± adjuvant radio/chemotherapy had a significantly greater survival when compared to patients in the same age group treated with only a biopsy ± adjuvant therapy.
References
Brandes AA, Monfardini S (2003) The treatment of elderly patients with high-grade gliomas. Semin Oncol 30:58–62
Brandes AA, Vastola F, Basso U, Berti F, Pinna G, Rotilio A, Gardiman M, Scienza R, Monfardini S, Ermani M (2003) A prospective study on glioblastoma in the elderly. Cancer 97:657–662
Fernandez PM, Brem S (1997) Malignant brain tumors in the elderly. Clin Geriatr Med 13:327–338
Kleinschmidt-DeMasters BK, Lillehei KO, Varella-Garcia M (2005) Glioblastomas in the older old. Arch Pathol Lab Med 129:624–631
Lowry JK, Snyder JJ, Lowry PW (1998) Brain tumors in the elderly: recent trends in a Minnesota cohort study. Arch Neurol 55:922–928
Lutterbach J, Bartelt S, Momm F, Becker G, Frommhold H, Ostertag C (2005) Is older age associated with a worse prognosis due to different patterns of care? A long-term study of 1346 patients with glioblastomas or brain metastases. Cancer 103:1234–1244
Mangiola A, Maira G, De Bonis P, Porso M, Pettorini B, Sabatino G, Anile C (2006) Glioblastoma multiforme in the elderly: a therapeutic challenge. J Neurooncol 76:159–163
Patwardhan RV, Shorter C, Willis BK, Reddy P, Smith D, Caldito GC, Nanda A (2004) Survival trends in elderly patients with glioblastoma multiforme: resective surgery, radiation, and chemotherapy. Surg Neurol 62:207–213; discussion 214–205
Pierga JY, Hoang-Xuan K, Feuvret L, Simon JM, Cornu P, Baillet F, Mazeron JJ, Delattre JY (1999) Treatment of malignant gliomas in the elderly. J Neurooncol 43:187–193
Whittle IR, Denholm SW, Gregor A (1991) Management of patients aged over 60 years with supratentorial glioma: lessons from an audit. Surg Neurol 36:106–111
Davis DL, Hoel D, Fox J, Lopez A (1990) International trends in cancer mortality in France, West Germany, Italy, Japan, England and Wales, and the USA. Lancet 336:474–481
Davis DL, Hoel D, Fox J, Lopez AD (1990) International trends in cancer mortality in France, West Germany, Italy, Japan, England and Wales, and the United States. Ann N Y Acad Sci 609:5–48
Davis DL, Hoel D, Percy C, Ahlbom A, Schwartz J (1990) Is brain cancer mortality increasing in industrial countries? Ann N Y Acad Sci 609:191–204
Deorah S, Lynch CF, Sibenaller ZA, Ryken TC (2006) Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus 20:E1
Claus EB, Black PM (2006) Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973–2001. Cancer 106:1358–1363
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198
Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, Lillehei KO, Bernstein M, Brem H, Sloan A, Berger MS, Chang S (2003) Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg 99:467–473
Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr., Albain KS (1999) Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 341:2061–2067
Prognostic factors for high-grade malignant glioma: development of a prognostic index (1990) A report of the medical research council brain tumour working party. J Neurooncol 9:47–55
Sawaya R (1999) Extent of resection in malignant gliomas: a critical summary. J Neurooncol 42:303–305
Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, Wildrick DM (1998) Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42:1044–1055; discussion 1055–1046
Lutterbach J, Sauerbrei W, Guttenberger R (2003) Multivariate analysis of prognostic factors in patients with glioblastoma. Strahlenther Onkol 179:8–15
Shaw EG (2004) Nothing ventured, nothing gained: treatment of glioblastoma multiforme in the elderly. J Clin Oncol 22:1540–1541
Nishihara M, Taomoto K, Kohmura E (2005) Prognostic factors in malignant glioma in elderly patients. Nippon Rinsho 63(Suppl 9):596–599
Counsell CE, Collie DA, Grant R (1996) Incidence of intracranial tumours in the Lothian region of Scotland, 1989–90. J Neurol Neurosurg Psychiatr 61:143–150
Greig NH, Ries LG, Yancik R, Rapoport SI (1990) Increasing annual incidence of primary malignant brain tumors in the elderly. J Natl Cancer Inst 82:1621–1624
Annegers JF, Schoenberg BS, Okazaki H, Kurland LT (1981) Epidemiologic study of primary intracranial neoplasms. Arch Neurol 38:217–219
Modan B, Wagener DK, Feldman JJ, Rosenberg HM, Feinleib M (1992) Increased mortality from brain tumors: a combined outcome of diagnostic technology and change of attitude toward the elderly. Am J Epidemiol 135:1349–1357
Piccirilli M, Bistazzoni S, Gagliardi FM, Landi A, Santoro A, Giangaspero F, Salvati M (2006) Treatment of glioblastoma multiforme in elderly patients. Clinico-therapeutic remarks in 22 patients older than 80 years. Tumori 92:98–103
Buckner JC (2003) Factors influencing survival in high-grade gliomas. Semin Oncol 30:10–14
Uzuka T, Tanaka R, Takahashi H (2005) Treatment of malignant gliomas in the elderly. Nippon Rinsho 63(Suppl 9):591–595
Polin RS, Marko NF, Ammerman MD, Shaffrey ME, Huang W, Anderson FA Jr., Caputy AJ, Laws ER (2005) Functional outcomes and survival in patients with high-grade gliomas in dominant and nondominant hemispheres. J Neurosurg 102:276–283
Nazzaro JM, Neuwelt EA (1990) The role of surgery in the management of supratentorial intermediate and high-grade astrocytomas in adults. J Neurosurg 73:331–344
Laws ER, Shaffrey ME, Morris A, Anderson FA Jr. (2003) Surgical management of intracranial gliomas–does radical resection improve outcome? Acta Neurochir Suppl 85:47–53
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukerji, N., Rodrigues, D., Hendry, G. et al. Treating high grade gliomas in the elderly: the end of ageism?. J Neurooncol 86, 329–336 (2008). https://doi.org/10.1007/s11060-007-9476-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9476-2