Abstract
Macrophages and monocytes migrate in response to chemotactic cytokines such as monocyte chemoattractant protein 1 (MCP-1/CCL2) in a variety of tissues including the central nervous system. Overexpression of MCP-1 has been reported in glioblastoma (GBM), which correlates to prominent macrophage infiltration characterized by this tumor type, but whether MCP-1 receptor is also expressed by the neoplastic cells remains unclear. Expression of MCP-1 and its receptor, CC chemokine receptor 2 (CCR2), were examined in GBM using cDNA microarrays and validated in two independent microarray datasets. We investigated the expression of the CCR2A isoform in human glioma cell lines and GBM, and found overexpression of CCR2A in most GBM specimens examined when compared to normal brain tissues. CCR2A is mainly localized in the cytoplasm of neoplastic cells, and pronounced neuronal cytoplasmic CCR2A immunoreactivity in tumor-infiltrating area was associated with prior chemo/radiation therapy. Glioma cells ectopically overexpressing CCR2A demonstrated increased migration compared to vector-transfected cells in vitro. Inhibition of MCP-1 synthesis suppressed migration of CCR2A-overexpressed glioma cells. Our data suggest that CCR2A might be associated with the pathobiology of GBM such as host response to treatment and tumor cell migration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM) is the most aggressive glioma subtype (WHO grade IV astrocytoma). Its infiltrative pattern of growth makes complete surgical removal impossible, and its resistance to conventional therapeutic modalities inevitably results in tumor recurrence [1]. GBM tumors are also characterized by marked inter-tumoral heterogeneity that is reflected in genetic, cytologic, and histologic variability [2]. Identification of gene products differentially expressed between GBM tumors not only defines molecular signatures but may also reflect the underlying biologic basis of tumor heterogeneity. This rationale is the basis of ongoing functional and mechanistic studies of many genes identified in GBM through a variety of genetic screening techniques.
Microarray-based analysis of tumor specimens identifies individual gene-expression patterns and allows characterization of differential gene expression between specimens in a high-throughput fashion. A recent study of gene-expression profiles derived from GBM tumors revealed that a cluster of the most differentially expressed genes among the specimens examined consisted of genes typically expressed in macrophages, microglia, and lymphocytes (designated as an “immune cell signature”) [3]. This cluster includes a group of inflammatory CC chemokine genes clustered on chromosome 17q (e.g. monocyte chemoattractant protein 1 (MCP-1), MCP-4, and SCYA11). Among this group of cytokines, MCP-1 is of particular interest for several reasons. A large number of cell types in the brain, such as astrocytes, neurons, and endothelial cells, express MCP-1, particularly in response to stimuli leading to tissue injury [4, 5]. Expression of MCP-1 is associated with higher astrocytoma tumor grade [6, 7]. From a functional perspective, MCP-1 expressed by glioma cells induces migration of monocytes in vitro [6]. Finally, an intracerebral animal model provided evidence that MCP-1 recruits microglia and promotes vascular density and tumor growth [8]. Overall, these findings suggest that increased expression of MCP-1 might be responsible for the presence of infiltrating macrophages observed in human GBM tumors and contribute to growth-permissive environment. Although infiltrating leukocytes, lymphocytes, and macrophages can contribute to a cytotoxic effect on neoplastic cells [9], the regulation of these effects are poorly defined. In particular, the tissue distribution and function of the MCP-1 receptor is not well characterized in human brain tumors.
Based on the high-affinity binding of CC chemokine receptor-2 (CCR2) to MCP-1 [10], as well as the common phenotypes shared by CCR2-deficient and MCP-1-deficient mice [11–15], it appears that CCR2 is the primary receptor for MCP-1. CCR2 belongs to the family of G protein-coupled receptors, and two alternatively spliced variants, CCR2A and CCR2B, have been isolated from human tissues [16]. The two isoforms differ structurally only in their cytoplasmic carboxyl termini. Differences in tissue distribution [17], subcellular localization [18], chemotactic activity, and induction of calcium mobilization [19] suggest that CCR2A and CCR2B have distinct functions.
Cytokine receptors in the central nervous system are likely to have other non-immunologic roles, such as promoting proliferation and directing migration of neuronal and glial precursor cells during development [20]. Human fetal astrocytes express functional CCR2 and demonstrate migratory response to MCP-1 [21]. Neurons in adult rat brain also express CCR2, and the animals display behavioral response to intra-cerebroventricular injection of MCP-1 [22]. Endothelial cells of brain microvessels respond to MCP-1 by decreasing tight-junction protein expression, which is associated with the increase of vessel permeability for leukocyte transmigration [23]. Although reactive astrocytic, neuronal, and endothelial elements within and adjacent to GBM tumors may express functional MCP-1 receptors, it has been unclear whether neoplastic astrocytes themselves express these receptors and whether they respond to MCP-1. In this study, we found that CCR2A is the predominant isoform of the MCP-1 receptor expressed in cultured glioma cells, and its expression is increased in most GBM specimens examined. CCR2A may also modulate glioma cell migration. Our data support a role for this cytokine receptor in the oncogenic phenotype of brain tumors.
Materials and methods
Tissue specimens
Frozen specimens were obtained from the Brain Tumor Research Center Tissue Bank at the University of California, San Francisco after approval from the Committee on Human Research. One gliotic and three normal brain tissues in our dataset (Database 1) were obtained from an epileptic patient and postmortem specimens, respectively. Non-neoplastic brain tissues used in the Database 2 were from epileptic patients as described by the authors. Normal brain total RNA used in the Database 3 was purchased from Stratagene (La Jolla, CA).
Microarray analysis
Sample preparation and microarray methods were described in our previous study [3]. Briefly, total RNA was extracted from frozen tissue specimens using Trizol (Invitrogen; Carlsbad, CA) followed by mRNA purification using FastTrack (Invitrogen). Messenger RNA was reverse transcribed to cDNA and directly labeled with Cy dyes (Amersham Biosciences; Piscataway, NJ) before hybridization. Other detailed protocols can be found in web supplement (http://microarray-pubs.stanford.edu/gbm/).
Cell culture
NHA/hTERT and NHA/hTRET/E6E7 cells were a gift from Dr. Russell Pieper (University of California, San Francisco). NHA/hTERT cells are normal human fetal astrocytes expressing human telomerase, while NHA/hTERT/E6E7 cells have been additionally transfected with human papillomavirus E6 and E7 genes and grow faster than NHA/hTERT cells in culture. Neither immortalized cell line shows tumorigenicity in vitro [24]. All malignant glioma cell lines (U87, U251, SF763, SF767, SF268, and SF295) were obtained from the Brain Tumor Research Center (BTRC) Tissue Bank at the University of California, San Francisco (UCSF). Glioma cell lines and immortalized human astrocytes were maintained in Eagle’s minimal essential medium with 10% fetal bovine serum and 5% CO2.
Antibodies
Dilution of antibodies against MCP-1, CCR2A, CCR2B (Santa Cruz Biotechnology, Santa Cruz, CA), and actin (GIBCO-BRL, Gaithersburg MD) for both immunoblotting and immunohistochemistry was 1:100, 1:200, 1:100, and 1:100, respectively. AIIB2 (Developmental Studies Hybridoma Bank, Iowa City, IA) was diluted to 1:20 and used for inhibition of glioma-cell migration. Peroxidase-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA, and Santa Cruz Biotechnology), biotinylated secondary antibodies (Vector Laboratories), fluorescine-conjugated anti-rabbit IgG and Rodamine-conjugated anti-goat IgG secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA), and species-specific normal sera (Vector Laboratories and Jackson ImmunoResearch Laboratories) were used according to manufacturer’s instructions.
Immunoblotting
Glioma cell lines and immortalized human astrocytes were lysed in 1% Triton X-100 buffer (in 50 mM Tris pH 7.5, 5 mM EDTA, and 150 mM NaCl) supplemented with 1 mM NaF, 1 mM Na3VO4, and Complete protease inhibitor cocktail tablets (Roche, Basel, Switzerland). Cell lysates from equal number of cells or with equal amount of proteins quantitated by using a Dc Protein Assay Kit (Bio-Rad, Hercules, CA) were separated by SDS-PAGE and transferred to nitrocellulose membranes, followed by 10% skim milk blocking and antibody incubation, and visualized using the Super Signal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL). Biotinylation of cell-surface proteins using EZ-Link™ Sulfo-NHS-LC-Biotin (Pierce) was performed according to the manufacturer’s instructions. The intensity of identified bands in arbitrary units was measured using Scion Image (Scion Corporation, Frederick, MD).
Immunocytochemistry and immunohistochemistry
U87 and U251 cells were plated overnight in multi-well slides (Cel-Line, Portsmouth, NH) pre-coated with poly-D-lysine for immunocytochemistry as described previously [25]. After fixation in 4% formaldehyde, cells were blocked with normal serum, incubated with the first antibody at 4°C overnight and with the secondary antibody at room temperature (RT) for 1 h, and finally covered with Vectashield (Vector Laboratories) to prevent fading of fluorescence. All frozen sections used for immunohistochemistry were fixed in 4% formaldehyde, blocked with normal serum, incubated with the first antibody at 4°C overnight, and then incubated with the biotinylated secondary antibody at RT for 1 h. A peroxidase-labeled streptavidin and DAB Reagent kit (KPL, Gaithersburg, MD) was used to visualize immunoreactivity. Immunohistochemistry of the specimens was evaluated by single neuropathologist (A.B.). The intensity of CCR2A immunoreactivity in tumor cells was scored from scale 1 to 3, and the percentage of CCR2A-positive cells was separately recorded.
Cloning and transfection
The CCR2A cDNA in a pcDNA3 vector was a gift from Dr. Oswald Quehenberger (University of California, San Diego). We subcloned the BamHI/XhoI fragment of this CCR2A construct into pcDNA3 to generate the CCR2A antisense construct. In all transient transfection experiments, 1 μg of DNA was incubated with a mixture of 3 μl of FuGENE (Roche, Indianapolis, IN) and 97 μl of serum-free medium at RT for 30 min. The DNA solution was then added to a suspension of 2 × 105 U87 or U251 cells, and the whole mixture was plated to a 35 mm dish. Permanent cell lines were selected from U87 cells transfected with either vector (U87-pcDNA3), the CCR2A-sense construct (U87-CCR2A), or the CCR2A antisense construct (U87-CCR2AAS) using G418, and the culture arose from polyclonal expansion of transfected cells.
Migration assay
The inserts of TransWell chambers (Corning, Acton, MA) with 8 μm pores were undercoated with approximately 100 μg/ml of rat-tail type 1 collagen (BD Biosciences, San Diego, CA) and incubated overnight at RT, and then washed with PBS. Transiently transfected U87 or U251 cells were subject to migration assay 2 days after transfection. Cells were resuspended in serum-free medium supplemented with 1% Insulin-Transferrin-Selenium Supplement (Invitrogen, Carlsbad, CA), and 1 × 104 cells were plated into each insert. The same supplemented serum-free medium was placed in the bottom well; in some cases, MCP-1 at different concentrations was added in the bottom well to examine the migratory response of the cells. Each migration assay had either triplicates or quadruplicates. After 4 h, cells that remained in the inserts were removed with cotton swabs, and migrated cells were fixed and stained using a HEMA 3 stain set (Fisher Diagnostics, Middletown, VA). For each insert, the numbers of migrated cells were counted from five randomly chosen fields under 200× magnification.
Antisense oligonucleotides and migration assay
The scrambled and antisense oligonucleotides used in this study were the “SC ODN” and “MCP-1 AS2”, respectively, described in a previous report [26], except that only the first three and the last three phosphodiester bonds were modified to phosphorothioate bonds to prevent degradation. Oligonucleotides were incubated with FuGENE at RT for 30 min, added to 4 ml of serum-free medium containing 5 × 105 U87-pcDNA3 or U87-CCR2A cells plated in a 60 mm dish to a final concentration of either 50 μM or 25 μM, and incubated for 18–20 h. During the migration assay, cells were incubated with the same concentration of the oligonucleotides/FuGENE mixture. The migration assay was performed as above.
Data analysis
Statistical significance of the difference between two data sets was analyzed using two-tailed Mann-Whitney U test in SPSS for Windows (Release 11.5.0). A P value ≤ 0.05 was considered statistically significant.
Results
Expression of genes encoding MCP-1 and its receptor is increased but highly variable in glioblastoma
We first examined expression of mcp-1 gene in GBM from our cDNA microarray dataset [3] that contained gene expression profiles from 32 GBM specimens, five oligoastrocytoma and oligodendroglioma specimens (Oligo), and four non-neoplastic brain tissues (NT). MCP-1 expression was increased in GBM in comparison to Oligo tumors (P = 0.005) and NT brain (P = 0.05) (Database 1 in Fig. 1a). This finding was further validated using two independent published microarray datasets [27, 28]. The first dataset (Database 2) contained 29 GBM specimens, 14 Oligo tumors, and four NT brain specimens, while the second dataset (Database 3) collected 81 GBM, 49 Oligo, and 23 NT brain samples. GBM once again demonstrated increased expression of MCP-1 compared to Oligo tumors (P = 1.2 × 10−5) and NT brain (P = 4.9 × 10−5) from the Database 2. MCP-1 expression in GBM from the Database 3 was also higher than in Oligo tumors (P = 2.4 × 10−6), but it only showed a trend of increased expression in GBM without reaching statistical significance when compared to NT brain specimens (P = 0.59, Fig. 1a). This might be due to unexpected up-regulated MCP-1 expression in some NT samples from the Database 3, as in this dataset MCP-1 expression in Oligo tumors was even lower than the NT brain (P = 0.00096). Overall, our analyses were consistent with previous findings on MCP-1 expression in GBM [6, 7].
Both MCP-1 and CCR2 genes demonstrate increased but highly variable expression in GBM compared to non-neoplastic (NT) brain tissues. (a) GBM had up-regulated MCP-1 expression compared to NT brain tissues in the Databases 1 and 2 (*, P = 0.05 and 4.9 × 10−5 for Databases 1 and 2). MCP-1 expression in GBM from the Database 3 showed a trend of increased expression despite no statistical significance (*, P = 0.59). (b) CCR2 expression in GBM was consistently higher than in NT brain tissues in all three datasets (*, P = 0.044, 0.041, and 0.002, for Databases 1, 2, and 3, respectively). For (a) and (b), Oligo, oligoastrocytoma and oligodendroglioma; A, astrocytoma; ◯, outliers; ●, extreme data points. (c) Expression of MCP-1 and CCR2 had marginal correlation in GBM (r = 0.44, 0.23, and 0.33 for Databases 1, 2, and 3, respectively). Database 1, ■; Database 2, ▲; Database 3, ●
Expression of the gene encoding MCP-1 receptor, ccr2, was also examined in the same three datasets. ccr2 gene demonstrated significantly higher expression in GBM specimens than in NT brain (P = 0.044, 0.041, and 0.002, for Databases 1, 2, and 3, respectively, Fig. 1b), and it also had a trend of increased expression in high grade GBM compared to Oligo tumors (P = 0.015 and 0.002 for Databases 1 and 3, and P = 0.088 for Database 2). Expression of both mcp-1 and ccr2 genes were only slightly correlated in the three datasets we examined (r = 0.44, 0.23, and 0.33 for Databases 1, 2, and 3, respectively by Pearson, Fig. 1c). Although up-regulation of MCP-1 by GBM is expected to induce infiltration of CCR2-expressing mononuclear cells from blood stream, the marginal correlation between MCP-1 and CCR2 suggests additional sources of CCR2 expression, possibly tumor cells.
CCR2A protein is overexpressed in GBM tumors compared to normal brain
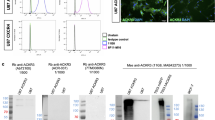
Two alternatively spliced variants from the ccr2 transcript were identified from human tissues. Because the probe sets used by the microarrays in the three datasets cannot distinguish these two splicing variants, we used isoform-specific polyclonal antibodies to examine the expression of CCR2A and CCR2B. We examined an immortalized astrocyte cell line (NHA/hTERT), a transformed human astrocyte cell line (NHA/hTRET/E6E7), and a panel of human glioma cell lines with immunoblotting, and found that CCR2A was readily detected in all cell lines (Fig. 2), whereas U87 and U251 glioma cells had the lowest abundance of CCR2A in all cell lines examined. CCR2B was barely detectable even after prolonged film exposure, and immunostaining of CCR2B in these cells also showed no detectable signal (data not shown).
CCR2A is universally expressed in immortalized astrocytes and glioma cell lines. Expression of CCR2A was examined in total cell lysates from immortalized normal human astrocytes (NHA/hTERT and NHA/hTERT/E6E7) and human glioma cells using immunoblotting. Expression of actin was used to normalize the intensity of CCR2A bands on immunoblot
To extend our in vitro results, we used immunohistochemistry to examine the expression and localization of CCR2A in a panel of non-neoplastic brain tissues and specimens from patients with GBM (Table 1). In normal brain tissue, scattered and weak cytoplasmic CCR2A immunoreactivity was seen in astrocytes, endothelial cells, and neurons but not in oligodendrocytes (Fig. 3a and data not shown). Reactive astrocytes in gliotic tissues that are strongly GFAP-positive were moderately positive for CCR2A (data not shown). Although CCR2A protein was expressed at a basal level in several cell types in non-neoplastic brain tissues, its expression was significantly increased in GBM but the expression patterns were highly variable in terms of the degree of immunoreactivity and the percentage of positive cells. Using a scoring system to categorize CCR2A staining intensity (summarized in Table 2), only two of the total group of 31 GBM specimens showed 1% of CCR2A-positive tumor cells with an intensity similar to what was observed in normal or gliotic brain (Fig. 3b). The other 29 specimens showed varying cytoplasmic CCR2A immunoreactivity in 10–100% of the neoplastic cells (Fig. 3c–f). Neurons (Fig. 3g) and endothelial cells (Fig. 3a–d) with moderately increased immunoreactivity for CCR2A were sometimes found in normal brain, inside the tumor tissues, or in regions infiltrated by neoplastic cells. The immunoreactivity of CCR2A in both normal (Fig. 3a, arrow) and neoplastic astrocytes (Fig. 3b–f) was mainly cytoplasmic, but was clearly on the plasma membrane in endothelial cells where elevated CCR2A expression was seen (Fig. 3c, inset, and d). No immunoreactivity was detected with secondary antibody alone (data not shown).
CCR2A is overexpressed in most GBM specimens examined. (a) In normal brain, astrocytes (arrow) and endothelial cells (arrowhead) were weakly positive. The intensity of CCR2A immunoreactivity in tumors was scored, and representative photomicrographs of each scale were selected. (b) Among 31 GBM samples examined, only two had a small number (approximately 1%) of CCR2A-positive cells. Neoplastic astrocytes in most tumors were strongly positive ((c) and (d), scale 1; (e), scale 2; ((f), scale 3). A magnified view of the boxed area at the right side of the panel (c) is shown in the inset, and the left boxed area is shown in the panel (d). CCR2A was predominantly localized in the cytoplasm of tumor cells, while its immunoreactivity at the cell membrane of endothelial cells of tumor vasculature is clearly seen (inset in (c) and arrowheads in (d)). Prominent CCR2A expression was seen in neurons (arrowheads) within the tumor-infiltrating area of a recurrent tumor from a patient receiving prior chemotherapy and radiation (g), but only minimal neuronal CCR2A immunoreactivity was detected (arrowheads) in tumors from patients without such history (h). Bar represents 100 μm in (a), (b), and (d)–(h), and 50 μm in (c)
Among the 31 cases whose GBM tumors were examined by immunohistochemistry, six patients had records of prior surgical removal for primary brain tumors followed by chemotherapy and/or radiation. CCR2A showed prominent expression in neurons within tumor-infiltrated gray matter from four of these cases (Fig. 3g), whereas neurons surrounded by neoplastic astrocytes had only minimally detectable CCR2A immunoreactivity from patients with no such history (Fig. 3h). χ2 test (P = 1.54 × 10−12) suggests that radiation/chemotherapy is associated with up-regulated neuronal expression of CCR2A.
Gene expression data indicated that MCP-1 had a positive but not statistically significant trend to associate with CCR2A in the abundance of their transcripts (Fig. 1c). We indeed observed by immunohistochemistry that the majority of CCR2A-overexpressing GBM (N = 22) specimens also showed intracellular MCP-1 immunoreactivity (Table 1), but CCR2A-positive tumor cells in a quarter of the samples (N = 7) were essentially negative for MCP-1 or only scattered MCP-1 positive cells could be detected.
Overexpression of CCR2A promotes glioma cell migration in vitro
We explored a role for CCR2A in glioma pathogenesis by transiently transfecting U87 and U251 glioma cells with a CCR2A-expressing construct, and found that cells transfected with CCR2A gene demonstrated increased migration compared to those transfected with vector alone (data not shown). We subsequently selected permanent cell lines from U87 cells transfected with the vector alone (U87-pcDNA3), the CCR2A gene (U87-CCR2A), and the CCR2A antisense construct (U87-CCR2AAS) (Fig. 4a). Compared to U87-pcDNA3 cells, U87-CCR2A cells showed greater and U87-CCR2AAS cells showed less migration (Fig. 4b). The growth rates of these cell lines did not differ significantly (data not shown). An independent round of transfection and permanent cell line selection was performed and the cell lines were tested with similar results (data not shown).
Glioma cell migration is promoted by ectopic CCR2A expression in an MCP-1-dependent manner. (a) The expression of CCR2A in U87-pcDNA3 cells, U87-CCR2A cells, and U87-CCR2AAS cells was compared using immunoblotting. The intensity of the CCR2A band was calibrated using the expression of actin in corresponding cell lines; ratios of calibrated CCR2A expression in U87-CCR2A/U87-pcDNA3 cells and U87-CCR2AAS/U87-pcDNA3 were plotted. Values represent the average from four different measurements. (b) Compared to U87-pcDNA3 cells, U87-CCR2A cells displayed greater migration (*, P = 0.05), and U87-CCR2AAS cells showed reduced migration (**, P = 0.034). (c) Conditioned media collected from identical number of U87-pcDNA3 and U87-CCR2A cells treated with either scrambled (sc) or antisense (as) oligonucleotides to the MCP-1 gene were analyzed using immunoblotting, and the intensity of the MCP-1 band in each cell type was separately quantitated based on the individual sc-treated cells. (d) U87-pcDNA3 and U87-CCR2A were treated with 50 μM of either sc (white column) or as oligonucleotides (black column) overnight, followed by the migration assay. MCP-1 reduction effectively decreased migration of the U87-CCR2A cells (*, P < 0.05). Migration of all cell types was abolished using the monoclonal antibody AIIB2 in the presence of sc or as oligonucleotides
We tested whether the CCR2A-induced U87 cell migration is dependent on self-produced MCP-1. When U87-pcDNA3 and U87-CCR2A cells were treated with 50 μM of MCP-1 antisense oligonucleotides, secretion of MCP-1 was inhibited by 60 to 75% compared to scrambled oligonucleotides-treated cells (Fig. 4c). U87-CCR2A cell migration was decreased by about 30% upon MCP-1 inhibition but the migration of U87-pcDNA3 cells appeared to be resistant to MCP-1 reduction. Migration of both types of cells was completely abolished using AIIB2, a monoclonal antibody against integrin β1, in the presence of either scrambled or antisense oligonucleotides. The oligonucleotide treatments did not change the amount of CCR2A (data not shown). These results suggest that CCR2A-induced U87 glioma cell migration is at least partially dependent upon self-produced ligand.
Discussion
Degenerative diseases of the central nervous system, traumatic injury, and ischemia are all associated with varying degrees of tissue inflammation. Activation and persistence of the inflammatory response is mediated by a large number of pro-inflammatory cytokines [29]. Although inflammation is not a homogeneous process, a number of common features are observed including migration and activation of astrocytes and microglia, an increase in the permeability of cerebral endothelial cells, and infiltration of macrophages and leukocytes. In most high-grade glial tumors, a robust inflammatory response is present both before and after initiation of treatment. Infiltration by immune cells, increased levels of activating cytokines, and changes in the tumor microenvironment either individually or collectively may modulate tumor growth [8].
Cytokines are known to directly contribute to tumor growth, although this has not been widely demonstrated in all tumor types [30]. Glioma cell lines have been reported to secrete stromal cell-derived factor-1, which in turn stimulates proliferation of tumor cells through activation of its receptor, CXC chemokine receptor 4 [31]. In human astrocytomas, one of the most commonly overexpressed chemokines is MCP-1 [32]. It is reasonable to speculate that tumor-derived MCP-1 may lead to excessive activation of inflammation beyond that triggered by normal cell populations such as activated astrocytes and endothelial cells, but a pro-tumorigenic effect attributed directly to this chemokine would be unexpected. Using an orthotopic tumor model in rodents, a recent report noted that overexpression of MCP-1 in glioma cells led to increased infiltration of microglial cells and tumors with a more aggressive phenotype [8]. The mechanism accounting for this response is unclear.
We demonstrated that the abundance of CCR2 mRNA is frequently higher in GBM than in lower grades of glioma and non-neoplastic brain tissues. The primary cell-surface receptor for MCP-1 is CCR2 [10], but no significant correlation between CCR2 mRNA and the expression of its ligand suggests an additional source of CCR2 expression other than blood mononuclear cells induced by MCP-1. Indeed, our immunohistochemical analysis of GBM specimens showed that CCR2A expression is increased in neoplastic cells in almost all cases, suggesting a direct or indirect role in tumor pathogenesis. The subcellular localization of CCR2A in both cultured glioma cells and primary tumor specimens is predominantly intracellular, which is consistent with other reports describing the distribution of the two CCR2 spliced variants in normal tissues [18]. Even though CCR2B is the primary receptor isoform in human cells that responds to MCP-1, it is more likely that CCR2A is the dominant isoform of MCP-1 receptor expressed in neoplastic astrocytes and has more important roles in pathogenesis. Although CCR2B could still be detected in a fraction of GBM specimens by immunohistochemistry (data not shown), it is not clear from this study why CCR2B is not overexpressed in conjunction with CCR2A in glioma cells. It is possible that regulation of splicing of the ccr2 gene favors CCR2A expression in transformed cells.
U87 glioma cells demonstrated lower migration compared to transfected cells overexpressing CCR2A. Furthermore, the baseline migration of vector-transfected cells can be further reduced by expressing an antisense construct to CCR2A (Fig. 4b). Although the function of CCR2A in normal cells is unknown, our data suggest that CCR2A overexpression affects glioma cell motility in a self-produced ligand-dependent manner. We did note that inhibition of endogenous MCP-1 did not reduce migration of CCR2A-overexpressing cells to the level of the control cells. This observation appears to parallel with the variable expression levels of CCR2A we observed in primary tumors, such that MCP-1 may induce migration of high CCR2A-expressing neoplastic astrocytes whereas CCR2A may modulate low CCR2A-expressing tumor cells independent of its ligand. Since there are many potential sources of MCP-1 in the tumor microenvironment (e.g. neoplastic cells, infiltrating macrophages and leukocytes, reactive astrocytes, neurons, and endothelial cells), regulation of CCR2A-mediated tumor cell migration, particularly when CCR2A expression in cells is greatly overexpressed, could be complicated. This type of mechanism is not without precedent; a recent study noted that MCP-1 induces proliferation and tumorigenicity of glioma cells only when these cells overexpress connexin 43 [33]. Our study warrants future validation of this differential ligand-dependent response of CCR2A in GBM and investigation of the mechanisms of CCR2A-regulated cell migration when its expression is low.
In normal cells, it would be expected that increased levels of ligand production would eventually result in decreased levels of the cognate receptor through negative feedback regulation in gene expression, but co-existence of MCP-1 production and overexpression of CCR2A in three quarters of the specimens we examined suggests that this negative autocrine loop might be deregulated in GBM tumors. It is our goal to directly test the interactions between MCP-1 and CCR2 in a transgenic intracranial tumor model to determine how this ligand-receptor pair affects the growth and invasive phenotype of brain tumors. Our in vitro migration data further suggest that a technique such as antibody neutralization targeting soluble MCP-1 or cell surface CCR2A might not be effective to control glioma cell migration. Alternatively, suppressing the synthesis of CCR2A by techniques such as antisense oligonucleotides or RNA interference could be a valid approach.
References
Prados MD, Levin V (2000) Biology and treatment of malignant glioma. Semin Oncol 27(3 Suppl 6):1–10
Nagane M, Huang HJ, Cavenee WK (1997) Advances in the molecular genetics of gliomas. Curr Opin Oncol 9(3):215–222
Liang Y, Diehn M, Watson N et al (2005) Gene expression profiling reveals clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci U S A 102(16):5814–5819
Che X, Ye W, Panga L, Wu DC, Yang GY (2001) Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res 902(2):171–177
Little AR, Benkovic SA, Miller DB, O’Callaghan JP (2002) Chemically induced neuronal damage and gliosis: enhanced expression of the proinflammatory chemokine, monocyte chemoattractant protein (MCP)-1, without a corresponding increase in proinflammatory cytokines(1). Neuroscience 115(1):307–320
Desbaillets I, Tada M, de Tribolet N et al (1994) Human astrocytomas and glioblastomas express monocyte chemoattractant protein-1 (MCP-1) in vivo and in vitro. Int J Cancer 58(2):240–247
Leung SY, Wong MP, Chung LP, Chan AS, Yuen ST (1997) Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathol (Berl) 93(5):518–527
Platten M, Kretz A, Naumann U et al (2003) Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann Neurol 54(3):388–392
Frei K, Siepl C, Groscurth P et al (1987) Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol 17(9):1271–1278
Monteclaro FS, Charo IF (1996) The amino-terminal extracellular domain of the MCP-1 receptor, but not the RANTES/MIP-1alpha receptor, confers chemokine selectivity. Evidence for a two-step mechanism for MCP-1 receptor activation. J Biol Chem 271(32):19084–19092
Lu B, Rutledge BJ, Gu L et al (1998) Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med 187(4):601–608
Boring L, Gosling J, Chensue SW et al (1997) Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 100(10):2552–2561
Kurihara T, Warr G, Loy J, Bravo R (1997) Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med 186(10):1757–1762
Kuziel WA, Morgan SJ, Dawson TC et al (1997) Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci U S A 94(22):12053–12058
Peters W, Charo IF (2001) Involvement of chemokine receptor 2 and its ligand, monocyte chemoattractant protein-1, in the development of atherosclerosis: lessons from knockout mice. Curr Opin Lipidol 12(2):175–180
Charo IF, Myers SJ, Herman A et al (1994) Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci U S A 91(7):2752–2756
Bartoli C, Civatte M, Pellissier JF, Figarella-Branger D (2001) CCR2A and CCR2B, the two isoforms of the monocyte chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta Neuropathol (Berl) 102(4):385–392
Tanaka S, Green SR, Quehenberger O (2002) Differential expression of the isoforms for the monocyte chemoattractant protein-1 receptor, CCR2, in monocytes. Biochem Biophys Res Commun 290(1):73–80
Sanders SK, Crean SM, Boxer PA et al (2000) Functional differences between monocyte chemotactic protein-1 receptor A and monocyte chemotactic protein-1 receptor B expressed in a Jurkat T cell. J Immunol 165(9):4877–4883
Tran PB, Miller RJ (2003) Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci 4(6):444–455
Andjelkovic AV, Song L, Dzenko KA, Cong H, Pachter JS (2002) Functional expression of CCR2 by human fetal astrocytes. J Neurosci Res 70(2):219–231
Banisadr G, Queraud-Lesaux F, Boutterin MC et al (2002) Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem 81(2):257–269
Stanimirovic D, Satoh K (2000) Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol 10(1):113–126
Sonoda Y, Ozawa T, Hirose Y et al (2001) Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res 61(13):4956–4960
Liang Y, Haring M, Roughley PJ, Margolis RK, Margolis RU (1997) Glypican and biglycan in the nuclei of neurons and glioma cells: presence of functional nuclear localization signals and dynamic changes in glypican during the cell cycle. J Cell Biol 139(4):851–864
Maus UA, Herold S, Schlingensiepen KH et al (2000) Antisense oligomers for selective suppression of MCP-1 synthesis in human pulmonary endothelial cells. Antisense Nucleic Acid Drug Dev 10(3):185–193
Sun L, Hui AM, Su Q et al (2006) Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 9(4):287–300
Bredel M, Bredel C, Juric D et al (2005) Functional network analysis reveals extended gliomagenesis pathway maps and three novel MYC-interacting genes in human gliomas. Cancer Res 65(19):8679–8689
Schwamborn J, Lindecke A, Elvers M et al (2003) Microarray analysis of tumor necrosis factor alpha induced gene expression in U373 human glioblastoma cells. BMC Genomics 4(1):46
Conti I, Rollins BJ (2004) CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol 14(3):149–154
Barbero S, Bonavia R, Bajetto A et al (2003) Stromal cell-derived factor 1alpha stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res 63(8):1969–1974
Khodarev NN, Labay E, Darga T et al (2004) Endothelial cells co-cultured with wild-type and dominant/negative p53-transfected glioblastoma cells exhibit differential sensitivity to radiation-induced apoptosis. Int J Cancer 109(2):214–219
Huang R, Lin Y, Wang CC et al (2002) Connexin 43 suppresses human glioblastoma cell growth by down-regulation of monocyte chemotactic protein 1, as discovered using protein array technology. Cancer Res 62(10):2806–2812
Acknowledgements
We thank the Neurosurgery Tissue Bank at the University of California, San Francisco for contributing tissue specimens in this study. We are grateful to Sharon Reynolds for commenting on the manuscript. Funding support was provided by the Department of Neurosurgery at UCSF. UCSF is an NCI-designated Specialized Program of Research Excellence for Brain Tumors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, Y., Bollen, A.W. & Gupta, N. CC chemokine receptor-2A is frequently overexpressed in glioblastoma. J Neurooncol 86, 153–163 (2008). https://doi.org/10.1007/s11060-007-9463-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9463-7