Abstract
Purpose of Review
Temozolomide is a first-line treatment for newly diagnosed glioblastoma. In this review, we will examine the use of temozolomide in other contexts for treating gliomas, including recurrent glioblastoma, glioblastoma in the elderly, diffuse low- and high-grade gliomas, non-diffuse gliomas, diffuse intrinsic pontine glioma (DIPG), ependymoma, pilocytic astrocytoma, and pleomorphic xanthoastrocytoma.
Recent Findings
Temozolomide improved survival in older patients with glioblastoma, anaplastic gliomas regardless of 1p/19q deletion status, and progressive ependymomas. Temozolomide afforded less toxicity and comparable efficacy to radiation in high-risk low-grade gliomas and to platinum-based chemotherapy in pediatric high-grade gliomas.
Summary
The success of temozolomide in promoting survival has expanded beyond glioblastoma to benefit patients with non-glioblastoma tumors. Identifying practical biomarkers for predicting temozolomide susceptibility, and establishing complementary agents for chemosensitizing tumors to temozolomide, will be key next steps for future success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment for glioblastoma has advanced in the last several decades with the inclusion of temozolomide to standard therapy with surgical resection and radiation, thereby establishing a well-tolerated, multi-modal regimen providing significant improvements to survival and outcomes. However, glioblastoma represents only a fraction of all gliomas, among which many subtypes lack an established treatment, especially those occupying inoperable central nervous system parenchyma, exhibiting aggressive features, or recurring despite prior therapy. Given the favorable efficacy and tolerability profile of temozolomide in patients with glioblastoma, the logical extension would be to incorporate temozolomide in treating other types of glial tumors. However, current evidence for temozolomide use in gliomas other than glioblastoma varies in scope and quality and lacks systematic appraisal. Accordingly, the aim of this review is not only to present the recent literature investigating the use of temozolomide in gliomas but also to define the lessons and therapeutic challenges derived from these studies to guide future treatment directions for a clinically and biologically heterogeneous group of tumors.
Temozolomide in Glioblastoma
External beam radiation treatment with concurrent and adjuvant temozolomide became the standard of care treatment for patients younger than 65 years old with newly diagnosed glioblastoma in 2005, after the landmark study published by Stupp et al. [1•]. The benefit of temozolomide in this setting has also been shown in other trials [2]. Temozolomide functions by methylating DNA to prevent tumor proliferation, as it depletes the repair enzyme O6-methylguanine-DNA methyltransferase (MGMT), making it especially effective in those patients whose tumors harbor a methylated MGMT promoter, rendering the enzyme ineffective [3]. It is an oral agent with good penetration through the blood–brain barrier [4, 5] and is generally well tolerated. Although it can cause hematologic toxicity and nausea, it has a more favorable side effect profile than other commonly used chemotherapy regimens like procarbazine, lomustine, and vincristine (PCV).
In addition to treatment of newly diagnosed glioblastoma, temozolomide has been tested in relapsed disease, but with variable response rates. Among 9 published phase 2 trials enrolling patients with recurrent glioblastoma, dosing varied greatly. Protocols ranged from 40 to 50 mg/m2/day continuously (metronomic or dose-intense regimens), to 75 to 100 mg/m2/day for 21 days in 28-day cycles or for 42 days in 70-day cycles, or up to 80 to 150 mg/m2/day in alternating weeks of 28-day cycles [6,7,8,9,10,11,12,13,14•, 15]. Progression-free survival at 6 months ranged from 19 to 35.7% for low-dose metronomic regimens, 11 to 30.3% for moderate-dose regimens, and 10 to 43.8% for cyclic high dose regimens. No survival benefit was conferred by the addition of bevacizumab [16, 17]. These studies together suggest limited benefit for retreatment with temozolomide, and overall no discernible differences in treatment efficacy dependent on dose strength or use in combination with anti-VEGF therapy. However, certain patient characteristics identified in these trials may be predictive for response rates to treatment. For example, in the 2010 RESCUE study, treatment benefit tended to favor patients with a treatment-free interval after completing standard adjuvant temozolomide, or with early progression during initial conventional therapy [13]. In the 2015 DIRECTOR trial, favorable response rates were overwhelmingly seen in patients with methylated MGMT status [14•]. In contrast, patients previously treated with bevacizumab consistently demonstrated poorer outcomes compared with bevacizumab-naïve patients [8, 12]. Despite these studies, there is no established optimal dosing regimen for treating recurrent disease. Future studies should aim to characterize additional biomarkers for responsiveness to temozolomide in recurrent disease.
Temozolomide in the Elderly with Glioblastoma
In elderly patients with glioblastoma, treatment is not well established, as much of the prior clinical research has excluded or under-represented this age group. Emerging data suggest temozolomide may be effective as monotherapy or in conjunction with hypofractionated radiotherapy. Maximal safe resection remains an appropriate first step in management, regardless of age [18].
To test the efficacy of chemotherapy in elderly patients, but limit the potential intolerability of combination treatment, several trials provided temozolomide as initial single-agent treatment. The Nordic trial randomized patients ≥ 60 years old to receive temozolomide monotherapy, hypofractionated radiotherapy (34 Gy in 10 fractions), or standard radiotherapy (60 Gy in 30 fractions). The median overall survival was longer with temozolomide compared with standard radiotherapy (8.3 vs 6.0 months), and equivalent to hypofractionated radiotherapy. In patients older than 70 years old, overall survival was similar for hypofractionated radiotherapy and temozolomide. The hypofractionated radiotherapy was better tolerated with a higher rate of completion and equivalent survival outcomes compared with standard radiotherapy. In the temozolomide monotherapy arm, patients with MGMT-methylated tumors had better survival compared to the unmethylated population. The MGMT methylation status did not affect survival outcomes in those treated with radiotherapy alone [19]. Similarly, the Methusalem (NOA-08) trial enrolled patients ≥65 years old and compared temozolomide monotherapy (100 mg/m2/day for 7 days, given every 2 weeks) versus standard radiotherapy (60 Gy in 30 fractions). Overall survival was similar between the two groups (8.6 vs 9.6 months), meeting the prespecified endpoint of noninferiority, and there was longer survival in those with methylated MGMT status [20]. A Cochrane review of these two studies showed similar progression-free survival and overall survival when comparing radiotherapy (standard or hypofractionated) with temozolomide monotherapy, and no significant difference in the quality of life among the groups. However, there was a higher rate of adverse events in the temozolomide arms, mostly hematologic (neutropenia, thrombocytopenia, or lymphocytopenia), but also infection and thromboembolic events [2].
Poor performance status is also a concern for some elderly patients. To address this, a phase 2 non-randomized study identified 70 patients with KPS < 70 (median = 60) that were treated with temozolomide monotherapy. It demonstrated improved functional status in one-third of patients (achieving a KPS ≥ 70), with increased survival compared with supportive care alone (25 weeks vs 12 to 16 weeks), especially in those with methylated MGMT. It was also well tolerated, and non-treatment-related toxicities led to discontinuation of therapy [21].
Ironside et al. reviewed 39 patients aged 70–83, with KPS 70–80 and histologically proven glioblastoma, who declined radiotherapy and were treated with 1–12 cycles of temozolomide (median, 5) as upfront monotherapy. These were patients who were otherwise eligible for radiotherapy. Treatment was commonly discontinued because of disease progression rather than toxicity. It showed similar survival outcome to trial populations treated with radiation, with a median overall survival of 36 weeks and median progression-free survival of 20 weeks, although MGMT-promoter methylation was not predictive of outcome [18]. Thus, hypofractionated radiotherapy or temozolomide monotherapy were deemed reasonable options in the treatment of elderly patients with glioblastoma.
Temozolomide has also been used in conjunction with radiotherapy in elderly patients, rather than as monotherapy. In a recent 2017 study (NCIC CE.6/EORTC 26062 trial), elderly patients ≥ 65 years old were randomly assigned to receive radiotherapy or radiotherapy with concomitant and adjuvant temozolomide. This study demonstrated that the addition of temozolomide to short-course radiotherapy (40 Gy in 15 fractions) resulted in longer survival than radiotherapy alone, with similar quality of life. In those with methylated MGMT, the addition of temozolomide nearly doubled overall survival (13.5 vs 7.7 months) [22•].
Temozolomide in Diffuse Low-Grade Glioma (Oligodendroglioma and Astrocytoma)
Most of the large studies that evaluated chemotherapy for infiltrating low-grade gliomas were conducted before the WHO 2016 update on the classification of central nervous system (CNS) tumors. Previously, grade II and III gliomas were classified by histologic morphology. Since then, several molecular markers have been shown to have superior prognostic and predictive value, leading to the current classification system [23]. In the 2016 classification of diffuse glioma, an oligodendroglioma requires the presence of an IDH mutation and chromosome 1p/19q co-deletion. Astrocytomas, which lack chromosome 1p/19q co-deletion, are divided into IDH-wild type and IDH-mutant tumors. Grading is then based on other subjective histological characteristics such as high cellularity, mitosis, nuclear atypia, endothelial proliferation, and necrosis. The trend across studies is that oligodendrogliomas show a better response to chemotherapy than astrocytomas [24, 25], and that co-deletion of chromosomes 1p and 19q and also IDH-1 and IDH-2 mutations are independent prognostic factors favoring a response to therapy [26, 27]. The presence of an IDH mutation can lead to downstream methylation of MGMT, which in theory is one mechanism explaining the sensitivity to chemotherapy [27].
The current National Comprehensive Cancer Network (NCCN) guidelines for infiltrative gliomas include maximal safe resection, usually followed by radiotherapy and chemotherapy for residual disease in grade III and high-risk grade II tumors [28]. Historically, chemotherapy has been used as a salvage therapy, often following surgical resection and radiation, for recurrent or anaplastic gliomas. Studies have been conducted to identify how chemotherapy can be used as upfront monotherapy in an effort to spare or delay the long-term side effects of radiotherapy, or concomitantly with radiotherapy [24].
RTOG 9802 was a phase III randomized, controlled study of radiation with or without adjuvant PCV chemotherapy regimen for high-risk, low-grade gliomas, which began prior to 2016 WHO molecular glioma classification. A total of 251 patients ≥ 40 years old, or under 40 years old with a subtotal resection or a biopsy, and histologically confirmed grade 2 astrocytoma, oligodendroglioma or oligoastrocytoma were enrolled from 1998 to 2002, and followed for a median of 11.9 years. In 2016, long-term results of this study were released, which demonstrated that there is both a longer progression-free survival, as well as overall survival for patients who received radiation plus PCV chemotherapy, in comparison to those who received radiation alone. The median overall survival was 13.3 versus 7.8 years, and the rate of progression-free survival at 10 years for those receiving multimodal therapy was 51% versus 21% in those who received radiation therapy alone. Furthermore, the patients who derived the most benefit from combined PCV and radiation therapy were patients with 1p/19q co-deleted tumors and those with IDH1 mutations. This was the springboard for additional studies targeting either oligodendrogliomas or astrocytomas specifically, and also highlighted the importance of long-term follow up in order to identify whether a delayed benefit exists for this population of patients, as the two initial interim analyses were negative. Although this study showed the importance of chemotherapy, the question of whether or not temozolomide could be as effective as PCV in the low-grade glioma population remained [29].
The EORTC 22033-26033 study looked at high-risk low-grade gliomas stratified by 1p/19q co-deletion status. These participants were randomized to either conformal radiation or dose-dense temozolomide. There was no significant difference in progression-free survival between the two groups, but the median overall survival had not been reached at the time of publication [30•]. It is likely that data will require many years to mature, as was the case with RTOG 9802.
RTOG 0424 is the only large prospective study to date that evaluated overall survival of high-risk low-grade gliomas treated with radiotherapy with concurrent and adjuvant temozolomide. This was a single arm phase II study that compared overall survival of those receiving radiation and temozolomide in comparison to historical controls who received radiotherapy alone. Between 2005 and 2009, 129 patients with high-risk low-grade glioma were identified, defined as high-risk with the presence of at least three of the following risk factors: age ≥ 40, bihemispheric tumor, tumor diameter ≥ 6 cm, astrocytoma histology, or moderate-to-severe neurologic impairment. Patients received radiation 54 Gy in 30 fractions with concurrent oral temozolomide (75 mg/m2/day) and up to 12 cycles of adjuvant temozolomide (150-200 mg/m2/day, 5/28 day cycles). Overall survival in those treated with radiation and temozolomide significantly exceed that of historical controls, with 3-year overall survival rate of 73.5% receiving treatment, compared with the historical control of 54% [31, 32]. Although MGMT status was not initially known in the 2015 report, post hoc analysis has subsequently revealed that MGMT methylation is an independent prognostic factor in high-risk low-grade glioma treated with both radiotherapy and temozolomide, with improved progression-free and overall survival [33•].
For chemotherapy options in low-grade gliomas, both temozolomide and PCV are established treatments for grade II and grade III gliomas. In a 2014 Cochrane review, Lecavalier-Barsoum et al. reviewed 931 participants with anaplastic oligodendrogliomas or anaplastic oligoastrocytomas across three randomized controlled trials and their overall survival with a variety of therapies: radiotherapy alone, sequential radiotherapy and PCV, PCV alone, and temozolomide alone. There was sufficient evidence to conclude that PCV (before or after RT) improves overall survival in patients with anaplastic oligodendrogliomas or oligoastrocytomas. This was particularly true in those with 1p/19q co-deletion [26].
Despite the demonstrated efficacy of either chemotherapy regimen for low-grade glioma, there remained uncertainty as to whether temozolomide or PCV was the superior treatment. To address this question, these chemotherapies were compared against each other within the 2009 NOA-04 trial, in which patients received either radiation therapy, temozolomide, or PCV, and permitted to switch from chemotherapy to radiation, or vice versa, if treatment toxicity became intolerable or if there was evidence of disease progression. The primary outcome consisted of time to disease progression after two sequential treatment modalities in any order. Although there was no significant difference in overall survival between the groups treated with PCV and temozolomide upfront, it was not powered for direct comparison, and ultimately, there was insufficient evidence to determine whether temozolomide carried a similar benefit in oligodendroglioma as PCV [34].
In addition to promoting progression-free and overall survival, it is also important to consider how each chemotherapy agent impacts tumor growth. Izquierdo et al. examined growth kinetics of 1p/19q co-deleted low-grade gliomas in patients who were treated with upfront temozolomide. They found that 27% had regrowth during treatment, and in the remaining patients, the mean time to regrowth after discontinuation of temozolomide was 12 months, with 6% of patients remaining without regrowth 5 years after discontinuing temozolomide. The low-grade glioma growth kinetics on PCV showed no patients with regrowth during treatment, duration of ongoing volume reduction lasted between 28 and 35 months, and at 5 years after treatment, 60% had no regrowth. The proposed explanation for this is that tumors treated with temozolomide acquire a hypermutation phenotype that leads to malignant progression [35•]. This warrants additional study.
The CATNON trial is an ongoing phase III, randomized, open-label study in which patients with 1p/19q non-co-deleted anaplastic astrocytomas are divided into four treatment arms: radiotherapy alone, radiation with adjuvant temozolomide, radiotherapy with concurrent temozolomide, or radiotherapy with concurrent and adjuvant temozolomide. The planned interim analysis has demonstrated clear overall survival, and progression-free survival benefit in patients who received radiotherapy with adjuvant temozolomide. Adjuvant temozolomide increased the median progression-free survival from 19.0 to 42.8 months, and the 5-year overall survival increased from 44 to 56%. This is the first study that demonstrates a clear benefit of temozolomide in non-co-deleted anaplastic glioma, despite previous evidence that these tumors are less chemotherapy-sensitive than co-deleted tumors. The role of concurrent (rather than adjuvant) temozolomide in these patients has not yet been determined, with inconclusive data based on the interim analysis [36•].
The CODEL study is also a phase III study, currently recruiting patients with 1p/19q co-deleted low-grade or anaplastic oligodendrogliomas, with the aim of determining whether radiation with concomitant and adjuvant temozolomide versus radiation with adjuvant PCV chemotherapy is more effective in this population.
It is anticipated that the CATNON and CODEL studies will provide much-needed clinical insight into the role of temozolomide in treating patients with low- or high-grade gliomas.
Temozolomide in Diffuse Intrinsic Pontine Glioma
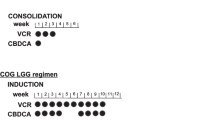
Diffuse intrinsic pontine gliomas (DIPG) remain a therapeutic challenge due to poor prognosis even with treatment, which typically consists of corticosteroids and radiation therapy, resulting in median overall survival of 10 to 11 months [37, 38]. Unfortunately, the use of temozolomide in pediatric DIPG has not provided any additional therapeutic benefit. Rizzo et al. studied children treated with focal radiotherapy with concurrent daily temozolomide, followed by adjuvant temozolomide. At median follow up of 15 months, the prognosis remained poor despite the addition of temozolomide, as only two out of 15 children were alive, and had progressive disease [39]. Multiple other studies have also failed to show improved outcomes in children with DIPG treated with temozolomide [39, 40]. To illustrate, three trials enrolled children with newly diagnosed DIPG using concurrent radiation with temozolomide dosing regimens of 75 to 90 mg/m2/day followed by adjuvant temozolomide (200 mg/m2/day for 5 days in 28-day cycles or 75-100 mg/m2/day for 21 days in 28-day cycles), with one trial including cis-retinoic acid to the adjuvant phase of treatment [41,42,43]. Treatment was overall well tolerated, however median overall survival in these trials was not significantly improved compared to historical cohorts, ranging from 9.5 to 13.5 months [41,42,43].
The physiologic basis for poor treatment response in DIPG is not completely understood, but one possible explanation may be regional differences in drug bioavailability: an in vivo non-human primate model found significantly lower levels of temozolomide in the pons compared with the cortex and cerebral spinal fluid, suggesting that the blood–brain barrier is not homogenous, and that variable penetration of drugs at different levels of the central nervous system may explain why temozolomide (and other chemotherapies) is ineffective in treating DIPG [44]. Others have argued for using temozolomide with radiotherapy in these cases because of lower toxicity in comparison with cisplatin-based polychemotherapies (which included combinations of cisplatin, etoposide, ifosfamide, and vincristine) [45•]. In parallel, and in part due to the lack of consistent or substantial clinical benefit of temozolomide for DIPG, ongoing clinical trials have re-directed focus to alternative mechanistic bases for treatment, including molecularly targeted therapies such as mTOR inhibitors, HDAC inhibitors, and CAR-T cells [46•, 47, 48].
For pontine gliomas in adults, the generalizability of DIPG treatment from pediatric patients is limited by multiple factors. First, several clinical, histologic, and molecular features separate adult pontine gliomas as biologically and prognostically distinct from their pediatric counterparts, with survival outcomes favoring adult-onset disease [49, 50]. Second, the absolute rarity of brainstem gliomas occurring in adult patients (~2% of all adult glioma cases) has led to an even starker paucity of data for all treatment modalities, let alone for chemotherapy [50, 51]. Nevertheless, in instances that include the use of temozolomide, results are promising: three studies showed improved survival in adult pontine glioma patients treated with temozolomide and concurrent radiation; however, all were limited by their retrospective nature, higher KPS scores in the treated patients, and small cohort sizes (n = 25, 15, and 7) [49, 52, 53]. Further studies with larger cohorts and prospective design are needed to determine if such results are reproducible and clinically meaningful for this rare variant of adult glioma.
Temozolomide in Non-diffuse Gliomas
Ependymoma
For patients with ependymoma, surgical resection followed by radiotherapy, remains the standard of care. A significant number recur and can have multiple relapses, usually locally, but spread via cerebral spinal fluid is also possible. This can prompt re-resection, reirradiation, or consideration of salvage chemotherapy. A variety of chemotherapy agents have been used, none proving superior to others, such that there is no standard salvage chemotherapy. Temozolomide is emerging as an agent that may have a role, given its historical efficacy in glioblastoma and overall tolerability. Freyschlag et al. provide a case study of a patient with multifocal recurrence after resection and adjuvant radiotherapy, who had a dramatic regression after 12 weeks and no radiographic progression after 5 months of surveillance imaging, when temozolomide was administered [54]. A retrospective study of 17 patients with grade III or IV ependymoma by Gramatzki et al. reviewed response to temozolomide, PCV, platinum-based chemotherapy, or epirubicin/ifosfamide. Most of the patients (n = 10) received temozolomide, with the best response being stable disease when used for recurrence (after resection and radiotherapy). Two cases that received a combination of radiotherapy and concurrent temozolomide followed by adjuvant temozolomide had low median progression-free survival (3 months) and median overall survival (27 months). The authors concluded that although temozolomide demonstrated some activity against intracranial ependymoma, other chemotherapies also produced a response [55].
Another retrospective study identified 18 patients with recurrent ependymoma who were treated with resection and radiotherapy at various points during their clinical course (sometimes multiple times) prior to receiving temozolomide [56•]. There was variability in response, ranging from complete remission (one of 18), partial response (three), stable disease (seven), and disease progression (seven), with a median progression-free survival of 9.69 months and median overall survival of 30.55 months. Notably, those with response to temozolomide were chemotherapy-naive, and some had a delayed response, which was felt to be cumulative over time. MGMT methylation status, when it was known (11 of 18 patients), did not correlate with outcome or response, unlike in glioblastoma [56•].
With these limited data, larger and prospective studies are needed to elucidate the role of chemotherapy, and specifically temozolomide, for patients with ependymoma after failure of surgery and radiation, but it appears to be a reasonable treatment agent, both in terms of effectiveness and tolerability.
Pilocytic Astrocytoma
Pilocytic astrocytomas (PAs) are well-circumscribed, low-grade neoplasms with favorable prognosis (5-year survival ~97%), and standard treatment consisting of gross total resection, essentially resulting in cure [28, 57]. However, the benefits of surgery can, at times, be limited by incompleteness of the initial resection, location of tumor in eloquent parenchyma, or progression due to aggressive disease at onset or anaplastic transformation [58,59,60]. In these uncommon circumstances, radiation therapy is typically employed [28, 59], but limited evidence suggests a potential role for chemotherapy with temozolomide. In one phase II prospective trial, two-year progression-free survival was 49% in children with progressive low-grade gliomas (including PAs), and 54% of these children had disease stabilization without significant toxicities (grade 2–4 cytopenia occurring in 23%) [61]. In one small retrospective study, event-free survival was 57%, and median time to disease progression was 6.7 months [62]. Further studies are warranted to clarify the best temozolomide scheduling, and if concurrent radiotherapy or combination chemotherapy potentiates anti-tumor activity.
Pleomorphic Xanthoastrocytoma
Pleomorphic xanthoastrocytomas (PXAs), like PAs, have a favorable prognosis and are typically curable with surgical resection [28]. For subtotal resections or recurrent disease, there have been no systematic investigations for the use of adjuvant or salvage temozolomide. The current published examples instead consist of case reports with anaplastic PXAs, including those with disseminated multifocal disease, co-treated with radiation therapy; one patient was without disease recurrence at 24 months, one demonstrated radiographic tumor regression at 10 months, but one patient with multifocal disease had continued progression and dissemination in the neuroaxis [63,64,65]. Nonetheless, there is at least some mechanistic basis for the use of temozolomide to inhibit tumor growth, as suggested by ex vivo experiments in a xenograft murine model of PXA [66].
The Changing Landscape of Glioma Treatment
More recent advances in cancer treatment have focused on “precision medicine”. This involves conforming an optimal treatment paradigm specific to each patient based on genomic, proteomic, epigenetic, and biomarker data—from both patients and molecular characterization of their tumor—that informs the molecularly-targeted treatment most likely to provide clinical benefit [67]. An excellent example of this success has been the use of BRAF-inhibitors in melanoma tumors with BRAF v600E mutations [68, 69]. Other recent advances involve immunotherapy, including immune checkpoint inhibitors, CAR-T cells, and bi-specific T cell engagers (BiTE antibodies) [70,71,72]. Despite this changing treatment landscape, there is still a role for temozolomide in primary CNS tumors. It has proven success in glioblastoma and other diffuse gliomas, as discussed above. Its success has been less certain in the non-diffuse gliomas, and targeted agents may ultimately prove to be more beneficial in these cases. This may be especially true in tumors that harbor easily targetable mutations. For example, > 70% of pilocytic astrocytomas harbor a BRAF mutation, raising the possibility that BRAF inhibitors could play a future role in targeted treatment for progressive or anaplastic disease [73]. However, temozolomide’s long history and favorable side effect profile still make it an attractive option.
Conclusion
While the role of temozolomide has been well established for glioblastoma, its use as a first-line or salvage agent for a variety of other gliomas has yet to be defined. With limited data across most tumor types, additional research is needed to delineate the potential role of temozolomide, as monotherapy, adjuvant therapy, or with concurrent radiation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
• Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J med. 2005;352:987–96. https://doi.org/10.1056/NEJMoa043330 This study demonstrated, both clinically and statistically, significant survival benefit with the addition of temozolomide to radiotherapy for glioblastoma, without significant toxicity. This has now become the standard of care following maximal safe surgical resection.
Hart MG, Garside R, Rogers G, Stein K, Grant R. Temozolomide for high grade glioma. Cochrane Database Syst Rev. 2013:Cd007415. https://doi.org/10.1002/14651858.CD007415.pub2.
Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev. 1997;23:35–61.
Agarwala SS, Kirkwood JM. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist. 2000;5:144–51.
Brindley CJ, Antoniw P, Newlands ES. Plasma and tissue disposition of mitozolomide in mice. Br J Cancer. 1986;53:91–7.
Abacioglu U, Caglar HB, Yumuk PF, Akgun Z, Atasoy BM, Sengoz M. Efficacy of protracted dose-dense temozolomide in patients with recurrent high-grade glioma. J Neuro-Oncol. 2011;103:585–93. https://doi.org/10.1007/s11060-010-0423-2.
Brandes AA, Tosoni A, Cavallo G, Bertorelle R, Gioia V, Franceschi E, et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from gruppo italiano cooperativo di neuro-oncologia (GICNO). Br J Cancer. 2006;95:1155–60. https://doi.org/10.1038/sj.bjc.6603376.
Han SJ, Rolston JD, Molinaro AM, Clarke JL, Prados MD, Chang SM, et al. Phase II trial of 7 days on/7 days off temozolmide for recurrent high-grade glioma. Neuro-Oncology. 2014;16:1255–62. https://doi.org/10.1093/neuonc/nou044.
Khan RB, Raizer JJ, Malkin MG, Bazylewicz KA, Abrey LE. A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro-Oncology. 2002;4:39–43. https://doi.org/10.1093/neuonc/4.1.39.
Kong DS, Lee JI, Kim JH, Kim ST, Kim WS, Suh YL, et al. Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma. Neuro-Oncology. 2010;12:289–96. https://doi.org/10.1093/neuonc/nop030.
Norden AD, Lesser GJ, Drappatz J, Ligon KL, Hammond SN, Lee EQ, et al. Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro-Oncology. 2013;15:930–5. https://doi.org/10.1093/neuonc/not040.
Omuro A, Chan TA, Abrey LE, Khasraw M, Reiner AS, Kaley TJ, et al. Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro-Oncology. 2013;15:242–50. https://doi.org/10.1093/neuonc/nos295.
Perry JR, Belanger K, Mason WP, Fulton D, Kavan P, Easaw J, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28:2051–7. https://doi.org/10.1200/jco.2009.26.5520.
• Weller M, Tabatabai G, Kastner B, Felsberg J, Steinbach JP, Wick A, et al. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res. 2015;21:2057–64. https://doi.org/10.1158/1078-0432.Ccr-14-2737 This study aimed to identify how dose-intense regimens of temozolomide may be beneficial in treatment of recurrent glioblastoma. There was no major difference in efficacy between one week on/one week off temozolomide compared with three weeks on/one week off. It did, however, indicate that temozolomide rechallenge should not be considered for patients with an unmethylated MGMT promoter status, although it is a viable option for methylated-MGMT tumors, as the median time to treatment failure, progression-free survival, and overall survival were longer versus unmethylated tumors.
Wick A, Felsberg J, Steinbach JP, Herrlinger U, Platten M, Blaschke B, et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol. 2007;25:3357–61. https://doi.org/10.1200/jco.2007.10.7722.
Desjardins A, Reardon DA, Coan A, Marcello J, Herndon JE 2nd, Bailey L, et al. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer. 2012;118:1302–12. https://doi.org/10.1002/cncr.26381.
Verhoeff JJ, Lavini C, van Linde ME, Stalpers LJ, Majoie CB, Reijneveld JC, et al. Bevacizumab and dose-intense temozolomide in recurrent high-grade glioma. Ann Oncol. 2010;21:1723–7. https://doi.org/10.1093/annonc/mdp591.
Ironside S, Das S, Sahgal A, Moroney C, Mainprize T, Perry JR. Optimal therapies for newly diagnosed elderly patients with glioblastoma. Curr Treat Options in Oncol. 2017;18:66. https://doi.org/10.1007/s11864-017-0508-7.
Malmstrom A, Gronberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–26. https://doi.org/10.1016/s1470-2045(12)70265-6.
Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13:707–15. https://doi.org/10.1016/s1470-2045(12)70164-x.
Gallego Perez-Larraya J, Ducray F, Chinot O, Catry-Thomas I, Taillandier L, Guillamo JS, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29:3050–5. https://doi.org/10.1200/jco.2011.34.8086.
• Perry JR, Laperriere N, O'Callaghan CJ, Brandes AA, Menten J, Phillips C, et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N Engl J Med. 2017;376:1027–37. https://doi.org/10.1056/NEJMoa1611977 Since previous trials implementing Stupp protocol largely excluded patients > 70 years of age, there were few data about the benefit of temozolomide for older patients with glioblastoma. This study was notable for showing that addition of temozolomide does provide survival benefit in elderly patients, even if overall prognosis is poorer compared to younger patients.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016(131):803–20. https://doi.org/10.1007/s00401-016-1545-1.
Drappatz J, Lieberman F. Chemotherapy of oligodendrogliomas. Prog Neurol Surg. 2018;31:152–61. https://doi.org/10.1159/000467376.
van den Bent MJ, Smits M, Kros JM, Chang SM. Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol. 2017;35:2394–401. https://doi.org/10.1200/jco.2017.72.6737.
Lecavalier-Barsoum M, Quon H, Abdulkarim B. Adjuvant treatment of anaplastic oligodendrogliomas and oligoastrocytomas. Cochrane Database Syst Rev. 2014:Cd007104. https://doi.org/10.1002/14651858.CD007104.pub2.
van den Bent MJ, Chang SM. Grade II and III oligodendroglioma and astrocytoma. Neurol Clin. 2018;36:467–84. https://doi.org/10.1016/j.ncl.2018.04.005.
Nabors LB, Portnow J, Ammirati M, Baehring J, Brem H, Butowski N, Fenstermaker RA, Forsyth P, Hattangadi-Gluth J, Holdhoff M, Howard S, Junck L, Kaley T, Kumthekar P, Loeffler JS, Moots PL, Mrugala MM, Nagpal S, Pandey M, Parney I, Peters K, Puduvalli VK, Ragsdale J 3rd, Rockhill J, Rogers L, Rusthoven C, Shonka N, Shrieve DC, Sills AK Jr, Swinnen LJ, Tsien C, Weiss S, Wen PY, Willmarth N, Bergman MA, Engh A. NCCN Clinical Practice Guidelines in Oncology. Central Nervous System Cancers (Version I.2018). Accessed November 1, 2018.
Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, et al. Radiation plus Procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374:1344–55. https://doi.org/10.1056/NEJMoa1500925.
• Baumert BG, Hegi ME, van den Bent MJ, von Deimling A, Gorlia T, Hoang-Xuan K, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033–26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17:1521–32. https://doi.org/10.1016/s1470-2045(16)30313-8 This was the first study to randomly assign patients with low-grade glioma and at least one high-risk feature to radiotherapy alone or temozolomide chemotherapy alone. Although there was no improvement in progression-free survival with chemotherapy alone, temozolomide may prevent or delay patients from the side effects of radiation by using a similarly effective therapy. Subgroup analysis suggested that IDH mutant, non-co-deleted tumors treated with radiotherapy had a longer progression-free survival than those treated with temozolomide (such that temozolomide treatment alone might be deleterious).
Fisher BJ, Hu C, Macdonald DR, Lesser GJ, Coons SW, Brachman DG, et al. Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: preliminary results of radiation therapy oncology group 0424. Int J Radiat Oncol Biol Phys. 2015;91:497–504. https://doi.org/10.1016/j.ijrobp.2014.11.012.
Fisher B, Zhang P, Macdonald D, Chakravarti A, Lesser G, Fox S, et al. ACTR-2. NRG Oncology/RTOG 0424: Long term results of a phase II study of temozolomide-based chemoradiotherapy regimen for high risk low-grade gliomas. Neuro-Oncology. 2018;20. https://doi.org/10.1093/neuonc/noy148.039.
• Bell EH, Zhang P, Fisher BJ, Macdonald DR, McElroy JP, Lesser GJ, et al. Association of MGMT promoter methylation status with survival outcomes in patients with high-risk glioma treated with radiotherapy and temozolomide: an analysis from the NRG oncology/RTOG 0424 trial. JAMA Oncol. 2018;4:1405–9. https://doi.org/10.1001/jamaoncol.2018.1977 This was the first study to demonstrate MGMT methylation as an independent prognostic factor in low-grade glioma treated with temozolomide and radiotherapy.
Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–80. https://doi.org/10.1200/jco.2009.23.6497.
• Izquierdo C, Alentorn A, Idbaih A, Simo M, Kaloshi G, Ricard D, et al. Long-term impact of temozolomide on 1p/19q-codeleted low-grade glioma growth kinetics. J Neurooncol. 2018;136:533–9. https://doi.org/10.1007/s11060-017-2677-4 This group examined the growth kinetics of co-deleted low-grade gliomas who were treated with upfront temozolomide, finding that most tumors resumed their growth within 3 years of treatment. They argued that volumetric analysis to identify early tumor progression can prevent unnecessary exposure to temozolomide, which could put patients at risk for malignant progression (based on mutational analyses proposed that TMZ exposure can lead to acquisition of a hypermutation, leading to progression).
• van den Bent MJ, Baumert B, Erridge SC, Vogelbaum MA, Nowak AK, Sanson M, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;390:1645–53. https://doi.org/10.1016/s0140-6736(17)31442-3 Before this trial, there had not been a well-designed study supporting adjuvant temozolomide for grade II and III gliomas. This study demonstrated that the less-chemosensitive 1p/19q non-co-deleted anaplastic gliomas still derived benefit from temozolomide therapy. It showed prolonged overall survival with 12 cycles of adjuvant temozolomide in addition to radiotherapy. The role of concurrent temozolomide therapy is not yet established.
Massimino M, Spreafico F, Biassoni V, Simonetti F, Riva D, Trecate G, et al. Diffuse pontine gliomas in children: changing strategies, changing results? A mono-institutional 20-year experience. J Neuro-Oncol. 2008;87:355–61. https://doi.org/10.1007/s11060-008-9525-5.
van Zanten SEM V, Lane A, Heymans MW, Baugh J, Chaney B, Hoffman LM, et al. External validation of the diffuse intrinsic pontine glioma survival prediction model: a collaborative report from the international DIPG registry and the SIOPE DIPG registry. J Neurooncol. 2017;134:231–40. https://doi.org/10.1007/s11060-017-2514-9.
Rizzo D, Scalzone M, Ruggiero A, Maurizi P, Attina G, Mastrangelo S, et al. Temozolomide in the treatment of newly diagnosed diffuse brainstem glioma in children: a broken promise? J Chemother. 2015;27:106–10. https://doi.org/10.1179/1973947814y.0000000228.
Hassan H, Pinches A, Picton SV, Phillips RS. Survival rates and prognostic predictors of high grade brain stem gliomas in childhood: a systematic review and meta-analysis. J Neuro-Oncol. 2017;135:13–20. https://doi.org/10.1007/s11060-017-2546-1.
Bailey S, Howman A, Wheatley K, Wherton D, Boota N, Pizer B, et al. Diffuse intrinsic pontine glioma treated with prolonged temozolomide and radiotherapy—results of a United Kingdom phase II trial (CNS 2007 04). Eur J Cancer. 2013;49:3856–62. https://doi.org/10.1016/j.ejca.2013.08.006.
Cohen KJ, Heideman RL, Zhou T, Holmes EJ, Lavey RS, Bouffet E, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the children's oncology group. Neuro-Oncology. 2011;13:410–6. https://doi.org/10.1093/neuonc/noq205.
Sirachainan N, Pakakasama S, Visudithbhan A, Chiamchanya S, Tuntiyatorn L, Dhanachai M, et al. Concurrent radiotherapy with temozolomide followed by adjuvant temozolomide and cis-retinoic acid in children with diffuse intrinsic pontine glioma. Neuro-Oncology. 2008;10:577–82. https://doi.org/10.1215/15228517-2008-025.
Warren KE. Beyond the blood:brain barrier: the importance of central nervous system (CNS) pharmacokinetics for the treatment of CNS tumors including diffuse intrinsic pontine glioma. Front Oncol. 2018;8:239. https://doi.org/10.3389/fonc.2018.00239.
• Seidel C, von Bueren AO, Bojko S, Hoffmann M, Pietsch T, Gielen GH, et al. Concurrent radiotherapy with temozolomide vs. concurrent radiotherapy with a cisplatinum-based polychemotherapy regimen: acute toxicity in pediatric high-grade glioma patients. Strahlenther Onkol. 2018:194:215–224. Doi:https://doi.org/10.1007/s00066-017-1218-6. This study identified that cisplatin-based chemotherapy demonstrated increased toxicity (mostly hematologic), with more interruptions of treatment, in comparison with temozolomide in pediatric high-grade gliomas. While the efficacy of chemotherapies for pediatric high-grade glioma are similar (and poor), the authors argued for temozolomide use with radiotherapy because of relatively lower toxicity.
• Flannery PC, JA DS, Amani V, Venkataraman S, Lemma RT, Prince EW, et al. Preclinical analysis of MTOR complex 1/2 inhibition in diffuse intrinsic pontine glioma. Oncol Rep. 2018;39:455–64. https://doi.org/10.3892/or.2017.6122 AZD2014 is a MTOR complex 1 and 2 inhibitor that was studied in three patient-derived DIPG cell lines. Its use potentiated greatly increased antitumor efficacy, via increased inhibition of cell self-renewal. It also demonstrated a synergistic relationship with dose-dependent radiotherapy, and also with other chemotherapies.
Hashizume R, Andor N, Ihara Y, Lerner R, Gan H, Chen X, et al. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat Med. 2014;20:1394–6. https://doi.org/10.1038/nm.3716.
Mount CW, Majzner RG, Sundaresh S, Arnold EP, Kadapakkam M, Haile S, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat Med. 2018;24:572–9. https://doi.org/10.1038/s41591-018-0006-x.
Reyes-Botero G, Giry M, Mokhtari K, Labussiere M, Idbaih A, Delattre JY, et al. Molecular analysis of diffuse intrinsic brainstem gliomas in adults. J Neuro-Oncol. 2014;116:405–11. https://doi.org/10.1007/s11060-013-1312-2.
Reyes-Botero G, Mokhtari K, Martin-Duverneuil N, Delattre JY, Laigle-Donadey F. Adult brainstem gliomas. Oncologist. 2012;17:388–97. https://doi.org/10.1634/theoncologist.2011-0335.
Salmaggi A, Fariselli L, Milanesi I, Lamperti E, Silvani A, Bizzi A, et al. Natural history and management of brainstem gliomas in adults. A retrospective Italian study. J Neurol. 2008;255:171–7. https://doi.org/10.1007/s00415-008-0589-0.
Babu R, Kranz PG, Karikari IO, Friedman AH, Adamson C. Clinical characteristics and treatment of malignant brainstem gliomas in elderly patients. J Clin Neurosci. 2013;20:1382–6. https://doi.org/10.1016/j.jocn.2012.12.011.
Theeler BJ, Ellezam B, Melguizo-Gavilanes I, de Groot JF, Mahajan A, Aldape KD, et al. Adult brainstem gliomas: correlation of clinical and molecular features. J Neurol Sci. 2015;353:92–7. https://doi.org/10.1016/j.jns.2015.04.014.
Freyschlag CF, Tuettenberg J, Lohr F, Thome C, Schmieder K, Seiz M. Response to temozolomide in supratentorial multifocal recurrence of malignant ependymoma. Anticancer Res. 2011;31:1023–5.
Gramatzki D, Roth P, Felsberg J, Hofer S, Rushing EJ, Hentschel B, et al. Chemotherapy for intracranial ependymoma in adults. BMC Cancer. 2016;16:287. https://doi.org/10.1186/s12885-016-2323-0.
• Ruda R, Bosa C, Magistrello M, Franchino F, Pellerino A, Fiano V, et al. Temozolomide as salvage treatment for recurrent intracranial ependymomas of the adult: a retrospective study. Neuro Oncol. 2016;18:261–8. https://doi.org/10.1093/neuonc/nov167 Before this study, there were few data to guide the addition of further chemotherapy to recurrent or progressive ependymomas despite initial resection and radiation. Although the efficacy of temozolomide among these select patients was variable, favorable results in chemotherapy-naïve patients suggested a role for temozolomide after failure of first-line treatments. Intriguingly, these benefits occurred independent of MGMT methylation status.
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Suppl 4):iv1–63. https://doi.org/10.1093/neuonc/nou223.
Bond KM, Hughes JD, Porter AL, Orina J, Fang S, Parney IF. Adult pilocytic astrocytoma: an institutional series and systematic literature review for extent of resection and recurrence. World Neurosurg. 2018;110:276–83. https://doi.org/10.1016/j.wneu.2017.11.102.
Dirven CM, Mooij JJ, Molenaar WM. Cerebellar pilocytic astrocytoma: a treatment protocol based upon analysis of 73 cases and a review of the literature. Childs Nerv Syst. 1997;13:17–23. https://doi.org/10.1007/s003810050033.
Krieger MD, Gonzalez-Gomez I, Levy ML, McComb JG. Recurrence patterns and anaplastic change in a long-term study of pilocytic astrocytomas. Pediatr Neurosurg. 1997;27:1–11. https://doi.org/10.1159/000121218.
Gururangan S, Fisher MJ, Allen JC, Herndon JE 2nd, Quinn JA, Reardon DA, et al. Temozolomide in children with progressive low-grade glioma. Neuro-Oncology. 2007;9:161–8. https://doi.org/10.1215/15228517-2006-030.
Khaw SL, Coleman LT, Downie PA, Heath JA, Ashley DM. Temozolomide in pediatric low-grade glioma. Pediatr Blood Cancer. 2007;49:808–11. https://doi.org/10.1002/pbc.21270.
Katayama K, Asano K, Shimamura N, Ogasawara Y, Naraoka M, Ohkuma H, et al. Case of pleomorphic xanthoastrocytoma with anaplastic features in the pineal gland. Brain Tumor Pathol. 2013;30:242–6. https://doi.org/10.1007/s10014-013-0137-1.
Suzuki Y, Akiyama Y, Kimura Y, Sugita S, Hasegawa T, Mikuni N. Pleomorphic xanthoastrocytoma with anaplastic features in the tectal region in a young adult patient: a case report. World Neurosurg. 2016;94(580):e11–5. https://doi.org/10.1016/j.wneu.2016.07.110.
Zhu M, Zhang C, Zhao K, Wang L, Sun J, Feng Y, et al. Anaplastic pleomorphic xanthoastrocytoma with disseminated growth pattern at the time of diagnosis as well as after treatment: case report and review of literature. Chinese Neurosurgical Journal. 2017;3:22. https://doi.org/10.1186/s41016-017-0087-2.
Thompson EM, Landi D, Ashley D, Keir ST, Bigner D. Bevacizumab, irinotecan, temozolomide, tyrosine kinase inhibition, and MEK inhibition are effective against pleomorphic xanthoastrocytoma regardless of V600E status. J Neuro-Oncol. 2018;140:261–8. https://doi.org/10.1007/s11060-018-2975-5.
Precision Medicine in Cancer Treatment. https://www.cancer.gov/about-cancer/treatment/types/precision-medicine. Accessed November 1, 2018.
Kaley T, Touat M, Subbiah V, Hollebecque A, Rodon J, Lockhart AC, et al. BRAF inhibition in BRAF(V600)-mutant gliomas: results From the VE-BASKET study. J Clin Oncol. 2018;Jco2018789990. https://doi.org/10.1200/jco.2018.78.9990.
Roskoski R Jr. Targeting oncogenic Raf protein-serine/threonine kinases in human cancers. Pharmacol Res. 2018;135:239–58. https://doi.org/10.1016/j.phrs.2018.08.013.
Krishnamurthy A, Jimeno A. Bispecific antibodies for cancer therapy: a review. Pharmacol Ther. 2018;185:122–34. https://doi.org/10.1016/j.pharmthera.2017.12.002.
Pianko MJ, Liu Y, Bagchi S, Lesokhin AM. Immune checkpoint blockade for hematologic malignancies: a review. Stem Cell Investig. 2017;4:32. https://doi.org/10.21037/sci.2017.03.04.
Zhao Z, Chen Y, Francisco NM, Zhang Y, Wu M. The application of CAR-T cell therapy in hematological malignancies: advantages and challenges. Acta Pharm Sin B. 2018;8:539–51. https://doi.org/10.1016/j.apsb.2018.03.001.
Collins VP, Jones DT, Giannini C. Pilocytic astrocytoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129:775–88. https://doi.org/10.1007/s00401-015-1410-7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Chua, J., Nafziger, E. & Leung, D. Evidence-Based Practice: Temozolomide Beyond Glioblastoma. Curr Oncol Rep 21, 30 (2019). https://doi.org/10.1007/s11912-019-0783-5
Published:
DOI: https://doi.org/10.1007/s11912-019-0783-5