Abstract
Seedling survival and successful forest restoration involves many silvicultural practices. One important aspect of a successful forest restoration program is planting quality seedlings with high survival capability. Thus the nursery needs to create seedlings with plant attributes that allow for the best chance of success once a seedling is field planted. Since the mid-twentieth century, research foresters have critically examined plant attributes that confer improved seedling survival to field site conditions. This review describes the value of commonly measured seedling quality material (i.e. shoot height, stem diameter, root mass, shoot to root ratio, drought resistance, mineral nutrient status) and performance (i.e. freezing tolerance and root growth) plant attributes defined as important in answering the question of why seedlings survive after planting. Desirable levels of these plant attributes can increase the speed with which seedlings overcome planting stress, become ‘coupled’ to the forest restoration site, thereby ensuring successful seedling establishment. Although planting seedlings with these desirable plant attributes does not guarantee high survival rates; planting seedlings with desirable plant attributes increases chances for survival after field planting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Why seedlings survive after planting has long been debated because seedling survival is pivotal in the initial success of a forest restoration program. During the early part of the twentieth century, programs planting nursery-grown seedlings in North America reached an annual size of 10–20 million (Toumey 1916). Due to this silvicultural investment, foresters began examining plantation failures and tried to discern reasons for seedling mortality (e.g. Tillotson 1915; Young 1921; Kittredge 1929; Rudolf 1939). Often seedling losses were attributed to environmental stress, animal grazing, disease, or insects. However, in many cases poor quality planting stock (Kittredge 1929) or the inability of planted seedlings to grow roots (Rudolf 1939) was defined as the cause of plantation failure. The work of Toumey (1916) and studies initiated on southern pines in the 1920s (reported by Wakeley 1954) were some of the initial attempts to grade nursery stock using morphological parameters to improve seedling establishment. Thus, early in the twentieth century researchers began to ask the question of how plant attributes influence seedling survival after field planting.
Nursery cultural and silvicultural practices have a strong influence on seedling performance immediately after planting. Effects of these practices on seedling performance need to be understood to make sound forest restoration decisions. Regeneration silviculture is a complex process and many factors go into making a successful forest restoration program. Implicit within a seedling production program is recognition of the inherent species characteristics when making the proper selection of the genetic source that is adapted to forest restoration site conditions (Zobel and Talbert 1984). Also silvicultural practices related to the regeneration process (e.g. storage, handling, planting date, planting practices, site preparation, and vegetation management) all have an effect on the success of a forest restoration program. Readers interested in understanding effects of these factors can examine a number of excellent texts on silvicultural practices and forest regeneration site performance of planted seedlings (e.g. Cleary et al. 1978a; Lavender et al. 1990; Duryea and Dougherty 1991; Hobbs et al. 1992; Grossnickle 2000; Wagner and Colombo 2001).
How recently planted seedlings initiate growth and become “coupled” into the forest ecosystem (Grossnickle 2005a), thereby avoiding water stress, are critical factors for success of a forest restoration program. It is the lack of coupling (i.e. due to a restricted rooting volume limiting access to soil water) that increases the possibility of excessive water stress in seedlings which can result in either carbon starvation or hydraulic failure, and subsequently seedling death (McDowell et al. 2008). Thus, seedling survival is related to their inherent growth potential and the degree to which field site environmental conditions limits or enhances this potential for seedlings to become established or coupled into the forest ecosystem (Grossnickle 2000).
In the mid-twentieth century, researchers started to critically examine plant attributes that conferred improved survival for bareroot (Wakeley 1948, 1954; Stone 1955) and container-grown (Tinus 1974) seedlings. This was the start of seedling quality programs based on the need for a better understanding of performance capabilities of nursery-grown seedlings in relation to the forest restoration site. Seedling quality assessment has evolved to include numerous morphological and physiological measurement procedures for defining field performance (Mattsson 1996; Grossnickle 2000). Subsequently, a wealth of information has been published on plant attributes that improve the odds of survival once a seedling is field planted.
Defining seedling quality comes from measurements of seedling properties that describe material (i.e. single point measures of individual plant parameters) and performance (i.e. plant measurements reflecting an integrated response of many material attributes to defined environmental conditions) attributes (Ritchie 1984). This review examines the seedling through commonly measured material (i.e. shoot height, stem diameter, root mass, shoot to root ratio, drought resistance, mineral nutrient status) and performance (i.e. freezing tolerance and root growth) plant attributes used to define seedling quality. The objective of this review is to conduct a comprehensive, though not exhaustive, examination of work describing the value of primary plant attributes that are important in answering the question of why seedlings survive after planting on forest restoration sites.
Material morphological attributes
…serve their purpose only so far as they actually separate seedlings with a high capacity for survival and growth after planting from those with a low capacity. (Wakeley 1948)
Extensive work since the 1950s shows desirable morphological attributes contribute to seedling survival after transplanting on to forest restoration sites. To summarize this work, a well-balanced shoot to root system, with a sturdy stem and a large fibrous root system provides the best chance for seedling survival (e.g. southern pines—Lantz 1985; South 2000, radiata pine (Pinus radiata D. Don)—Menzies et al. 1985, Pacific Northwest and Northern British Columbia tree species—Scagel et al. 1993, temperate zone deciduous hardwoods—Wilson and Jacobs 2006). Morphological attributes are considered a reliable measure of seedling quality (Puttonen 1997) because they retain their mark on the seedling identity for extended timeframes after seedlings are field planted and start to grow. These plant structural features play a key role in defining their hydraulic architecture (i.e. potential water balance), thus helping to determine whether plants live or die during exposure to drought (McDowell et al. 2008).
Even so, historical work has found morphological attributes to be an inconsistent measure of seedling survival (Wakeley 1954; Thompson 1985; Mexal and Landis 1990). This stems from the fact that morphological parameters only measure overall seedling size, growth potential and shoot to root balance, not seedling physiological quality. Thus measures of morphological parameters are only part of the equation of plant attributes required for successful seedling survival (Wakeley 1948, 1954; Tinus 1974; Ritchie 1984; Mexal and Landis 1990). With this caveat in mind, the following discussion focuses on the influence morphological parameters have on seedling survival.
Shoot height
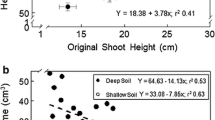
Large seedlings have been recommended for planting on sites where there is little environmental stress but there is the potential for excessive competition (Toumey 1916). As black spruce (Picea mariana (Mill.) B.S.P.) seedlings increased in shoot size they had a greater exposure to growing season available light on sites with high competition, resulting in greater survival and shoot growth (Jobidon et al. 1997, 2003). Taller bareroot loblolly pine (Pinus taeda L.) seedlings at planting confer higher survival on sites with little environmental stress (Fig. 1). Numerous studies show large stock on sites where competition is prevalent can improve survival and growth (Newton et al. 1993; Mason et al. 1996; Mohammed et al. 1998; South and Mitchell 1999; Puértolas et al. 2003; Villar-Salvador et al. 2004a).
The relationship between survival (year 2) and initial seedling height for bareroot loblolly pine (Pinus taeda L.) seedlings on adverse and non-adverse sites (Tuttle et al. 1987)
Shoot height is a general measure of photosynthetic and transpirational capacity and also a reflection of potential height growth (Armson and Sadreika 1979; Cleary et al. 1978b; Mexal and Landis 1990). As the shoot system initiates growth, larger seedlings produce greater absolute amounts of new shoot biomass (Thiffault 2004; Grossnickle 2005b) and occupy a greater area within the planting spot than smaller seedlings, thereby capturing more incoming solar radiation. This is critical because the effect of competition on limiting sunlight, thus seedlings net photosynthetic carbon gain, is related to survival (Johnson and Smith 2005). Shoot system size is important because on sites with available soil water and nutrients, competition for light between planted seedlings and the site vegetation complex is a main factor limiting seedling performance (Grossnickle 2000).
In contrast, planting seedlings with greater height can result in lower survival on droughty sites (Larsen et al. 1986; Boyer and South 1987; Tuttle et al. 1988; McTague and Tinus 1996). For example, shorter bareroot loblolly pine seedlings had higher survival on sites with limited soil water and greater environmental stress (Fig. 1). Under dry soil conditions, larger, compared to smaller, conifer seedlings can have greater water stress (Rose et al. 1993; Stewart and Bernier 1995), lower photosynthesis (Lamhamedi et al. 1997) and reduced growth (Baer et al. 1977; Hahn and Smith 1983). As the shoot system reaches a certain size, increased foliar mass can increase the seedling’s susceptibility to planting stress because a newly planted seedling’s root system cannot supply enough water to transpiring foliage to maintain a proper water balance (Grossnickle 2005a). The susceptibility of larger seedlings being exposed to water stress at planting is mitigated if they have the capability to quickly develop new roots (see Root Growth).
Stem diameter and root mass
Seedling stem diameter is a general measure of seedling sturdiness, root system size, and protection against drought and heat damage (Cleary et al. 1978b; Mexal and Landis 1990). Thus, it is difficult to separate the relationship between these two parameters, with a greater root system size occurring as stem diameter increases in both bareroot (Ritchie 1984) and container-grown (Grossnickle 2000) seedlings. Greater root mass is an indicator of root absorptive surface (Thompson 1985) conferring a greater seedling drought avoidance capability after field planting.
Johnson and Cline (1991) considered stem diameter the single most useful morphological measure of seedling quality. Numerous studies show larger stem diameter seedlings tend to survive better than small stem diameter seedlings (e.g. bareroot; van den Driessche 1980, 1984; South and Mexal 1984; Long and Carrier 1993; McGrath and Duryea 1994; South 1993; Zwolinski et al. 1996; South and Mitchell 1999; South et al. 2001, 2005; Rose and Ketchum 2003; Morrissey et al. 2010) (e.g. container-grown; Hines and Long 1986; Bayley and Kietzka 1997; Mexal et al. 2008; South et al. 2005; Oliet et al. 2009b; Morrissey et al. 2010). This is why Mexal and Landis (1990) felt stem diameter can best forecast field survival because it indirectly confers a number of desirable plant attributes (i.e. water absorption—roots, water transport—stem) that are considered important parameters of the plant hydraulic architecture that play a role in plant survival during drought (McDowell et al. 2008).
The relationship of larger seedling stem diameter, and by extension root development, and survival is not universal. In a review of bareroot pine seedlings spanning many trial sites in the southeastern US, survival was related to initial stem diameter at planting in most, but not all cases (Fig. 2). As seedling stem diameter increased in size the probability that a plantation would have survival at <75 % declined from a high of 88 % for seedlings with a small stem diameter (i.e. <2.4 mm) to only 9 % for seedlings with a very large stem diameter (i.e. >6.3 mm). Seedlings with a larger stem diameter had a greater probability of high survival, but not in all field situations.
The probability of seedling survival in the field <75 % for bareroot loblolly pine (Pinus taeda L.) and slash pine (Pinus elliottii Engelm.) seedlings graded into stem diameter classes (adapted from South et al. 1985). Note: Number within a bar for a stem diameter class defines the number of field sites from where data was collected
Seedlings with more roots have better survival potential (Toumey 1916) and seedlings with a good quality root system have better establishment capability after field planting (Davis and Jacobs 2005). Greater root system size at planting can result in greater seedling survival (Larsen et al. 1986; Rose et al. 1997). Seedlings with a larger stem diameter and more roots exhibit fewer symptoms of planting stress (Haase and Rose 1993) and have better survival than seedlings with a smaller stem diameter and fewer roots (South et al. 1985; Carlson 1986; Blake et al. 1989; Hobbs et al. 1989; Long and Carrier 1993). Greater root system size can confer greater root growth capability (see Root Growth). On harsh sites, a greater capability for absorption and transport of water from roots through the stem to the transpiring shoot system gives seedlings a better chance of overcoming planting stress (Grossnickle 2005a).
Shoot to root ratio
Measures of shoot to root (S:R) balance define seedlings drought avoidance potential (Thompson 1985). The need for seedlings to have root systems in proper proportion to the shoot system has long been recognized as a desirable seedling attribute (Toumey 1916) because seedling water status is directly tied to their S:R (Parker 1949). An imbalance in the shoot transpirational surface to the root absorbing surface (i.e. S:R > 3.0) can result in water stress for bareroot seedlings (Baldwin and Barney 1976). A S:R between 1.0 and 3.0 gave bareroot seedlings a better chance of survival (Hermann 1964; Foiles and Curtis 1973; Hobbs 1982), with survival increasing as S:R decreases (Tanaka et al. 1976; Lopushinsky and Beebe 1976; Larsen et al. 1986; Boyer and South 1987; Chamshama and Hall 1987; Kainer and Duryea 1990; Haase and Rose 1993; Généré and Garriou 1999) (Fig. 3a). Thompson (1985) found bareroot seedlings with lower S:R on average had a 29 % higher level of survival. The bareroot nursery cultural practices of undercutting (reviewed by South and Donald 2002) and top pruning (South and Blake 1994) are commonly used to decrease the S:R and produce seedlings with better survival capability. Even though data showing the importance of S:R for bareroot seedling survival is compelling, Hobbs (1984) warns that assessing potential survival based on the S:R without also judging the quality of the root system (i.e. fibrous root system with many growing tips) can limit its reliability to forecast survival.
a The effect of shoot to root ratio of bareroot loblolly pine (Pinus taeda L.) seedlings on the survival under a simulated drought environment (adapted from Mexal and Dougherty 1983). b The effect of shoot to root ratio of container-grown Patula pine (Pinus patula Schiede ex Schltdl. & Cham.) seedlings on survival 1 month after field planting (adapted from Bayley and Kietzka 1997)
Studies have also found the survival of container-grown seedlings to be greater with lower S:R under droughty field conditions (Zida et al. 2008; del Campo et al. 2010) (Fig. 3b). While these reports show the importance of initial S:R to enhance survival for container-grown seedlings, S:R based on total shoot and root weights may be limited in forecasting survival except under harsh field conditions. Bernier et al. (1995a) found little evidence that the S:R based on total shoot and root weights forecast survival for container-grown seedlings on sites lacking severe environmental stress. Bernier et al. (1995a) argued that the root plug-soil interface is the primary limiting factor rather than the overall root system in affecting seedling performance. Container-grown seedlings are typically grown in a medium that has desirable characteristics for root growth in the nursery (i.e. increased aeration and water holding capacity, low bulk density) (Tinus and McDonald 1979). These media characteristics limit movement of water into the root plug, after field planting, due to physical constraints of low density peat plugs (Bernier 1992; Bernier et al. 1995b) causing an imbalance of soil matric potential between plug media and field site soil (Day and Skoupy 1971). The ratio of root development outside of the container plug to total shoot dry weight best represented container-grown seedling drought avoidance potential; seedlings with minimal root development outside the plug having the greatest level of water stress (Grossnickle and Reid 1984). This is because the root plug-soil interface is the point of greatest resistance to water flow for newly planted container-grown seedlings (Örlander and Due 1986). A measure of root development out of the plug and into the soil in relation to shoot mass is a logical description of S:R balance of container-grown seedlings that better reflects their drought avoidance, thus survival potential.
Material and performance physiological attributes
…seedlings must be produced in such a way as to be physiologically ready to outplant into the field environment. (Lavender and Cleary 1974)
Survival is determined, in part, by the ability of seedlings to respond to environmentally stressful conditions that can occur after being planted. The most dramatic of these site conditions are alterations in the heat exchange processes and site-water relations limiting seedling performance (Miller 1983), while site nutrient dynamics are also considered rate limiting to young forest stands (Troth et al. 1986). The following discussion focuses on physiological attributes that allow seedlings to survive conditions of drought, frost and nutrient dynamics just after planting on a forest restoration site.
Cultural practices that provide an improved “physiological quality” to seedlings have long been considered important in increasing their chances for survival just after field planting (Wakeley 1948, 1954). This is because nonhardened seedlings (Rowe 1964; Tinus 1974; Hobbs 1984) or seedlings lacking the proper nutrient balance (see Nutrient Status) lack the physiological capability to become rapidly established after planting on forest restoration sites. Acclimation of seedlings is based on the concept of “slowly increasing stresses to induce physiological adjustments in plants” (Kozlowski and Pallardy 2002) thereby developing protection from potentially stressful field site conditions. Thus, plant acclimation or nursery cultural hardening practices (Wakeley 1954; Lavender and Cleary 1974; Landis et al. 1999) are applied to increase the odds in favor of seedling survival to field site conditions. Nursery practitioners have used cultural practices of reduced daylength, temperature, watering and fertilization regime modification to harden container-grown seedlings (Tinus and McDonald 1979; Landis et al. 1999), with watering and fertilization regime, plus shoot and root culturing modification used to harden (also improve S:R) bareroot seedlings (Duryea 1984; Mexal and South 1991). These treaties provide a detailed explanation of cultural practices used to harden seedlings. The following sections discuss physiological attributes, adjusted by hardening practices, which can improve seedlings chances of survival after transplanting to forest restoration sites.
Drought resistance
Drought stress causes tree species to develop drought resistance (Abrams 1988). Nurseries apply cultural practices that create water stress events at the end of the growing season to trigger budset or the cessation of shoot growth, and initiate stress resistance in seedlings. Periodic moderate water stress is used as a nursery cultural practice to induce bud formation (Lavender and Cleary 1974; Timmis and Tanaka 1976; Young and Hanover 1978; Macey and Arnott 1986; Calmé et al. 1993) as well as improve drought resistance (i.e. tolerance and avoidance) through osmotic adjustment (Kandiko et al. 1980; Hennessey and Dougherty 1984; Buxton et al. 1985; Ritchie and Roden 1985; Seiler and Johnson 1985; Grossnickle et al. 1991a; Major et al. 1994; Villar-Salvador et al. 2004b), stomatal sensitivity to drought (Unterschuetz et al. 1974; Roberts and Dumbroff 1986; Zwiazek and Blake 1989; Villar-Salvador et al. 1999), reduce susceptibility to xylem cavitation in some species (Beikircher et al. 2010), but not all species (Harvey and van den Driessche 1999; Beikircher et al. 2010), and create seedlings with lower S:R (Timmer and Miller 1991; Bayley and Kietzka 1997; Biel et al. 2004; Thomas 2009; Verdauger et al. 2011). Adjustment of the fertilization regime (i.e. from a high to lower N concentration plus improving the P and K status) in combination with drought hardening can improve drought resistance in hybrid poplars (Populus spp.) (Harvey and van den Driessche 1997, 1999). Short-day treatments that initiate budset can enhance drought resistance in the summer (Grossnickle and Folk 2003; Tan 2007) and fall (Colombo 1987; Grossnickle et al. 1991a; Major et al. 1994) crops of temperate zone tree species, though an extended short-day regime can be counterproductive causing increased mortality of summer-planted white spruce (Picea glauca (Moench) Voss) seedlings (Tan et al. 2008).
The use of hardening practices can improve the performance of field planted seedlings (Kozlowski et al. 1991). For example, drought hardening allowed seedlings to have a faster recovery of photosynthetic capability after transplanting (Kaushal and Aussenec 1989), better control of water loss (Christersson 1972; Clemens and Jones 1978; Johnson et al. 1985; Timmer and Miller 1991; Villar-Salvador et al. 1999, 2004b), and greater root regeneration after planting into soils having limiting edaphic conditions (i.e. droughted soils—Kaushal and Aussenec 1989, and cold soils—Hennessey and Dougherty 1984; Arnott et al. 1994). This improved performance has, in some cases, resulted in drought hardened seedlings having increased survival (Blake et al. 1979; van den Driessche 1991a, 1992) (Fig. 4) and growth when planted in xeric soil conditions (Johnson et al. 1985).
Drought avoidance response of control and drought hardened (mild and severe drought (DR) culture) Eucalyptus pilularis Sm. seedlings to drought conditions in a glasshouse (adapted from Thomas 2009) (Insert data shows the shoot to root ratio of seedlings after drought hardening cultural conditions with different letters indicating treatment differences (p = 0.05))
Drought hardening cultural practices does not always have beneficial effects on seedling field survival. This cultural practice can have either no effect (Grossnickle et al. 1991b; Folk et al. 1994; Royo et al. 2001; Biel et al. 2004; Villar-Salvador et al. 2004b) or a negative effect (Jospon and Paul 1985; O’Reilly et al. 1994) on survival in the field. This discrepancy probably stems from stress intensity during nursery application (Kozlowski and Pallardy 2002), timing of hardening practices (Duryea and McClain 1984; Landis et al. 1999), or the fact that stress resistance is not required to ensure seedling survival when exposed to optimum field conditions.
Freezing tolerance
Drought hardening practices can improve freezing tolerance in some conifer species (Timmis and Tanaka 1976; Blake et al. 1979; Grossnickle et al. 1991a). However, this phenomenon has not been observed in other conifers (van den Driessche 1969; D’Aoust and Cameron 1982; Menzies et al. 1981; Arnott et al. 1994). Drought hardening can improve seedling survival after field planting into frost prone conditions (Mexal et al. 1979).
Most tree species undergo many morphological and physiological changes during an annual phenological cycle in response to seasonal environmental conditions (Fuchigami and Nee 1987; Ritchie and Tanaka 1990; Burr 1990) with freezing tolerance (Burr 1990; Bigras et al. 2001) shifting to its highest level in the winter in many tree species. This phenomenon has been related to budset or the cessation of shoot growth, leaf maturation and seasonal shifts in temperature (Grossnickle 2000). Short-day treatments are an effective means to initiate budset (Vaartaja 1960; Lavender and Wareing 1972; Williams et al. 1972), control dormancy patterns (Lavender and Cleary 1974; Lavender 1985) and enhance freezing tolerance in the fall (Colombo et al. 2001) for temperate zone tree species. Proper fall hardening of temperate zone tree species ensures they can be lifted and stored for extended periods to maintain a high level of seedling quality (Colombo et al. 2001) without a depletion of carbohydrate reserves below a critical level that can affect survival (Ritchie 1982; Marshall 1985). These practices result in increased seedling survival (McKay and Mason 1991) (Fig. 5), improved capability to overcome planting stress (Grossnickle 2000) and become established when planted into the spring planting window (Grossnickle et al. 1991a, b, c; Jacobs et al. 2008).
Relationship between first year field survival and pre-storage freezing tolerance (measured as the temperature at which 50 % needle electrolyte leakage occurred: LT50) for lodgepole pine (Pinus contorta Dougl.) seedlings (Simpson 1990)
However, short-day treated spruce (Picea spp.) seedlings can show earlier budbreak setting up the potential for damage of actively growing seedlings by early-spring frost (Grossnickle 2000). Terminal bud damage of short-day treated spruce seedlings is attributed to early growing season frost, although this only occurs on frost-prone sites (Krasowski et al. 1993). This is a possible limitation of this cultural practice to maintain desired freezing tolerance levels, thereby limiting damage or mortality when planting seedlings into frost prone conditions.
Nutrient status
Proper nutrient balance
During the nursery acclimation process, fertilization practices are shifted in concert with other cultural parameters to slow and then cause growth cessation in container-grown (Landis et al. 1989) and bareroot (Duryea 1984; Lantz 1985) seedlings. This approach was developed under long held fertilization adjustment practices (bareroot seedlings—Wakeley 1954, container-grown seedlings—Lavender and Cleary 1974) to ensure seedlings had budset or the cessation of shoot growth at the proper time to develop hardiness and still go to the field with sufficient nutrient levels for good survival and growth. Sufficient nutrient levels at planting are critical because seedlings have a limited ability to access required nutrients from the field site during the establishment process (Tinus 1974).
Nursery fertilization can affect seedling survival after planting. In a review of 22 trials van den Driessche (1991b) found that “suitable nursery fertilization” resulted in 57 % of trials showing an increase in survival, 30 % of trials showing no effect and 13 % reported a decrease in seedling survival in the field. In a series of trials, survival of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) seedlings was related to N concentration at planting, with a 2 % (±0.5 %) N concentration resulting in the highest rate of survival 1 and 2 (Fig. 6), and 3 years (van den Driessche 1984, 1988) after planting.
Seedling survival at 1 and 2 years after field planting in relation nitrogen concentration for Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco)) seedlings at planting (van den Driessche 1980)
Nutrient loading
The shift of fertilization practices after budset or the cessation of shoot growth to increase nutrient concentration (i.e. fall nutrient loading) with little effect on seedling development has long been considered a beneficial nursery cultural practice (Benzian et al. 1974; Brix and van den Driessche 1974). Fall fertilization after seedlings have ceased growth, can increase the nutrient status without delaying progression of the bud dormancy cycle (Williams and South 1992), while not affecting (Luoranen et al. 2008) or even improving (Islam et al. 2009; Andivia et al. 2012) the fall development of freezing tolerance. Ingestad and Lund (1986) theorized that nutrient loading in the nursery provided seedlings with greater nutrient reserves to utilize after field planting, while Binkley (1986) considered increasing nutrient reserves through nursery fertilization a very energy-efficient approach to nutrient acquisition for recently planted seedlings, compared to the uptake of nutrients from the soil. Fall nutrient loading can also contribute to enhanced stress resistance (Timmer 1997), shoot growth potential (i.e. increased needle primordial in buds) (Colombo et al. 2003; Islam et al. 2009) and a lower S:R at planting (Timmer et al. 1991). Fall nutrient loading increases the availability of nutrient reserves that are rapidly remobilized to support nutrient demand of new growth once seedlings are planted.
Application of fall nutrient loading has a number of effects on the physiological response of seedlings after field planting. Reports show fall nutrient loading results in earlier shoot flush (Margolis and Waring 1986; Floistad and Kohmann 2004; Oliet et al. 2011), increased new root growth (van den Driessche 1985, 1988, 1992; Malik and Timmer 1996, 1998; Boivin et al. 2004; Villar-Salvador et al. 2004a; Oliet et al. 2009a, 2011; Andivia et al. 2011, 2012), increased nutrient uptake (Timmer and Aidelbaum 1996) and shoot growth (van den Driessche 1985, 1992; Margolis and Waring 1986; Malik and Timmer 1996; VanderSchaaf and McNabb 2004; Puértolas et al. 2003; Boivin et al. 2004; Close et al. 2005; Salifu et al. 2009; Oliet et al. 2009a, 2011). Nutrient loaded seedlings can have a greater capability to overcome planting stress on harsh sites and they can be an effective stocktype on forest restoration sites with high levels of competition (van den Driessche 1991b; Timmer 1997).
In some instances, fall nutrient loading improves seedling survival in the field (van den Driessche 1980, 1984, 1992; Irwin et al. 1998; Oliet et al. 2009b; del Campo et al. 2010), while in other field trials minimal survival benefits were noted (Benzian et al. 1974; van den Driessche 1988; Gleason et al. 1990; Birchler et al. 2001; South and Donald 2002; VanderSchaaf and McNabb 2004; Salifu et al. 2009; Andivia et al. 2011). In a few instances nutrient loading reduced seedling survival in the field (Benzian et al. 1974; South and Donald 2002; Boivin et al. 2004) and Boivin et al. (2004) attributed this phenomenon to excessive nutrient loading causing toxic nutrient concentrations. Variability in the response to nutrient loading indicates that this practice may only be beneficial when site nutrient restrictions are limiting seedling establishment.
Seedlings loaded with nutrient reserves can have unintended consequences to forest restoration programs. Higher mortality for nursery-grown spruce seedlings by grazing from snowshoe hares (Lepus americanus Erxleben) was attributed to preferential feeding on nursery-grown than naturally regenerated seedlings (Sullivan and Moses 1986; Rodgers et al. 1993). A similar phenomenon of herbivores feeding occurs in conifers (Bergquist and Örlander 1998; Burney and Jacobs 2011) and hardwoods (Close et al. 2004; Paul et al. 2012) where the frequency of browsing by herbivores was greater with higher N concentration. However, increased browsing was not always associated with N levels (Burney and Jacobs 2011; Paul et al. 2012) and on sites with high browse pressure seedlings with both high and low N levels were browsed (Close et al. 2004). Increasing nutrient reserves, through nursery fertilization, may decrease seedling field survival due to browsing damage of herbivorous animals.
Shift in physiological attributes
Since effects of drought and cold hardening can be tied to a plants normal phenological cycle, these hardening benefits are ephemeral in nature. As tree species initiate shoot growth in the spring, drought tolerance (Teskey and Hinckley 1986; Abrams 1988; Grossnickle 2000) and freezing tolerance (Burr 1990; Bigras et al. 2001) can be lost in rapid fashion. For example, interior spruce (Picea glauca (Moench) Voss × Picea engelmannii Parry) seedlings lost a good portion of their stress resistance within weeks of initiating growth (Fig. 7).
Shift in physiological attribute status of nutrient loaded spring planted black spruce (Picea mariana (Mill.) B.S.P.) seedlings (NUT Load; adapted from Malik and Timmer 1998), and drought tolerance (DR TOL—osmotic potential at turgor loss point), drought avoidance (DR AVD—cuticular transpiration) and freezing tolerance (FR TOL—Index of Injury at −6 °C), of spring planted interior spruce (Picea glauca (Moench) Voss × Picea engelmannii Parry) seedlings (adapted from Grossnickle and Folk 2007) at time of planting and 5–7 weeks after planting. Note: Seedlings had budbreak 2–3 weeks after planting
Any potential benefit of increased fertility in the nursery in terms of improved seedling performance in the field is also short-lived. Seedling nutrient reserves decline after planting, due to dilution in tissue nutrient concentrations if external nutrient sources cannot meet demands of new growth (Munson and Bernier 1993; Kim et al. 1999). Nutrient loaded black spruce seedlings lost 26 % of their N concentration 7 weeks after they resumed growth (Fig. 7) and N concentration of nutrient loaded and control seedlings were comparable by the end of the growing season (Malik and Timmer 1998).
Seedlings ability to utilize improved physiological plant attributes to overcome planting stress and become established is a very narrow window, making it very difficult to quantify benefits of these hardening and nutrient loading practices on survival. For this reason, seedling survival and successful establishment is not only predicated on their hardiness and nutrient status, but also on their morphological attributes (see above discussion) and capability to grow roots (see below discussion) after planting.
Performance attribute: root growth
If the root system did not increase in size at a fairly rapid rate…the seedling would die of drought…. (Stone 1955)
Seedlings that develop a root system after planting establish a proper water balance because they are coupled into the hydrologic cycle whereby water flows from the soil to plant roots, through the plant and into the atmosphere (Grossnickle 2005a). If sufficient root development does not occur just after field planting, seedlings can be exposed to stress because they do not have access to soil water. This planting stress can lead to a cycle of root growth being limited by the lack of water and photosynthates, and in turn photosynthesis being limited by water stress due to a lack of root growth (Burdett 1990; Grossnickle 2000). Alternatively, seedlings that establish roots quickly after planting develop a favorable water status which continues the cycle of root growth supported by photosynthesis (Guehl et al. 1989) and photosynthesis supported by root growth (Burdett 1990). This is why survival is predicated on sufficient root growth coupling the newly planted seedling to the site, thereby maintaining a proper plant water balance (Margolis and Brand 1990; Grossnickle 2005a).
This view that root growth is critical for seedling survival is why root growth capability is reported to be a common measurement tool used in operational programs worldwide to define seedling quality (Simpson and Ritchie 1997). This assessment approach is determined through a testing procedure called root growth capacity or root growth potential. Numerous reviews have discussed merits of measuring root growth within a seedling quality assessment approach for determining seedling performance (Ritchie and Dunlap 1980; Ritchie 1985; Burdett 1987; Ritchie and Tanaka 1990; Sutton 1990). This assessment approach is considered a direct indicator of a seedlings ability to grow roots and is a general indicator that all physiological systems are functioning properly and thus provides a measure of seedling performance potential (Ritchie 1984; Burdett 1987). This is why root growth in newly planted seedlings has long been recognized as important to ensure successful survival and establishment (Toumey 1916; Rudolf 1939; Wakeley 1948, 1954; Stone 1955; Tinus 1974).
Due to the necessity of root growth in successful seedling establishment, a critical aspect of nursery cultural programs is to produce seedlings with the capability to rapidly grow roots after field planting. Factors of seedling size, root system fibrosity and stocktype affect their capability to grow roots.
Seedling size
Seedling size affects a seedling’s ability to grow roots. Greater initial root mass is related to greater root growth (Brissette and Roberts 1984; Johnsen et al. 1988; Williams et al. 1988; van den Driessche 1992; Grossnickle and Major 1994; Villar-Salvador et al. 2004a; Grossnickle 2005b; Chirino et al. 2008; Cuesta et al. 2010a, b). Greater root growth in larger seedlings after planting can reduce plant water stress and increase survival (Hines and Long 1986; Luis et al. 2009).
Root system fibrosity
Greater root system fibrosity (i.e. branchiness) in bareroot seedlings (i.e. through undercutting—Faulkner 1953; Tanaka et al. 1976; Stupendick and Shepherd 1980; Schultz and Thompson 1996) has been related to increased root growth capability (Rook 1969; Bacon and Bachelard 1978; Hallgren and Tauer 1989) and in certain instances increased survival (Shoulders 1959; Tanaka et al. 1976; Kormanik 1986; Hallgren and Tauer 1989; Muse and Hatchell 1992; Schultz and Thompson 1996; Li et al. 2011). Greater root system fibrosity can lead to greater water movement capability through the root system (Carlson 1986) thereby reducing seedling water stress (Nambiar 1984). Conversely, stripping lateral roots during the lifting of bareroot seedlings, thus reducing root system fibrosity and root growth capability (South and Stumpff 1990), can reduce survival after field planting (Dierauf et al. 1992).
Manipulation of the container surface can increase root system fibrosity of container-grown seedlings (i.e. copper treatment on container walls; Burdett and Martin 1982; McDonald et al. 1984 and side slit containers for air pruning; Whitcomb 1984). These container treatments redistribute primary roots more evenly along the plug length (Wenny et al. 1988; Jones et al. 2002; Sword Sayer et al. 2011) and increase the number of first order lateral roots (McDonald et al. 1982; Smith and Mccubbin 1992; Nelson 1999). This can result in greater root growth capability (Arnold and Struve 1989; Dumroese 2000; Moore 2002; Tsakaldimi and Ganatsas 2006) and increased seedling survival (Barnett and McGilvray 1974).
Stocktype influence
Stocktype selection can influence survival because bareroot and container-grown seedlings can have differing root growth capabilities. Extensive comparisons between container and bareroot stocktypes across a range of sites lack no clear consensus favouring survivability of one particular stocktype (Hobbs 1984; Owston 1990; Grossnickle 2000). Trials that detected any survival differences, found container-grown, compared to bareroot, seedlings had greater initial survival on droughty sites (Arnott 1981; Dixon et al. 1981; Hahn and Smith 1983; Hobbs and Wearstler 1983; Burdett et al. 1984; South and Barnett 1986; Becker et al. 1987; Barnett and McGilvray 1993; Nilsson and Örlander 1995; Wilson et al. 2007).
Container-grown, compared to bareroot, seedlings can have greater root growth during the first field growing season (Dixon et al. 1981; Burdett et al. 1984; Johnson et al. 1984; Becker et al. 1987; Wilson et al. 2007). This improved root growth for container-grown seedlings reduces their resistance to water flow through the soil–plant-atmosphere-continuum (Dixon et al. 1983; Grossnickle and Blake 1987) thereby minimizing plant water stress (Burdett et al. 1984; Becker et al. 1987; Blake and Sutton 1987). Increased survival of container-grown, compared to bareroot, seedlings on drought prone sites has been related to lower plant water stress (Hobbs and Wearstler 1983; Nilsson and Örlander 1995).
Superior survival capability of container-grown, over bareroot, seedlings has also been attributed to their intact, undisturbed and multidimensional root systems that minimizes stress and leads to a quick root growth response (Tinus 1974). A growing media based plug surrounding the root system can improve seedling water status, compared to bareroot seedlings, independent from soil water availability (Jutras et al. 2007). Encasing roots of container-grown seedlings in a plug acts as a protective barrier against root desiccation, thereby creating a favorable plant water status to support photosynthesis and root growth (Burdett 1990; Mena-Petite et al. 2001).
Restoration site performance
Root growth, though critical for survival and (or) growth of seedlings is not a perfect predictor of seedling performance on forest restoration sites (Simpson and Ritchie 1997). The relationship between root growth capability and field performance varies. Many studies have shown that when seedlings grow roots they survive in the field (Stone 1955; Sutton 1980; Burdett et al. 1983, 1984; Feret and Kreh 1985; Hines and Long 1986; Larsen et al. 1988; Simpson 1990; Simpson and Vyse 1995; McTague and Tinus 1996; Mena-Petite et al. 2001; del Campo et al. 2007). Reviews on this issue (Ritchie and Dunlap 1980—26 trials, Ritchie and Tanaka 1990—12 trials) found ~80 % of trials reported a positive relationship between root growth capability and seedling survival. Though most work has found a positive relationship between root growth and survival, the lack of consistent trend has led to operational assessments questioning the importance of root growth to seedling survival (South and Hallgren 1997). An extensive assessment of operational plantings in British Columbia Canada, found that root growth potential failed to display a strong relationship with field survival (Binder et al. 1988).
A reassessment of the Binder et al. (1988) data base using boundary line analysis might reveal how root growth relates to seedling survival capability across a range of forest restoration sites. Field data collected across a range of environmental conditions tends to have a scatter of biological response to these conditions. On careful examination, normally a series of biological response points occur within an upper boundary of response to the dependent variable. Jarvis (1976) stated that this upper boundary is a maximum biological response one can expect in the way of plant performance, while Webb (1972) credited the scatter of biological response points below the boundary line to errors in measurement, variability of biological data and the interaction with other site environmental factors. Several researchers (Chambers et al. 1985; Grossnickle and Arnott 1992) used this premise to create upper boundary biological response data sets (i.e. systematic selection of 20–25 % of data from across the dependent variable range) to quantify the maximum biological response expected with a given dependent variable. When used on the Binder et al. (1988) data, the boundary line shows that low root growth capability equates with the chance of low survival, while high root growth capability equates with the chance of high survival when these seedlings were field planted (Fig. 8). A further examination of this data showed that if seedlings had low root growth capability (i.e. Index of Root Growth or IRG < 1) the probability was 52 % that the field plantation had <75 % survival, while some seedling populations with low root growth capability had high field survival rates. As the root growth capability of sample seedlings increased there was a greater chance of survival. Seedlings with very high root growth capability (i.e. IRG > 4) had <10 % chance of a plantation having <75 % survival.
Second year seedling survival of field planted seedlings to mean index of root growth potential (adapted from Binder et al. 1988). Data is from operational testing of 540 samples of seedlings. Data includes 12 species and numerous stocktypes tested by the British Columbia Ministry of Forests. Mean index root growth potential (IRG) classes are: 0 no new root growth, 1 some new roots, but none over 2 cm, 2 1–3 new roots over 1 cm long, 3 4–10 new roots over 1 cm long, 4 11–30 new roots over 1 cm long, 5 and 6 >35 new roots over 1 cm long. Boundary layer analysis (data defined by solid triangles) and the regression equation were determined by analysis procedures of Grossnickle and Arnott (1992)
Whether or not newly planted seedlings initially require new root growth for proper field performance is related to the planting stress phenomenon. One way planting stress is relieved is when root growth occurs and seedling water stress is reduced (Grossnickle 2005a). Simpson and Ritchie (1997) believe that root growth is strongly related to field performance when seedlings have an inherently low level of stress resistance and/or when site environmental conditions become more severe. These are conditions that lead to planting stress. However, if seedlings are not exposed to planting stress, then initial root growth is not essential for good field performance (Simpson and Ritchie 1997). This view is exemplified by Stone et al. (2003), where critical root growth capacity (i.e. the minimum root growth required for seedling survival on a given planting site) was twice as high for harsh sites compared to gentle sites. Thus, survival of newly planted seedlings improves when they can extend new roots into the soil, ensuring that water intake equals or exceeds water loss (Wakeley 1948, 1954) and forest restoration site conditions dictate the amount of root growth required to overcome planting stress and ensure survival.
Conclusions
Morphological plant attributes influence a seedlings ability to survive after being planted on to a forest restoration site because a plants susceptibility to drought induced mortality can be due to their hydraulic architecture (McDowell et al. 2008). Thus, nursery cultural practices that alter seedling morphological characteristics (i.e. hydraulic architecture) can limit their susceptibility to planting stress (i.e. water stress). This is why morphological attributes such as greater seedling stem diameter and root system size confer a higher chance of survival. Seedling balance between the shoot and root systems, and seedling overall size need to be adjusted in relation to potential forest restoration site environmental conditions. Greater shoot system height is important if competition for light within the vegetation complex is the potential site limiting factor. A somewhat smaller shoot system and/or lower S:R are critical attributes if dry soils and high evaporative demand are potential site limiting factors. However, morphological parameters are only measures that help define overall seedling size, growth potential and balance, while seedling physiological quality and root growth capability also have a major influence on survival.
Factors affecting seedling physiological quality such as stress resistance and nutritional status have a major influence on survival. Improved survival is attributed to greater stress resistance (i.e. through nursery cultural hardening practices that enhance drought resistance and freezing tolerance) and improved seedling nutrition at planting (i.e. through fall nutrient loading) that increases the speed with which seedlings can overcome planting stress and become established on the forest restoration site. However, direct benefits from improved seedling physiological attributes are ephemeral. Thus the primary purpose of these improved physiological attributes is in aiding root growth, displaying a shoot system of sufficient size within the forest restoration site complex and conferring improved seedling establishment within months of planting. Ultimately the combination of a well-established seedling having desirable shoot and root development is what ensures high survival.
Improved survival can be attributed to greater root growth immediately after seedlings are field planted. Greater root growth is the result of greater root system size, fibrosity, plus greater seedling stress resistance and improved nutrient status. Stocktype selection can also influence survival, especially on droughty sites, because container-grown, compared to bareroot, seedlings can have better root growth that is related to plug protection of roots against desiccation and greater root growth capability that reduces potential planting stress.
These conclusions reiterate long held beliefs within the forest restoration community that desirable morphological attributes (Toumey 1916), root growth (Stone 1955) and physiological attributes (Wakeley 1948, 1954; Tinus 1974) improve chances for increased seedling survival. Work conducted during the past half century has confirmed that these historical beliefs were correct, while defining species specific ranges for these plant attributes. Planting seedlings with these desirable plant attributes does not guarantee high survival; rather planting seedlings with desirable attributes increases chances for survival within a forest restoration program.
References
Abrams MD (1988) Sources of variation in osmotic potentials with special reference to North American tree species. For Sci 34:1030–1046
Andivia E, Fernandez M, Vázquez-Piqué J (2011) Autumn fertilization of Quercus ilex ssp. ballota (Desf.) Samp. nursery seedlings: effects on morpho-physiology and field performance. Ann For Sci 68:543–553
Andivia E, Márquez-García B, Vázquez-Piqué J, Córdoba F, Fernandez M (2012) Autumn fertilization with nitrogen improves nutritional status, cold hardiness and oxidative stress response of Holm oak (Quercus ilex ssp. ballota (Desf.) Samp.) nursery seedlings. Trees 26:311–320
Armson KA, Sadreika V (1979) Forest nursery soil management and related practices. Ontario Ministry of Natural Resources, Toronto, ON
Arnold MA, Struve DK (1989) Growing green ash and red oak in CuCO3-treated containers increases root regeneration and shoot growth following transplant. J Am Soc Hort Sci 114:402–406
Arnott JT (1981) Survival and growth of bullet, styroplug and bareroot seedlings on mid-elevation sites in coastal British Columbia. For Chron 57:65–70
Arnott JT, Grossnickle SC, Puttonen P, Mitchell AK, Folk RS (1994) Influence of nursery culture on growth, cold hardiness and drought resistance of yellow cypress. Can J For Res 23:2537–2547
Bacon GJ, Bachelard EP (1978) The influence of nursery conditioning treatments on some physiological responses of recently transplanted seedlings of Pinus caribaea Mor. var. honduriensis B. and G. Aust For Res 8:171–183
Baer N, Ronco F, Barney CW, Baer NW (1977) Effects of watering, shading and size of stock on survival of planted lodgepole pine. USDA Forest Service Res Note RM-347
Baldwin VC, Barney CW (1976) Leaf water potential in planted ponderosa and lodgepole pines. For Sci 22:344–350
Barnett JP, McGilvray JM (1974) Copper screen controls root growth and increases survival on containerized southern pine seedlings. Tree Planters’ Notes 25:11–12
Barnett JP, McGilvray JM (1993) Performance of container and bareroot loblolly pine seedlings on bottomlands in South Carolina. South J Appl For 17:80–83
Bayley AD, Kietzka JW (1997) Stock quality and field performance of Pinus patula seedlings produced under two nursery growing regimes during seven different nursery production periods. New For 13:341–356
Becker CA, Mroz GD, Fuller LG (1987) The effects of plant moisture stress on red pine (Pinus resinosa) seedling growth and establishment. Can J For Res 17:813–820
Beikircher B, Florineth F, Mayr S (2010) Restoration of rocky slopes based on planted gabions and use of drought-preconditioned woody specis. Ecol Eng 36:421–426
Benzian B, Brown RM, Freeman SCR (1974) Effect of late-season topdressing of N (and K) applied to conifer transplants in the nursery on their survival and growth on British forest sites. Forestry 47:153–184
Bergquist J, Örlander G (1998) Browsing damage by roe deer on Norway spruce seedlings planted on clearcuts of different ages: 2. Effect of seedling vigour. For Ecol Manage 105:295–302
Bernier PY (1992) Soil texture influences seedling water stress in more ways than one. Tree Planters’ Notes 43:39–42
Bernier PY, Lamhamedi MS, Simpson DG (1995a) Shoot:root ratio is of limited use in evaluating the quality of container conifer stock. Tree Planters’ Notes 46:102–106
Bernier PY, Stewart JD, Gonzalez A (1995b) Effects of the physical properties of sphagnum peat on water stress in containerized Picea mariana seedlings under simulated field conditions. Scand J For Res 10:184–189
Biel C, Savé R, Habrouk A, Espelta JM, Retana J (2004) Effects of restricted watering and CO2 enrichment in the morphology and performance after transplanting of nursery-grown Pinus nigra seedlings. HortScience 39:535–540
Bigras FJ, Ryyppo A, Linderstrom A, Stattin E (2001) Cold acclimation and deacclimation of shoots and roots of conifer seedlings. In: Bigras FJ, Colombo SJ (eds) Conifer cold hardiness. Kluwer, The Netherlands, pp 57–88
Binder WD, Scagel RK, Krumlik GJ (1988) Root growth potential: facts, myths, value? USDA Forest Service Gen. Tech. Rep. RM-167, pp 111–118
Binkley D (1986) Forest nutrition management. Wiley, New York
Birchler TM, Rose R, Haase DL (2001) Fall fertilization with N and K: effects on Douglas-fir seedlings quality and performance. West J Appl For 16:71–79
Blake TJ, Sutton RF (1987) Variation in water relations of black spruce stock types planted in Ontario. Tree Physiol 3:331–344
Blake J, Zaerr J, Hee S (1979) Controlled moisture stress to improve cold hardiness and morphology of Douglas-fir seedlings. For Sci 25:576–582
Blake JL, Teeter LD, South DB (1989) Analysis of economic benefits from increasing uniformity in Douglas-fir nursery stock. In: Mason WL, Deans JD, Thompson S (eds) Producing uniform conifer planting stock, vol 26, pp 251–262 (for suppl)
Boivin JR, Salifu KF, Timmer VR (2004) Late season fertilization of Picea mariana seedlings: intensive loading and outplanting response on greenhouse bioassays. Ann For Sci 61:737–745
Boyer JN, South DB (1987) Excessive seedling height, high shoot-to-root ratio and benomyl dip reduce survival of stored loblolly pine seedlings. Tree Planters’ Notes 37:19–21
Brissette JC, Roberts TC (1984) Seedling size and lifting date effects on root growth potential of loblolly pine from two Arkansas nurseries. Tree Planters’ Notes 35:34–38
Brix H, van den Driessche R (1974) Mineral nutrition of container-grown tree seedlings. In: Tinus RW, Stein WI, Balmer WE (eds) Proceedings of the North American containerized forest tree seedling symposium. Great Plains Ag. Council Publ. No. 68, pp 77–84
Burdett AN (1987) Understanding root growth capacity: theoretical considerations in assessing planting stock quality by means of root growth tests. Can J For Res 17:768–775
Burdett AN (1990) Physiological processes in plantation establishment and the development of specifications for forest planting stock. Can J For Res 20:415–427
Burdett AN, Martin PAF (1982) Chemical root pruning of coniferous seedlings. HortScience 17:622–624
Burdett AN, Simpson DG, Thompson CF (1983) Root development and plantation establishment success. Plant Soil 71:103–110
Burdett AN, Herring LJ, Thompson CF (1984) Early growth of planted spruce. Can J For Res 14:644–651
Burney OT, Jacobs DF (2011) Ungulate herbivory of regenerating conifers in relation to foliar nutrition and terpenoid production. For Ecol Manage 262:1834–1845
Burr KE (1990) The target seedling concept: bud dormancy and cold-hardiness. In: Rose R, Campbell SJ, Landis TD (eds) Target seedling symposium: proceedings of the western forest nursery associations. USDA Forest Service Gen. Tech. Rep. RM-200, pp 79–90
Buxton GF, Cyr DR, Dumbroff EB (1985) Physiological responses of three northern conifers to rapid and slow induction of moisture stress. Can J Bot 63:1171–1176
Calmé S, Margolis HA, Bigras FJ (1993) Influence of cultural practices on the relationship between frost tolerance and water content of containerized black spruce, white spruce, and jack pine seedlings. Can J For Res 23:503–511
Carlson WC (1986) Root system considerations in the quality of loblolly pine seedlings. South J Appl For 10:87–92
Chambers JL, Hinckley TM, Cox GS, Metcalf CL, Aslin RG (1985) Boundary-line analysis and models of leaf conductance for four oak-hickory forest species. For Sci 31:437–450
Chamshama SAO, Hall JB (1987) Effects of nursery treatments on Eucalyptus camaldulensis field establishment and early growth at Mafiga, Morogoro, Tanzania. For Ecol Manage 21:91–108
Chirino E, Vilagrosa A, Hernández EI, Matoc A, Vallejoa VR (2008) Effects of deep container on morpho-fuctional characteristics and root colonization in Quercus suber L. seedlings for reforestation in Mediterranean climate. For Ecol Manage 256:779–785
Christersson L (1972) The transpiration rate of unhardened, hardened, and dehardened seedlings of spruce and pine. Physiol Plant 26:258–263
Cleary BD, Greaves RD, Hermann RK (eds) (1978a) Regenerating Oregon’s forests: a guide for the regeneration forester. Oregon State University Extension Service, Corvallis, OR
Cleary BD, Greaves RD, Owsten PW (1978b) Seedlings. In: Cleary BD, Greaves RD, Hermann RK (eds) Regenerating Oregon’s forests: a guide for the regeneration forester. Oregon State University Extension Service, Corvallis, OR, pp 63–97
Clemens J, Jones PG (1978) Modification of drought resistance by water stress conditioning in Acacia and Eucalyptus. J Exp Bot 29:895–904
Close DC, McArthur C, Pietrzykowski E, Fitzgerald H, Paterson S (2004) Evaluating effects of nursery and post-planting nutrient regimes on leaf chemistry and browsing of eucalypt seedlings in plantations. For Ecol Manage 200:101–112
Close DC, Bail I, Hunter S, Beadle CL (2005) Effects of exponential nutrient-loading on morphological and nitrogen characteristics and on after planting performance of Eucalyptus globulus seedlings. For Ecol Manage 205:397–403
Colombo SJ (1987) Changes in osmotic potential, cell elasticity, and turgor relationships of 2nd-year black spruce container seedlings. Can J For Res 17:365–369
Colombo SJ, Menzies MI, O’Reilly C (2001) Influence of nursery cultural practices on cold hardiness of coniferous forest tree seedlings. In: Bigras FJ, Colombo SJ (eds) Conifer cold hardiness. Kluwer, The Netherlands, pp 223–252
Colombo SJ, Glerum C, Webb DP (2003) Daylength, temperature and fertilization effects on desiccation resistance, cold hardiness and root growth potential of Picea mariana seedlings. Ann For Sci 60:307–317
Cuesta B, Vega J, Villar-Salvador P, Rey-Benayas JM (2010a) Root growth dynamics of allepo pine (Pinus halipensis Mill.) seedlings in relation to shoot elongation, plant size and tissue nitrogen concentration. Trees 24:899–908
Cuesta B, Vega J, Villar-Salvador P, Puértolas J, Jacobs DF, Rey-Benayas JM (2010b) Why do large, nitrogen rich seedlings better resist stressful transplanting conditions? A physiological analysis in two contrasting Mediterranean forests. For Ecol Manage 20:71–78
D’Aoust AL, Cameron SE (1982) The effects of dormancy induction, low temperature and moisture stress on cold hardening of containerized black spruce seedlings. In: Scarrett JB, Glerum C, Plexman CA (eds) Proceedings of the Canadian containerized tree seedling symposium. Can. For. Serv. Great Lakes For. Cent. COJFRC Symp. Proc. O-P-10, pp 153–161
Davis AD, Jacobs DF (2005) Quantifying root system quality of nursery seedlings and relationship to outplanting performance. New For 30:295–311
Day RJ, Skoupy J (1971) Moisture storage capacity and post-planting patterns of moisture movement from seedling containers. Can J For Res 1:151–158
del Campo AD, Navarro RM, Hermoso J, Ibáńez AJ (2007) Relationship between root growth potential and field performance in Aleppo pine. Ann For Sci 64:541–548
del Campo AD, Navarro RM, Ceacero CJ (2010) Seedling quality and field performance of commercial stocklots of containerized holm oak (Quercus ilex) in Mediterranean Spain: an approach for establishing a quality standard. New For 39:19–37
Dierauf TD, Chandler LA, Hixson DL (1992) Stripping of lateral roots from loblolly pine seedlings during lifting—effect on field performance. Virginia Dept. For. Occ. Rep. 105
Dixon RK, Garrett HE, Cox GS, Johnson PS, Sander IL (1981) Container- and nursery-grown black oak seedlings inoculated with Pisolithus tinctorius: growth and ectomycorrhizal development following outplanting on an Ozark clear-cut. Can J For Res 11:492–496
Dixon RK, Pallardy SG, Garrett HE, Cox GS (1983) Comparative water relations of container-grown and bare-root ectomycorrhizal and nonmycorrhizal Quercus velutina seedlings. Can J Bot 61:1559–1565
Dumroese KR (2000) Changes in interior Douglas-fir root development in containers after copper and auxin treatments. West J Appl For 15:213–216
Duryea ML (1984) Nursery cultural practices: impacts on seedling quality. In: Duryea ML, Landis TD (eds) Forest nursery manual: production of bareroot seedlings. Martinus Nijhoff/Dr. W. Junk Publishers, The Hague, pp 143–164
Duryea ML, Dougherty PM (eds) (1991) Forest regeneration manual. Kluwer, Dordrecht
Duryea ML, McClain KM (1984) Altering seedling physiology to improve reforestation success. Corvallis, OR. In: Duryea ML, Brown GH (eds) Seedling physiology and reforestation success: proceedings of the physiology working group technical session. Martinus Nijhoff/Dr. W. Junk Publishers, Boston, pp 77–114
Faulkner R (1953) Early observations on the root development of one-year-old Corsican pine seedlings following root pruning. Scott For 7:23–26
Feret RP, Kreh RE (1985) Seedling root growth potential as an indicator of loblolly pine field performance. For Sci 31:1005–1011
Floistad IS, Kohmann K (2004) Influence of nutrient supply spring frost hardiness and time of bud break in Norway spruce (Picea abies (L.) Karst.) seedlings. New For 27:1–11
Foiles MW, Curtis JD (1973) Regeneration of ponderosa pine in the northern Rocky Mountain-Intermountain region. USDA Forest Service Res. Paper INT-145, 45 pp
Folk RS, Grossnickle SC, Major JE, Arnott JT (1994) Influence of nursery culture on western red cedar. II. Freezing tolerance of fall-planted seedlings and morphological development of fall- and spring-planted seedlings. New For 8:231–247
Fuchigami LH, Nee CC (1987) Degree growth stage model and rest-breaking mechanisms in temperate woody perennials. HortScience 22:836–845
Généré B, Garriou D (1999) Stock quality and field performance of Douglas-fir seedlings under varying degrees of water stress. Ann For Sci 56:501–510
Gleason JF, Duryea M, Rose R, Atkinson M (1990) Nursery and field fertilization of 2+0 ponderosa pine seedlings: the effect on morphology, physiology and field performance. Can J For Res 20:1766–1772
Grossnickle SC (2000) Ecophysiology of northern spruce species: the performance of planted seedlings. NRC Research Press, Ottawa
Grossnickle SC (2005a) Importance of root growth in overcoming planting stress. New For 30:273–294
Grossnickle SC (2005b) Seedling size and reforestation success. How big is big enough? In: Colombo SJ (Compiler) The thin green line: a symposium on the state-of-the-art in reforestation. Ont. For. Res. Inst., Ontario Ministry of Natural Resources. For. Res. Info. Paper 160, pp 138–144
Grossnickle SC, Arnott JT (1992) Gas exchange response of western hemlock seedlings from various dormancy induction treatments to reforestation site environmental conditions. For Ecol Manage 49:177–193
Grossnickle SC, Blake TJ (1987) Comparison of water relation patterns for newly planted bare-root and container jack pine and black spruce seedlings on boreal cut-over sites. New For 1:101–116
Grossnickle SC, Folk RS (2003) Spring versus summer spruce stocktypes of western Canada: nursery development and field performance. West J Appl For 18:267–275
Grossnickle SC, Folk RS (2007) Field performance potential of a somatic interior spruce seedlot. New For 34:51–72
Grossnickle SC, Major JE (1994) Interior spruce seedlings compared to emblings produced from somatic embryogenesis. III. Physiological response and morphological development on a reforestation site. Can J For Res 24:1397–1407
Grossnickle SC, Reid CPP (1984) Water relations of Engelmann spruce seedlings on a high-elevation mine site: an example of how reclamation techniques can alter microclimate and edaphic conditions. Reclam Reveg Res 3:199–221
Grossnickle SC, Arnott JT, Major JE, Tschaplinski TJ (1991a) Influence of dormancy induction treatment on western hemlock seedlings. 1) Seedling development and stock quality assessment. Can J For Res 21:164–174
Grossnickle SC, Major JE, Arnott JT, LeMay VM (1991b) Stock quality assessment through an integrated approach. New For 5:77–91
Grossnickle SC, Arnott JT, Major JE (1991c) Influence of dormancy induction treatments on western hemlock seedlings. 2) Physiological and morphological response during the first growing season on a reforestation site. Can J For Res 21:175–185
Guehl JM, Aussenac G, Kaushal P (1989) The effects of transplanting stress on photosynthesis, stomatal conductance and leaf water potential in Cedrus atlantica: role of root regeneration. Ann Sci For 46S:464–468
Haase DL, Rose R (1993) Soil moisture stress induces transplant shock in stored and unstored 2+0 Douglas-fir seedlings of varying root volumes. For Sci 39:275–294
Hahn PF, Smith AJ (1983) Douglas-fir planting stock performance. Comparison after the third growing season. Tree Planters’ Notes 34:33–39
Hallgren SW, Tauer CG (1989) Root growth potential, first-year survival, and growth of shortleaf pine seedlings show effects of lift date, storage, and family. South J Appl For 13:163–169
Harvey HP, van den Driessche R (1997) Nutrition, xylem cavitation and drought resistance in hybrid poplars. Tree Physiol 17:647–654
Harvey HP, van den Driessche R (1999) Nitrogen and potassium on xylem cavitation and water-use efficiency in poplars. Tree Physiol 19:943–950
Hennessey TC, Dougherty PM (1984) Characterization of the internal water relations of loblolly pine seedlings in response to nursery cultural treatments: implications for forest regeneration success. In: Duryea ML, Brown GN (eds) Seedling physiology and reforestation success. Martinus Nijhoff Dr W. Junk Publishers, Dordrecht, pp 225–244
Hermann RK (1964) Importance of top-root ratios for survival of Douglas-fir seedlings. Tree Planters’ Notes 64:7–11
Hines FD, Long JN (1986) First-and second-year survival of containerized Engelmann spruce in relation to initial seedling size. Can J For Res 16:668–670
Hobbs SD (1982) Stocktype selection and planting techniques for Douglas-fir on skeletal soils in southwest Oregon. In: Hobbs SD, Helgerson OT (eds) Proceeding of reforestation of skelatal soils workshop. Forest Research Laboratory, Oregon State University, Corvallis, pp 92–96
Hobbs SD (1984) The influence of species and stocktype selection on stand establishment: an ecophysiological perspective. In: Duryea ML, Brown GH (eds) Seedling physiology and reforestation success: proceedings of the physiology working group technical session. Martinus Nijhoff/Dr. W. Junk Publishers, Boston, pp 180–224
Hobbs SD, Wearstler KA (1983) Performance of three Douglas-fir stocktypes on a skeletal soil. Tree Planters’ Notes 34:11–14
Hobbs SD, Crawford MS, Yelczyn BA (1989) Early development of three Douglas-fir stocktypes on a droughty skeletal soil. West J Appl For 4:21–24
Hobbs SD, Tesch SD, Owston PW, Stewart RE, Tappeiner JC II, Wells GE (1992) Reforestation practices in Southwestern Oregon and Northern California. Forest Research Laboratory, Oregon State University, Corvallis, OR
Ingestad T, Lund AB (1986) Theory and techniques for steady state mineral nutrition and growth of plants. Scand J For Res 1:439–453
Irwin KM, Duryea ML, Stone EL (1998) Fall-applied nitrogen improves performance of 1-0 slash pine nursery seedlings after outplanting. South J Appl For 22:111–116
Islam MA, Apostol KG, Jacobs DF, Dumroese RK (2009) Fall fertilization of Pinus resinosa seedlings: nutrient uptake, cold hardiness, and morphological development. Ann For Sci 66:704–713
Jacobs DF, Davis AD, Wilson BC, Dumroese RK, Goodman RC, Salifu KF (2008) Short-day treatment alters Douglas-fir seedling dehardening and transplant root proliferation at varying rhizosphere temperatures. Can J For Res 38:1526–1535
Jarvis PG (1976) The interpretation of the variations in leaf water potential and stomatal conductance found in canopies in the field. Philos Trans R Soc Lond Ser B 273:593–610
Jobidon R, Charette L, Bernier PY (1997) Initial size and competing vegetation effects on water stress and growth of Picea mariana (Mill.) BSP seedlings planted in three different environments. For Ecol Manage 103:295–308
Jobidon R, Roy V, Cyr G (2003) Net effect of competing vegetation on selected environmental conditions and performance of four spruce seedling stock sizes after eight years in Quebec (Canada). Ann For Sci 60:691–699
Johnsen KH, Feret PP, Seiler JR (1988) Root growth potential and shoot activity of northern and southern provenances of 1-0 eastern white pine seedlings grown in a Virginia nursery. Can J For Res 18:610–614
Johnson JD, Cline ML (1991) Seedling quality of southern pines. In: Duryea ML, Dougherty PM (eds) Forest regeneration manual. Kluwer, Dordrecht, pp 143–162
Johnson DM, Smith WK (2005) Refugial forests of the southern Appalachians: photosynthesis and survival in current-year Abies fraseri seedlings. Tree Physiol 25:1379–1387
Johnson PS, Novinger SL, Mares WG (1984) Root, shoot, and leaf area growth potentials of nortern red oak planting stock. For Sci 30:1017–1026
Johnson JD, Seiler JR, McNabb KL (1985) Manipulation of pine seedling physiology by water stress conditioning. In: South DB (ed) Proceedings of the international symposium on nursery manage. Practices for the Southern Pines, IUFRO Sub. Grp. S3.202-03, pp 290–302
Jones MD, Kiiskila S, Flanagan A (2002) Field performance of pine stock types: two-year results of a trial on interior lodgepole pine seedlings grown in Styroblocks™, Copperblocks™, or AirBlocks™. BC J Ecosyst Manag 2:1–12
Jospon TM, Paul JL (1985) Influence of fall fertilization and moisture stress on growth and field performance on container-grown Douglas-fir seedlings. USDA Forest Service Gen. Tech. Rep. INT-185, pp 14–19
Jutras S, Thiffault N, Munson AD (2007) Comparing large bareroot and container stock: water stress as influenced by peat and soil water availability. Tree Planters’ Notes 52:15–18
Kainer KA, Duryea ML (1990) Root wrenching and lifting date of slash pine: effects on morphology, survival and growth. New For 4:207–221
Kandiko RA, Timmis R, Worrall J (1980) Pressure-volume curves of shoots and roots of normal and drought-conditioned western hemlock seedlings. Can J For Res 10:10–16
Kaushal P, Aussenec G (1989) Drought preconditioning of Corsican pine and Cedar of Atlas seedlings: photosynthesis, transpiration and root regeneration after transplanting. Acta Oecol 11:61–78
Kim YT, Colombo SJ, Hickie DF, Noland TD (1999) Amino acid, carbohydrate, glutathione, mineral nutrient and water potential changes in non-water-stressed Picea mariana seedlings after transplanting. Scand J For Res 14:416–424
Kittredge J (1929) Forest planting in the lake states. USDA Bull. 1497
Kormanik PP (1986) Lateral root morphology as an expression of sweetgum seedling quality. For Sci 32:595–604
Kozlowski TT, Pallardy SG (2002) Acclimation and adaptive response of woody plants to environmental stress. Bot Rev 68:270–334
Kozlowski TT, Kramer PJ, Pallardy SG (1991) The physiological ecology of woody plants. Academic Press, New York
Krasowski MJ, Letchford T, Eastham AM (1993) Growth of short-day treated spruce seedlings planted throughout British Columbia. Forestry Canada and British Columbia Ministry of Forests, Victoria, BC, FRDA Rep. 209
Lamhamedi MS, Bernier PY, Hérbert C (1997) Effect of shoot size on the gas exchange and growth of containerized Picea mariana seedlings under different watering regimes. New For 13:209–223
Landis TD, Tinus RW, McDonald SE, Barnett JP (1989) Seedling nutrition and irrigation. The container tree nursery manual, vol 4. USDA Forest Service Agric. Handb. 674, Washington, DC, 87 pp
Landis TD, Tinus RW, Barnett JP (1999) Seedling propagation. The container tree nursery manual, vol. 6. USDA Forest Service Agric. Handb. 674, Washington, DC, 167 pp
Lantz CW (ed) (1985) Southern pine nursery handbook. USDA Forest Service Cooperative Forestry, Southern Region
Larsen HS, South DB, Boyer JM (1986) Root growth potential, seedling morphology and bud dormancy correlate with survival of loblolly pine seedlings planted in December in Alabama. Tree Physiol 1:253–263
Larsen HS, South DB, Boyer JM (1988) Foliar nitrogen content at lifting correlates with early growth of loblolly pine seedlings from 20 nurseries. South J Appl For 12:181–185
Lavender DP (1985) Bud dormancy. In: Duryea ML (ed) Evaluating seedling quality: principles, procedures, and predictive ability of major tests. Forest Research Laboratory, Oregon State University, Corvallis, OR, pp 7–15
Lavender DP, Cleary BD (1974) Coniferous seedling production techniques to improve seedling establishment. In: Tinus RW, Stein WI, Balmer WE (eds) Proceedings of the North American containerized forest tree seedling symposium. Great Plains Ag. Council Publ. No. 68, pp 177–180
Lavender DP, Wareing PF (1972) The effects of daylength and chilling on the response of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco.) seedlings to root damage and storage. New Phytol 71:1055–1067
Lavender DP, Parish R, Johnson CM, Montgomery G, Vyse A, Willis RA, Winston D (eds) (1990) Regenerating British Columbia’s forests. University of British Columbia Press, Vancouver, BC
Li GL, Lui Y, Yang J, Sun HY, Jia ZK, Ma LY (2011) Influence of initial age and size on the field performance of Larix olgensis seedlings. New For 42:215–226
Long AJ, Carrier BD (1993) Effects of Douglas-fir 2+0 seedling morphology on field performance. New For 7:19–32
Lopushinsky W, Beebe T (1976) Relationship of shoot-root ratio to survival and growth of outplanted Douglas-fir and ponderosa pine seedlings. USDA Forest Service Res. Note, PNW-274
Luis VC, Puértolas J, Climent J, Peters J, González-Rodríguez AM, Morales D, Jimenéz MS (2009) Nursery fertilization enhances survival and physiological status in Canary Island pine (Pinus canariensis) seedlings planted in a semiarid environment. Eur J For Res 128:221–229
Luoranen J, Lahti M, Rikala R (2008) Frost hardiness of nutrient-loaded two-year-old Picea abies seedlings in autumn and at the end of freezer storage. New For 35:207–220
Macey DE, Arnott JT (1986) The effect of moderate moisture and nutrient stress on bud formation and growth of container-grown white spruce seedlings. Can J For Res 16:949–954
Major JE, Grossnickle SC, Folk RS, Arnott JT (1994) Influence of nursery culture on western red cedar. I. Measurement of seedling attributes before fall and spring planting. New For 8:211–229
Malik V, Timmer VR (1996) Growth, nutrient dynamics, and interspecific competition of nutrient-loaded black spruce seedlings on a boreal mixedwood site. Can J For Res 26:1651–1659
Malik V, Timmer VR (1998) Biomass partitioning and nitrogen retranslocation in black spruce seedlings on competitive mixedwood sites: a bioassay study. Can J For Res 28:206–215
Margolis HA, Brand DG (1990) An ecophysiological basis for understanding plantation establishment. Can J For Res 20:375–390
Margolis HA, Waring RH (1986) Carbon and nitrogen allocation patterns of Douglas-fir seedlings fertilized with nitrogen in autumn. II. Field performance. Can J For Res 16:903–909
Marshall JD (1985) Carbohydrate status as a measure of seedling quality. In: Duryea ML (ed) Evaluating seedling quality: principles, procedures, and predictive ability of major tests. Forest Research Laboratory, Oregon State University, Corvallis, OR, pp 49–56
Mason EG, South DB, Weizhong Z (1996) Performance of Pinus radiata in relation to seedling grade, weed control, and soil cultivation in the central North Island of New Zealand. N Z J For Sci 26:173–183
Mattsson A (1996) Predicting field performance using seedling quality assessment. New For 13:223–248
McDonald SE, Tinus RW, Reid CPP (1982) Root development control measures in containers: recent findings. In: Scarrett JB, Glerum C, Plexman CA (eds) Proceedings of the Canadian containerized tree seedling symposium. Can. For. Serv. Great Lakes For. Cent. COJFRC Symp. Proc. O-P-10, pp 207–214
McDonald SE, Tinus RW, Reid CPP, Grossnickle SC (1984) Effect of CuCO3 container wall treatments and mycorrhizae fungi inoculation of growing medium on pine seedling growth and root development. J Environ Hortic 2:5–8
McDowell N, Pockman WT, Allen CD, Bershears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
McGrath DA, Duryea ML (1994) Initial moisture stress, budbreak and two-year field performance of three morphological grades of slash pine seedlings. New For 8:335–350
McKay HM, Mason WL (1991) Physiological indicators of tolerance to cold storage in Sitka spruce and Douglas-fir seedlings. Can J For Res 21:890–910
McTague JP, Tinus RW (1996) The effects of seedling quality and forest site weather on field survival of ponderosa pine. Tree Planters’ Notes 47:16–32
Mena-Petite A, Ortega-Lasuen U, González-Moro M, Lacuesta M, Muňoz-Rueda A (2001) Storage duration and temperature effects on the functional integrity of container and bare-root Pinus radiata D. Don stock-types. Trees 15:289–296
Menzies MI, Holden DG, Green LM, Rook DA (1981) Seasonal changes in frost tolerance of Pinus radiata seedlings raised in different nurseries. N Z J For Sci 11:100–111
Menzies MI, van Dorsser JC, Balneaves JM (1985) Seedling quality—radiata pine as a case study. In: South DB (ed) Proceedings of the international symposium on nursery manage. Practices for the Southern Pines, IUFRO Sub. Grp. S3.202-03, pp 385–415
Mexal JG, Dougherty PM (1983) Growth of loblolly pine seedlings. IV. Performance in a simulated drought environment. Weyerhaeuser Co. Hot Springs, AK. Tech Rep. 050-1422/6, 26 pp
Mexal JG, Landis TD (1990) Target seedling concepts: height and diameter. In: Rose R, Campbell SJ, Landis TD (eds) Target seedling symposium: proceedings of the western forest nursery associations. USDA Forest Service Gen. Tech. Rep. RM-200, pp 17–36
Mexal JG, South DB (1991) Bareroot seedling culture. In: Duryea ML, Dougherty PM (eds) Forest regeneration manual. Kluwer, Dordrecht, pp 89–115
Mexal JG, Timmis R, Morris WG (1979) Cold hardiness of containerized loblolly pine seedlings. South J Appl For 3:15–19
Mexal JG, Cuevas Rangel RA, Landis TD (2008) Reforestation success in central Mexico: factors determining survival and early growth. Tree Planters’ Notes 53:16–22
Miller PC (1983) Comparison of water balance characteristics of plant species in “natural” versus modified ecosystems. In: Mooney HA, Godron M (eds) Disturbance and ecosystems. Components of response. Springer, Berlin, pp 188–212
Mohammed GH, Noland TL, Wagner RG (1998) Physiological perturbation in jack pine (Pinus banksiana Lamb.) in the presence of competing herbaceous vegetation. For Ecol Manage 103:77–85
Moore DG (2002) Some new research into container design. Proc Int Plant Prop Soc 52:105–108
Morrissey RC, Jacobs DF, Davis AS, Rathfon RA (2010) Survival and competitiveness of Quercus rubra regeneration associated with planting stocktype and harvest opening intensity. New For 40:273–287
Munson AD, Bernier PY (1993) Comparing natural and planted black spruce seedlings. II. Nutrient uptake and efficiency of use. Can J For Res 23:2435–2442
Muse DH, Hatchell GE (1992) A preliminary identification of morphological indicators of field performance in bare-root nursery stock. USDA Forest Service Res. Pap. SE-283
Nambiar EKS (1984) Significance of first-order lateral roots on the growth of young radiata pine under environmental stress. Aust For Res 14:187–199
Nelson WR (1999) Root pruning can influence first order lateral root development of containerised plants. Proc Int Plant Prop Soc 49:96–103
Newton M, Cole EC, White DE (1993) Tall planting stock for enhanced growth and domination of brush in the Douglas-fir region. New For 7:107–121
Nilsson U, Örlander G (1995) Effects of regeneration methods on drought damage to newly planted Norway spruce seedlings. Can J For Res 25:790–802
Oliet JA, Tejada M, Salifu KF, Collazos A, Jacobs DF (2009a) Performance and nutrient dynamics of holm oak (Quercus ilex L.) seedlings in relation to nursery nutrient loading and post-transplant fertility. Eur J Forest Res 128:253–263
Oliet JA, Planelles R, Artero F, Valverde R, Jacobs DF, Segura M (2009b) Field performance of Pinus halepensis planted in Mediterranean arid conditions: relative influence of seedling morphology and mineral nutrition. New For 37:313–331
Oliet JA, Salazar JM, Villar R, Robredo E, Valladares F (2011) Fall fertilization of Holm oak affects N and P dynamics, root growth potential, and post-planting phenology and growth. Ann For Sci 68:647–656
O’Reilly C, Owens JN, Arnott JT, Dunsworth BG (1994) Effect of nursery culture on morphological development of western hemlock seedlings during field establishment. II. Survival, shoot length components, and needle length. Can J For Res 24:61–70
Örlander G, Due K (1986) Location of hydraulic resistance in the soil-plant pathway in seedlings of Pinus sylvestris L. grown in peat. Can J For Res 16:115–123
Owston PW (1990) Target seedling specifications: Are stocktype designations useful? In: Rose R, Campbell SJ, Landis TD (eds) Target seedling symposium: proceedings of the western forest nursery associations. USDA Forest Service Gen. Tech. Rep. RM-200, pp 9–16
Parker J (1949) Effects of variation in the root-leaf ratio on transpiration rate. Plant Physiol 24:739–743
Paul GS, Montagnini F, Berlyn GP, Craven DJ, van Breugel M, Hall JS (2012) Foliar herbivory and leaf traits of five native tree species in a young plantation of Central Panama. New For 43:9–87
Puértolas J, Gil L, Pardos JA (2003) Effects of nutritional status and seedling size on field performance of Pinus halepensis planted in former arable land in the Mediterranean basin. Forestry 76:159–168
Puttonen P (1997) Looking for the “silver bullet”—can one test do it all? New For 13:9–27
Ritchie GA (1982) Carbohydrate reserves and root growth potential in Douglas-fir seedlings before and after cold storage. Can J For Res 12:905–912
Ritchie GA (1984) Assessing seedling quality. In: Duryea ML, Landis TD (eds) Forest nursery manual: production of bareroot seedlings. Martinus Nijhoff/Dr. W. Junk Publishers, The Hague, pp 243–266
Ritchie GA (1985) Root growth potential: principles, procedures, and predictive ability. In: Duryea ML (ed) Evaluating seedling quality: principles, procedures, and predictive ability of major tests. Oregon State University, Forestry Research Laboratory, Corvallis, OR, pp 93–106
Ritchie GA, Dunlap JR (1980) Root growth potential: its development and expression in forest tree seedlings. N Z J For Sci 10:218–248
Ritchie GA, Roden JR (1985) Comparison between two methods of generating pressure-volume curves. Plant, Cell Environ 8:49–53
Ritchie GA, Tanaka Y (1990) Root growth potential and the target seedling. In: Rose R, Campbell SJ, Landis TD (eds) Target seedling symposium: proceedings of the western forest nursery associations. USDA Forest Service Gen. Tech. Rep. RM-200, pp 37–51
Roberts DR, Dumbroff EB (1986) Drought resistance, transpiration rates and ABA levels of three northern conifers. Tree Physiol 1:161–178
Rodgers AR, Williams D, Sinclair ARE, Sullivan TP, Andersen RJ (1993) Does nursery production reduce antiherbivore defenses of white spruce? Evidence from feeding experiments with snowshoe hares. Can J For Res 23:2358–2361
Rook DA (1969) Water relations of wrenched and unwrenched Pinus radiata seedlings on being transplanted into conditions of water stress. N Z J For 14:50–58
Rose R, Ketchum JS (2003) Interaction of initial seedling diameter, fertilization and weed control on Douglas-fir growth over the first four years after planting. Ann For Sci 60:625–635
Rose R, Gleason JF, Atkinson M (1993) Morphological and water-stress characteristics of three Douglas-fir stocktypes in relation to seedling performance under different soil moisture conditions. New For 7:1–17
Rose R, Haase DL, Kroiher F, Sabin T (1997) Root volume and growth of ponderosa pine and Douglas-fir seedlings: a summary of eight growing seasons. West J Appl For 12:69–73
Royo A, Gil L, Pardos JA (2001) Effects of water stress conditioning on morphology, physiology and field performance of Pinus halepensis Mill. seedlings. New For 21:127–140
Rowe SJ (1964) Environmental preconditioning with special reference to forestry. Ecology 45:399–403
Rudolf PO (1939) Why forest plantations fail. J For 37:377–383
Salifu KF, Jacobs DF, Birge ZKD (2009) Nursery nitrogen loading improves field performance of bareroot oak seedlings planted on abandon mine lands. Restor Ecol 17:339–349
Scagel R, Bowden R, Madill M, Kooistra C (1993) Provincial seedling stock type selection and ordering guidelines. British Columbia Ministry of Forests, Victoria, BC 75
Schultz RC, Thompson JR (1996) Effect of density control and undercutting on root morphology of 1+0 bareroot hardwood seedlings: five-year field performance of root-graded stock in central USA. New For 13:297–310
Seiler JR, Johnson JD (1985) Photosynthesis and transpiration of loblolly pine seedlings as influenced by moisture-stress conditioning. For Sci 31:742–749
Shoulders E (1959) Root pruning boosts longleaf pine survival. Tree Planters’ Notes 36:15–19
Simpson DG (1990) Frost hardiness, root growth capacity, and field performance relationships in interior spruce, lodgepole pine, Douglas-fir, and western hemlock seedlings. Can J For Res 20:566–572
Simpson DG, Ritchie GA (1997) Does RGP predict field performance? A debate. New For 13:253–277
Simpson DG, Vyse A (1995) Planting stock performance: site and RGP effects. For Chron 71:739–742
Smith IE, McCubbin PD (1992) Effect of copper tray treatment on Eucalyptus grandis (Hill ex Maiden) seedling growth. Acta Hortic 319:371–376
South DB (1993) Rationale for growing southern pine seedlings at low bed densities. New For 7:63–92
South DB (2000) Planting morphologically improved pine seedlings to increase survival and growth. Ala. Agric. Exp. Sta. Auburn Univ. For. and Wildlife Res. Ser. No. 1
South DB, Barnett JP (1986) Herbicides and planting affect early performance of container-grown and bare-root loblolly pine seedlings in Alabama. New For 1:17–27
South DB, Blake JI (1994) Top-pruning increases survival of pine seedlings. Ala Agric Exp Sta Highlights Agric Res 41:9
South DB, Donald DGM (2002) Effect of nursery conditioning treatments and fall fertilization on survival and early growth of Pinus taeda seedlings in Alabama, U.S.A. Can J For Res 32:1171–1179
South DB, Hallgren SW (1997) Research versus operational correlations between seedling survival and root growth potential of shortleaf pine. New For 13:357–365
South DB, Mexal JG (1984) Growing the “best” seedling for reforestation success. Ala Agric Exp Stn Auburn Univ For Dep Ser No. 12
South DB, Mitchell RJ (1999) Determining the “optimum” slash pine seedling size for use with four levels of vegetative management on flatwoods site in Georgia, USA. Can J For Res 29:1039–1046
South DB, Stumpff NJ (1990) Root stripping reduces root growth potential of loblolly pine seedlings. South J Appl For 14:196–199
South DB, Boyer JN, Bosch L (1985) Survival and growth of loblolly pine as influenced by seedling grade: 13 year results. South J Appl For 9:76–81
South DB, Rakestraw JL, Lowerts GA (2001) Early gains from planting large diameter seedlings and intensive management are additive for loblolly pine. New For 21:97–110
South DB, Harris SW, Barnett JP, Hainds MJ, Gjerstad DH (2005) Effect of container type and seedling size on survival and early height growth of Pinus palustris seedlings in Alabama, U.S.A. For Ecol Manage 204:385–398
Stewart JD, Bernier PY (1995) Gas exchange and water relations of 3 sizes of containerized Picea mariana seedlings subjected to atmospheric and edaphic water stress under controlled conditions. Ann For Sci 52:1–9
Stone EC (1955) Poor survival and the physiological condition of planting stock. For Sci 1:89–94
Stone EC, Cavallaro JI, Norberg EA (2003) Critical RGC-Expected survival models for predicting survival of planted white fir (Abies concolor Lindl.) seedlings. New For 26:65–82
Stupendick JAT, Shepherd KR (1980) Root regeneration of root-pruned Pinus radiata seedlings. II. Effects of root-pruning on photosynthesis and translocation. N Z J For Sci 10:148–158
Sullivan TP, Moses RA (1986) Demographic and feeding responses of a snowshoe hare population to habitat alteration. J Appl Ecol 23:53–63
Sutton RF (1980) Planting stock quality, root growth capacity, and field performance of three boreal conifers. N Z J For Sci 10:54–71
Sutton RF (1990) Root growth capacity in coniferous forest trees. Hortic Sci 25:259–266
Sword Sayer MA, Sung SJ, Haywood JD (2011) Longleaf pine root system development and seedling quality in response to copper root pruning and cavity size. South J Appl For 35:5–11
Tan W (2007) Impacts of nursery cultural treatments on stress tolerance in 1+0 container white spruce (Picea glauca [Moench] Voss) seedlings for summer-planting. New For 33:93–107
Tan W, Blanton S, Bielech JP (2008) Summer planting performance of white spruce 1+0 container seedlings affected by nursery short-day treatment. New For 35:187–205
Tanaka Y, Walstad JD, Borrecco JE (1976) The effect of wrenching on morphology and field performance of Douglas-fir and loblolly pine seedlings. Can J For Res 6:453–458
Teskey RO, Hinckley TM (1986) Moisture: effects of water stress on trees. In: Hennessey TC, Dougherty PD, Kossuth SV, Johnson JD (eds) Stress physiology and forest productivity. Martinus Nijhoff Publishers, The Netherlands, pp 9–33
Thiffault N (2004) Stock type in intensive silviculture: a (short) discussion about roots and size. For Chron 80:463–468
Thomas DS (2009) Survival and growth of drought hardened Eucalyptus pilularis Sm. seedlings and vegetative cuttings. New For 38:245–259
Thompson BE (1985) Seedling morphological evaluation: what you can tell by looking. In: Duryea ML (ed) Evaluating seedling quality: principles, procedures, and predictive ability of major tests. Forest Research Laboratory, Oregon State University, Corvallis, OR, pp 59–72
Tillotson CR (1915) Forest planting in the eastern United States. USDA Bull. 153
Timmer VR (1997) Exponential nutrient loading: a new fertilization technique to improve seedling performance on competitive sites. New For 13:279–299
Timmer VR, Aidelbaum AS (1996) Manual for exponential nutrient loading of seedlings to improve outplanting performance on competitive forest sites. NODA/NFP Tech. Rep. TR-25
Timmer VR, Miller BD (1991) Effects of contrasting fertilization and irrigation regimes on biomass, nutrients, and water relations of container grown red pine seedlings. New For 5:335–348
Timmer VR, Armstrong G, Miller BD (1991) Steady-state nutrient preconditioning and early out-planting performance of containerized black spruce seedlings. Can J For Res 21:585–594
Timmis R, Tanaka Y (1976) Effects of container density and plant water stress on growth and cold hardiness of Douglas-fir seedlings. For Sci 22:167–172
Tinus RW (1974) Characteristics of seedlings with high survival potential. In: Tinus RW, Stein WI, Balmer WE (eds) Proceedings of the North American containerized forest tree seedling symposium. Great Plains Ag. Council Publ. No. 68, pp 276–282
Tinus RW, McDonald SE (1979) How to grow tree seedlings in containers in greenhouses. USDA Forest Service Gen. Tech. Rep. RM-60
Toumey JW (1916) Seeding and planting. Wiley, New York
Troth JL, Campbell RG, Allen HL (1986) Nutrients: use of forest fertilization and nutrient efficient genotypes to manage nutrient stress in conifer stands. In: Hennessey TC, Dougherty PD, Kossuth SV, Johnson JD (eds) Stress physiology and forest productivity. Martinus Nijhoff Publishers, The Netherlands, pp 61–99
Tsakaldimi MN, Ganatsas PP (2006) Effects of chemical pruning on stem growth, root morphology and field performance on the Mediterranean pine Pinus halepensis Mill. Sci Hortic 109:183–189
Tuttle CS, South DB, Golden MS, Meldahal RS (1987) Relationship between initial seedling height and survival and growth of loblolly pine seedlings planted during a droughty year. South J Appl For 11:139–143
Tuttle CS, South DB, Golden MS, Meldahal RS (1988) Initial Pinus taeda seedling height relationships with early survival and growth. Can J For Res 18:867–871
Unterschuetz PF, Ruetz W, Geppert R, Ferrell WK (1974) The effect of age, pre-conditioning, and water stress in the transpiration rates of Douglas-fir (Pseudotsuga menziesii) seedlings of several ecotypes. Physiol Plant 32:214–221
Vaartaja O (1960) Effect of photoperiod on drought resistance of white spruce seedlings. Can J Bot 38:597–599
van den Driessche R (1969) Influence of moisture supply, temperature, and light on frost hardiness changes in Douglas-fir seedlings. Can J Bot 47:1765–1772
van den Driessche R (1980) Effects of nitrogen and phosphorus fertilization on Douglas-fir nursery growth and survival after outplanting. Can J For Res 10:65–70
van den Driessche R (1984) Relationship between spacing and nitrogen fertilization of seedlings in the nursery, seedling mineral nutrition, and outplanting performance. Can J For Res 14:431–436
van den Driessche R (1985) Late-season fertilization, mineral nutrient reserves, and retranslocation in planted Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) seedlings. For Sci 31:485–496
van den Driessche R (1988) Nursery growth of conifer seedlings using fertilizers of different solubilities and application time, and their forest growth. Can J For Res 18:172–180
van den Driessche R (1991a) Influence of container nursery regimes on drought resistance of seedlings following planting: survival and growth. Can J For Res 21:555–565
van den Driessche R (1991b) Effects of nutrients on stock performance in the forest. In: van den Driessche R (ed) Mineral nutrition of conifer seedlings. CRC Press, Boca Raton, FL, pp 229–260
van den Driessche R (1992) Changes in drought resistance and root growth capacity of container seedlings in response to nursery drought, nitrogen, and potassium treatments. Can J For Res 22:740–749
VanderSchaaf C, McNabb K (2004) Winter nitrogen fertilization of loblolly pine seedlings. Plant Soil 265:295–299
Verdauger D, Vilagran J, Lloansi S, Fleck I (2011) Morphological and physiological acclimation of Quercus coccifera L. seedlings to water availability and growing medium. New For 42:363–381
Villar-Salvador P, Ocana L, Penuelas J, Carrasco I (1999) Effects of water stress conditioning on the water relations, root growth capacity, and nitrogen and non-structural carbohydrate concentration of Pinus halepensis Mill. seedlings. Ann For Sci 56:459–465
Villar-Salvador P, Penuelles R, Enriquez E, Penuelas J, Rubira JL (2004a) Nursery cultivation regimes, plant functional attributes, and field performance relationship in Mediterranean oak Quercus ilex L. For Ecol Manage 196:257–266
Villar-Salvador P, Penuelles R, Oliet J, Enriquez E, Penuelas JL, Jacobs DF, Gonzalez M (2004b) Drought tolerance and transplanting performance of holm oak (Quercus ilex) seedlings after drought hardening in the nursery. Tree Physiol 24:1147–1155
Wagner B, Colombo SJ (eds) (2001) Regenerating Ontario’s forests. Fitzhenry & Whiteside Ltd., Markham, ON
Wakeley PC (1948) Physiological grades of southern pine nursery stock. Soc Am For Proc 1948:311–322
Wakeley PC (1954) Planting the southern pines. Agric. Monogr. No. 18. USDA Forest Service Washington, DC, 233 pp
Webb RA (1972) Use of boundary line in the analysis of biological data. J Hortic Sci 97:309–319
Wenny DL, Liu Y, Dumroese RK, Osborne HL (1988) First year field growth of chemically root pruned containerized seedlings. New For 2:111–118
Whitcomb CE (1984) Plant production in containers. Lacebark Publications, Stillwater, OK
Williams HM, South DB (1992) Effects of fall fertilizer applications on mitotic index and bud dormancy of loblolly pine seedlings. For Sci 38:336–349
Williams BJ, Pellett NE, Klein RM (1972) Phytochrome control of growth cessation and initiation of cold acclimation in selected woody plants. Plant Physiol 50:262–265
Williams HM, South DB, Webb A (1988) Effects of fall irrigation on morphology and root growth potential of loblolly pine seedlings growing in sand. S Afr For J 147:1–5
Wilson BC, Jacobs DF (2006) Quality assessment of temperate zone deciduous hardwood seedlings. New For 31:417–433
Wilson ER, Vitols K, Park A (2007) Root characteristics and growth potential of container and bare-root seedlings of red oak (Quercus rubra L.) in Ontario Canada. New For 34:163–176
Young LJ (1921) Forest planting in southern Michigan. J For 19:1–8
Young E, Hanover JW (1978) Effects of temperature, nutrient, and moisture stresses on dormancy of blue spruce seedlings under continuous light. For Sci 24:458–467
Zida D, Tigabu M, Sawadogo L, Odén PC (2008) Initial seedling morphological characteristics and field performance of two Sudanian savannah species in relation to nursery production period and watering regimes. For Ecol Manage 255:2151–2162
Zobel BJ, Talbert JT (1984) Applied forest tree improvement. Wiley, New York
Zwiazek JJ, Blake TJ (1989) Effects of preconditioning on subsequent water relations, stomatal sensitivity, and photosynthesis in osmotically stressed black spruce. Can J Bot 67:2240–2244
Zwolinski JB, South DB, Cunningham L, Christie S (1996) Weed control and large bare-root stock improve early growth of Pinus radiata in South Africa. N Z J For Sci 26:163–172
Acknowledgments
I thank Dr. Steve Colombo for the wide ranging discussions that lead to the initial structure and focus of this paper. I also thank Drs. John Mexal and Dave South, and the Editor-in-Chief and three anonymous reviewers whose review of draft versions of this manuscript kept me on topic and within context of the published literature.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grossnickle, S.C. Why seedlings survive: influence of plant attributes. New Forests 43, 711–738 (2012). https://doi.org/10.1007/s11056-012-9336-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-012-9336-6