Abstract
Genetic improvement of Eucalyptus genotypes for drought and frost resistance is essential for successful intensive management of commercial plantations. Understanding the physiological mechanisms that relate water use and frost resistance for highly deployed genotypes may allow for better prediction of their future performance, genetic selection and seedling management for site specific purposes. We studied whether instantaneous water use efficiency (WUE i ) may serve as drought, freezing and photoinhibition tolerance predictor by studying its response on six E. globulus clones (Eg1–Eg6) and four E. globulus × E. nitens hybrid seedlings (Egn1–Egn4) under drought and irrigated (control) treatments. Net photosynthesis (A) and transpiration (E) were studied using a gas exchange system in order to calculate WUE i (A/E). Simultaneous chlorophyll a fluorescence measurements were performed to assess the non photochemical quenching components. Frost tolerance of plants under control and drought treatments were evaluated by measuring temperatures that exert 50% photoinactivation of photosystem II. Finally, drought tolerance was evaluated by plant survival within each genotype after rehydration. Our results showed significant genotype variability in the rate of soil and xylem water potential decrease during drought. While most of the genotypes reached −4.0 MPa in about 35 days of drought, genotypes Eg6 and Egn4 required 56 days of drought to reach this xylem water potential. WUE i exhibited significant differences among genotypes and irrigation treatments. Genotypes Eg5 and Egn4 increased their WUE i between 70 and 80% after drought. This was associated with a more conservative control of water loss at the stomatal level combined with maintenance of relatively higher rates of net photosynthesis than the other genotypes under drought conditions. Plants exposed to drought were more freezing tolerant than control plants, having in average 3°C lower LT50 than well irrigated ones. There was no a clear correlation between WUE i and drought tolerance or drought-induced photoinhibition, however WUE i was inversely correlated with LT50. Our results suggest that WUE i is not suitable by itself to select drought tolerant genotypes, but may provide evidence for discarding drought sensitive genotypes. In addition, it could provide valuable information to select for freezing tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eucalyptus is one of the most widely planted exotic genera in tropical and Mediterranean climates (Cromer et al. 1993; Stape et al. 2001; Almeida et al. 2004b). Considerable concerns exist about the expansion of Eucalyptus plantations to colder or drier environments, not only given the reduced base of available land for forestation at countries where the species are extensively being planted, but also on new challenging regions such as southern Brazil and southeast USA (Dwivedi and Alavalapati 2009). Over the last 40 years, intensive management of these species have focused on improving productivity by selection of genetic material for clonal plantations obtaining large increases in biomass and pulp yield (Bison et al. 2007; Vellini et al. 2008). Current major efforts on species and genotypes selection rely on strategies aimed to maximize survival and improve early growth rates via enhancing cold and/or drought resistance to establish new plantings on harsher environments (Bison et al. 2007). However, most genetic selections have been discriminated only focusing on empirical growth rates and fiber productivity from field trials, without taking into account a clear understanding of plant physiological responses to potential changes in environmental conditions such as water availability, frost events and low fertility (Fernandez et al. 2007; Vellini et al. 2008).
Cold and drought resistance requires an understanding of physiological stress mechanisms and the ability of a specific genotype to respond to these changes in terms of the intensity and duration of cold or drought stresses. Tissue damage under low temperatures affects metabolic processes triggering changes at the membrane level and also on plant biochemistry (protein synthesis) and photosynthesis (Kratsch and Wise 2000). Fast reductions in temperature usually cause severe damage involving plant death to critical temperatures (Nishida and Murata 1996). However, plant acclimation involve gradual reductions in temperature allowing plant physiological adjustments that increase plant resistance to damage of critical temperatures that affect plant physiology and growth. Drought is one of the most important factors that limit growth and forest production (Pepper et al. 2008; Stape et al. 2010). Several models predict that global warming will change distribution of precipitations affecting crops and forestry production (Baesso et al. 2010; Yang et al. 2010). This is especially relevant in Mediterranean climates where intensively managed plantations grow experiencing long dry seasons during summer and where soil water availability affects largely forest productivity (Gurovich et al. 1996; Flores and Allen 2004; White et al. 2009). In the literature, it is frequently argued that species that are able to adjust transpiration (E) to a minimum allowing carbon fixation under drought conditions are able to better withstand this unfavorable condition (Osorio and Pereira 1994; Gindaba et al. 2005; Stape et al. 2008).
Critical on these adjustments are cross-linked effects between drought and cold resistance that share similar physiological processes such as osmotic adjustment and protein synthesis (Loveys et al. 2006; Cernusak et al. 2007; Fernandez et al. 2007; Coopman et al. 2010; Loveys et al. 2010). Experimental work has shown that water stress may induce morphological changes such as reduction in shoot growth and leaf/root ratio, and physiological changes such as reduction in predawn water potentials, increases in osmolytes, and increases in antioxidant and xanthophyll cycle pigments in leaves and stems. Water stress may also induce an increase in ABA affecting the novo synthesis of LEA proteins (Valentini et al. 1990; Ares and Fownes 2000; Coopman et al. 2008; Costa e Silva et al. 2009; Castillo et al. 2010; Loveys et al. 2010). All these responses may favor cold resistance and photoprotection in Eucalyptus (Close et al. 2000) and some of these adaptations may trigger changes in carbon partitioning, leaf area development and plant water use (Boyden et al. 2008). On this regard, water use efficiency has been identified as one of the key physiological parameters to consider for evaluating Eucalyptus adaptation to water use and drought stress (Li 2000). Water use efficiency (total dry mass accumulated per transpired water) has been shown as a physiological parameter useful to assess plant drought adaptation (Martin et al. 1999; Li 2000). However, there is less consensus regarding the instantaneous measurements of water use efficiency (WUE i ), which is the rate of net photosynthesis per unit of transpired water (A/E) (Warren and Adams 2006).
We hypothesized that genotypes with higher WUE i would have higher resistance to drought, drought induced photoinhibition and freezing stress. Similar to clones, little understanding exists on how hybrid genotypes compare to their parents species on physiological mechanisms (Almeida et al. 1994, 2004a, 2007; Mokotedi et al. 2009). Considering this hypothesis we evaluated WUE i as a physiological indicator in assessing drought, drought induced photoinhibition and cold stress resistance within clones of E. globulus and E. globulus × E. nitens hybrids. Both species are extensively planted on Mediterranean climates where E. globulus is selected for drier environments and E. nitens for frost prone and cold sites (White et al. 1996; White et al. 1998; Close et al. 2000; Costa e Silva et al. 2009; Mokotedi 2010).
Methods
Rooted cuttings and genetic material
Eucalyptus globulus clones (Eg1, Eg2, Eg3, Eg4, Eg5, Eg6) and E. globulus × E. nitens hybrids (Egn1, Egn2, Egn3, Egn4) plants were produced from containerized rooted cuttings (110 cm3), allowing them to grow at open air on a pine bark growing media with no water or nutrient limitations following standard nursery practices. All plants were produced at Forestal Mininco S.A. nursery located in Los Angeles, Chile. After fourth months of development 32 plants from each genotype were selected considering similar initial height (30 ± 1 cm) and free of diseases and transported to University of Concepción experimental nursery facility, Concepción, Chile. Selected plants from each genotype were transferred from nursery containers into 0.033 m3 styrofoam block containers (38 × 32 × 27.5 cm) filled with commercial pine bark substrate packed to a density of approximately 540 kg m−3. Two containers of 16 plants each (8 cm × 6.8 cm spacing per plant) were established for each genotype in a closed transparent roof nursery and grown for 2 weeks combining irrigation every 2 days and fertirrigation once a week with 0.2 g l−1 Phostrogen (NPK 14-4.4-22.4, S, Mg, Ca and microelements such as Fe, B, Mn, Cu and Mo, Bayer garden, Cambridge, UK) allowing the substrate to saturate. These conditions were provided in order to acclimate and homogenize the plants initial conditions to the new nursery growing environment.
Irrigation treatments
Irrigation treatments were applied after transplanting, once plant acclimation to the new nursery growing environment occurred. The experimental design tested the effect of a progressive drought treatment (drought) versus well irrigated rooted cutting plants (control) for the selected Eucalyptus genotypes. One container per genotype, containing 16 plants each, were selected for the application of each irrigation treatment. The control treatment applied continuous irrigation maintaining a predawn xylem water potential (PD-Ψ x ) levels ranging from 0 to −0.5 MPa, while the progressive drought treatment allowed rooted cuttings to dry under ambient conditions until they reached a target PD-Ψ x level ranging from −2.0 to −2.7 MPa for detailed physiological determinations.
Environmental and physiological assessments
Hourly mean, minimum and maximum temperatures at the nursery were recorded daily during the study using copper-constantan thermocouples and retrieved via an USB data acquisition system DAQ/56 (IOtech Inc., OH, USA). Daily averages calculated for each parameter are presented in Table 1.
Weekly photosynthetic active radiation (PAR, μmol m−2 s−1) assessments were obtained inside the nursery facility at midday using a LI-191 quantum sensor and a LI-250A data light meter (LI-COR, Nebraska, USA). Soil water potential (Ψ s ) assessments were obtained weekly between 0700 and 0900 h using a series of thermocouple psychrometers (PST-55, Wescor Inc., UT, USA) located at 20 cm depth on each container and measured using a PSΨRO equipment (Wescor Inc., UT, USA). PD-Ψ x was measured weakly using a Scholander pressure bomb (Soil Moisture Equipment Corp., CA, USA) for all genotypes after Ψ s levels dropped below −2.5 MPa. Estimates were obtained by destructively sampling one plant at random from each container.
Detailed physiological assessments comparing rooted cuttings under each irrigation treatment (drought and control) were performed once the target PD-Ψ x (−2.0 to −2.7 MPa) was attained for each genotype under the drought treatment (21–42 days). Leaf gas exchange measurements including net photosynthesis (A), stomatal conductance (g s ) and transpiration (E) were evaluated on fully expanded leaves of six randomly selected plants from each genotype and irrigation treatment. Measurements were made from 0900 to 1300 h considering 500 μmol m−2 s−1 light intensity, 20 ± 1°C of temperature and CO2 saturation of 370 ± 10 ppm using an infrared gas analyzer (Ciras-2, PP Systems, Hitchin, UK). Instantaneous water use efficiency (WUE i ) was estimated as the ratio between A and E (e.g. WUE i = A/E).
Frost, drought and photoinhibition tolerances
Frost tolerance of plants under control and drought treatments was evaluated exposing detached leaves of each genotype to 90 min temperature treatments of −3, −6, −9, −12 and −15°C. Five leaves were collected for each genotype. Leaf photosynthetic damage was evaluated considering the temperature at which 50% of the leaf tissue was damaged (LT50) by estimating the (F v /F m ) maximum photochemical efficiency determined by fluorescence of the photosystem II (PSII) at pre and post treatment conditions. Sampled leaves were maintained in the dark during 30 min, and initial (F v /F m )1 was estimated. Leaves were incubated at each temperature treatment and later maintained at 4°C during 24 h and final (F v /F m )2 was obtained. All (F v /F m ) determinations were obtained using a Plant Efficiency Analyzer (Hansatech Instruments, King’s Lyn, UK). The LT50 by photoinactivation (Phi) was estimated as:
Drought tolerance among genotypes was evaluated by comparing survival rates of plants under drought treatments that were allowed to rehydrate for a week after reaching a critical PD-Ψ x level of −4.0 ± 0.5 MPa. All determinations were obtained by selecting one plant at random from each container. Plants were considered alive if the tip of each plant recovered a turgid state.
NPQ components were evaluated essentially as previously described by Bravo et al. (2007). The fluorescence parameters F o , F mo , \( F_{m}^{\prime } \), F s , y \( F_{o}^{\prime } \) were obtained using a modulated fluorimeter (MFSII, Hansatech Instrument, King’s Lyn, UK). Three leaves of each genotype were exposed to oversaturating light conditions (1,600 μmol m−2 s−1) for 40 min and at 15°C temperature using a LS2H external lamp and LD2/3 cell (Hansatech Instrument, King’s Lyn, UK). The dark relaxation kinetic of F m was used to determine the non-photochemical quenching (NPQ) fast and slow relaxation components (NPQ f , NPQ s ). F mr was obtained by interpolation in a semi-logarithmic relationship that was established between the maximum fluorescence versus time after darkness as described by Maxwell and Johnson (2000) according to the equations:
where
- NPQ s :
-
Slow non-photochemical quenching
- NPQ f :
-
Fast non-photochemical quenching
- F mo :
-
Maximum fluorescence in the dark-adapted state
- \( F_{m}^{\prime } \) :
-
Maximum fluorescence in the light
- F mr :
-
Calculated F m assuming only slowly relaxing quenching present in the light
Tolerance to chronic photoinhibition induced by high light and drought was assessed evaluating the magnitude of the slowly relaxing component (NPQ s ) as an index of damage for each genotype.
Statistical analyses
ANOVA analyses were used to evaluate genotype and irrigation treatment effects on WUE i , gas exchange parameters, freezing temperatures and NPQ components. Data was checked for normality and heterocedasticity using Shapiro–Wilk (Shapiro and Wilk 1965) and Levene tests (Levene 1960) and logarithmic transformations were applied as required. Non-parametric Kruskall–Wallis analyses tests (Kruskal and Wallis 1952) were used when data showed lack of normality or heterocedasticity. Statistical ANOVA analyses were performed using SAS version 9.0 software (SAS Institute Inc., NC, USA). To determine specific genotype effects among treatment combinations when sample sizes were equal, a post hoc Scheffé test among the means was used (Scheffé 1959) and student t tests were used for analyses of two means when unequal sample sizes were obtained. To test our hypotheses, linear and logarithmic regression analyses were performed between WUE i and survival to drought conditions, freezing temperatures and photoinhibition. All regression analyses were made using SigmaPlot 10.0 software (SPSS® Inc., IL, USA).
Results
Soil (Ψ s ) and predawn xylem (PD-Ψ x ) water potentials
Ψ s of control treatment remained stable within the expected range of −0.1 to −0.4 MPa for all genotypes (Fig. 1). Drought treatment caused a rapid decrease in Ψ s from 0 to −2.0 MPa after 21 days for five genotypes (Eg1, Eg3, Egn1, Egn2 and Egn3) and three genotypes maintained Ψ s higher than −1.5 MPa (Eg2, Eg4 and Eg6). After 35 days of drought, three genotypes (Eg4, Egn2 and Egn3) further desiccated the soil reaching Ψ s −3.0 MPa at 35 days of treatment. Genotype Eg6 exhibited the slowest and less intense soil desiccation, reaching only −1.0 MPa at 35 days of treatment (Fig. 1). PD-Ψ x assessed after 21 days of drought, suggested that majority of evaluated genotypes showed PD-Ψ x values ranging from −1.7 to −2.5 MPa, except for genotype Eg6 which exhibited about −1.0 MPa (Fig. 2). PD-Ψ x decreased very rapidly after day 21 and reached values lower than −4.0 MPa for most of the genotypes at day 35. Two genotypes, Eg6 and Egn4, dehydrated slowly and reached about −4.0 MPa after 56 days of drought (Fig. 2).
Instantaneous water use efficiency (WUE i )
Instantaneous water use efficiency exhibited significant differences among genotypes at each drought treatment. WUE i did not vary with drought treatment in genotypes Eg1, Eg2, Eg3, Eg4, Egn2 and Egn3. Genotypes Eg5 and Egn4 increased by 70 to almost 80% respectively in their WUE i under drought. Conversely, genotype Eg6 and Egn1 exhibited a decrease in WUE i under drought conditions (Fig. 3).
Instantaneous water use efficiency (WUE i ) for each Eucalyptus genotype under control (dark bar) and drought (grey bar) treatments. Letters inside each bar indicates significant differences among genotypes within each treatment (Scheffé test P < 0.05). Asterisks symbols above bars indicate significant differences between drought treatments for each genotype (Student t test P < 0.05). Values represent the average of n = 6 ± SE
Leaf gas exchange measurements
Large differences in gas exchange parameters were found between drought and control treatments (P < 0.05) (Table 2). Most genotypes exhibited less than 5% of control plants net photosynthesis values. Genotypes Eg5 and Egn4 were able to maintain about 25% of irrigated controls net photosynthesis (Table 2). Gas exchange results suggested differences in physiological behavior of Eucalytpus genotypes WUE i under drought conditions. Genotypes Eg3 and Egn1 showed poor stomatal control causing high rates of transpiration compared to other genotypes under drought. Conversely, genotypes Eg5 and Egn4 showed an increase in WUE i under drought by maintaining better stomatal control and increased rates of net photosynthesis (Table 2). Other genotypes (Eg1, Eg2, Eg4, Eg6, Egn2 and Egn3) maintained lower rates of transpiration and net photosynthesis, therefore WUE i was the same or decreased under drought.
Drought tolerance
Plant survival was evaluated after severe drought (PD-Ψ x reached about −4 MPa) and re-watering. Genotypes with lower survival were Egn3 with 54% and Egn4 with 46%. Genotype Eg5 showed 100% survival after reaching PD-Ψ x of −4.1 MPa (Fig. 4). There was no clear correlation between genotype drought tolerance and WUE i (P = 0.8554).
Freezing tolerance
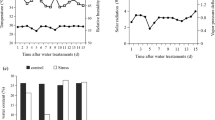
No significant differences in freezing tolerance were found among genotypes (Fig. 5). However, drought caused a significant decrease in LT50 for all genotypes except for Egn4 which did not showed a significant difference between control and drought treatments. Genotypes Eg2 and Eg4 exhibited a very limited freezing tolerance under well irrigated conditions, with more than 50% of damage at the lowest tested freezing temperature (−3°C). In the case of Eg5, Eg6 and Egn3, they also exhibited a large difference of LT50 between control and drought treatments (Fig. 5). WUE i was not significantly correlated with genotypes LT50 freezing tolerance (Fig. 6). However, a significant negative correlation (P < 0.01) was found when those genotypes in which WUE i decreased or remained unchanged after drought treatments were removed from the correlation (insert Fig. 6). Genotypes that were able to increase WUE i after drought also showed increased freezing tolerance.
Photoinhibition tolerance
The slowly relaxing component of thermal dissipation of PSII (NPQs) has been reported as an indicator of long term photoinhibition, or even irreversible photodamage. In general, well irrigated plants, regardless of the genotype, exhibited NPQs values lower than 0.4 (Fig. 7). When the same photoinhibitory treatment was applied under drought conditions, a significant increase in NPQs was observed for genotypes Eg1, Eg4, Egn2, Egn3 and Egn4, where Egn2 was the most photodamaged under drought conditions. Surprisingly, the Eg2 genotype exhibited a decrease in NPQs under drought (Fig. 7). No significant correlation was found between WUE i and the index of photoinhibition (NPQs) for all studied genotypes (P = 0.0849).
Mean slowly relaxing component of thermal dissipation of PSII (NPQs) of evaluated Eucalyptus genotypes. Letters above bars indicates significant differences among genotypes (Scheffé test P < 0.05). Asterisks indicate significant differences between treatments (Scheffé test P < 0.05). Values are the average of n = 3 ± SE
Discussion
The focus of our work was to study whether WUE i is a good indicator of stress tolerance for different genotypes of Eucalyptus subjected to drought. Our results suggest that E. globulus and E. globulus × E. nitens hybrids genotypes may present a large range of WUE i , but also differential responses in WUE i due to drought. A first group of the studied genotypes (Eg5 and Egn4) increased their WUE i under drought (Fig. 3). This response was explained by the capability of these genotypes to double A rates respect to the average genotypes under drought while maintaining similar rates of E and g s . This physiological response has been related to an increase in the efficiency in carbon acquisition under water limiting conditions (Li 2000). WUE i differences generated under drought are mainly attributed to a reduction in the rate of photosynthesis “A” (Piper et al. 2007). Photosynthesis is seriously impaired by drought at several steps such as thylakoidal electron transport chain (Noctor et al. 2002), metabolically at the Calvin cycle (Taiz and Zeiger 1998) and additionally by decreasing the stomata and mesophyll conductances (Flexas et al. 2009). Therefore carbon fixation and water loss depend both on stomatal and mesophyll conductances under a tradeoff between photosynthetic capacity and water loss control (Chaves et al. 2003). A second group of Eucalyptus genotypes showed a reduction in WUE i under drought (Eg6 and Egn1) by limiting stomatal control and maintaining relatively high rates of transpiration (Table 2). It is likely that this genotype was adapted to improved water availability conditions. It has been argued that photosynthesis is very stable in a wide range of stomatal openness and it is limited by CO2 diffusion only when stomatal conductance decline below a threshold of 100 mmol H2O m−2 s−1 in mediterranean species (Flexas et al. 2009). In our experiment most genotypes reduced drastically A, E and g s , with g s observed values below 100 mmol H2O m−2 s−1 except for Eg3 that showed only a half way reduction in g s but a significant decrease in A. These results suggest that Eucalyptus do respond to such a threshold similarly to the mediterranean species. A third group of genotypes of Eucalyptus varied largely in WUE i , but their values were not affected by drought (Eg1, Eg2, Eg3, Eg4, Egn2 and Egn3). In our genotypes the reduction in transpiration was concomitant with a reduction in A.
Trees that are able to cope with severe drought are capable to respond to this environmental constraint by keeping minimal water loss but maintaining themselves metabolically functional and alive (Osorio and Pereira 1994). Lower survival was observed for E. globulus × E. nitens hybrid genotypes under drought. White et al. (1996) indicated better drought tolerance for E. globulus compared to E. nitens which may explain the lower drought tolerance evaluated for hybrids. Drought tolerance, measured as survival after a severe drought, was not correlated with WUE i . In fact, several genotypes exhibiting high survival to drought (>80%) showed a large variation in WUE i ranging from 1 to 11 μmol CO2 mmol−1 H2O. It was interesting that several genotypes that showed little change in WUE i , and those that showed an increase in WUE i after drought, except for Egn4, exhibited higher rates of survival. On the other hand, genotypes with reductions in WUE i under drought (Eg6, Egn1) exhibited lower survival. Our results suggest that stability of WUE i may be a good predictor of tolerant or sensitive genotypes of Eucalyptus to drought. In addition, our results suggest that an increase in WUE i under water stress, together with other physiological and genetic tests, could be used as a tool to select tolerant genotypes to drought. An interesting result was obtained with genotype Eg6 which took a long time to dehydrate its substrate (Fig. 1) and plant tissue (Fig. 2), being consistent with its very low transpiration rate. However, Eg6 WUE i was also very low (Fig. 3), suggesting that probably stomata were too close to sustain carbon influx and reduced dramatically its photosynthesis rate. This pattern may not explain Egn4 genotype, which showed the lowest survival and the highest PD-Ψ x . High E and A values for this genotype suggests lack of response to water loss signals.
As expected, most Eucalyptus genotypes exhibited greater freezing tolerance after drought. It is well established that drought may trigger similar metabolic and physiological responses to those observed during cold acclimation (Coopman et al. 2010; Valentini et al. 1990). Typical responses include dehydrins accumulation (Close 1997; Bravo et al. 2003), osmotic adjustment by synthesis and accumulation of sugars and proline, and activation of efficient antioxidant systems (Shvaleva et al. 2006). On the other hand, drought resistant clones of E. globulus have shown a higher capacity to cold acclimate (Costa e Silva et al. 2009). In this study, LT50 decreased in average 3ºC after drought exposure (Fig. 5). Unexpectedly, E. globulus × E. nitens hybrids were not significantly more freezing tolerant than E. globulus genotypes. This suggests that their freezing tolerance, induced by the drought treatment, was similar to what was observed for other genotypes. This probably implies that these hybrids share more genetic background with E. globulus than E. nitens, at least regarding to freezing tolerance. Larger capability to withstand frost by E. globulus × E. nitens hybrids observed in the field may be gained by a hardening process of exposure to chilling temperatures (Valentini et al. 1990). Our results suggest also that managed drought stress may be used in nursery regimes to improve seedlings frost resistance.
A significant inverse correlation between WUE i and LT50 was found for genotypes that showed an increase in WUE i after drought (Fig. 6). Therefore, increased WUE i after drought may be used to select for increased freezing tolerance in Eucalyptus. Our results also suggest that drought will not trigger non-specific cross resistance against other studied environmental factors such as excess light. For instance, photoprotection was not significantly improved in drought treated genotypes. In fact three genotypes (Egn2, Egn3 and Egn4) were more photo-inhibited after drought than under well irrigated control conditions. This is inconsistent with the increase in xanthophyll cicle pigments observed in other E. globulus clones under drought (Shvaleva et al. 2006). There was only one genotype Eg2 that was less photo-inhibited after drought (Fig. 7). No significant correlation was found between NPQs and WUE i , which probably means that even though WUE i increases, stomatal closure does not totally impair photosynthesis. These genotypes may have also enough photoprotective mechanisms to deal with the excess absorbed energy and the over-excitation of PSII imposed by the combined effect of drought and high light, a non-photochemical energy dissipation reported elsewhere in Eucalyptus (Close et al. 2000), or an strong antioxidant system (Costa e Silva et al. 2009).
Obvious differences exist between WUE i and absolute water use efficiency. WUE i is measured at the leaf level and a typical adaptive drought response is leaf abscission, which limits plant transpiration by decreasing total plant leaf area but not necessarily by limiting transpiration per unit of leaf area (Raison et al. 1992; Pook et al. 1997; Williams et al. 1997; Munne-Bosch et al. 2001). This may lead to a completely different scenario when comparing WUE i to absolute WUE, which is based on the whole long term plant response. Therefore, under well irrigated or mild stress, WUE i may vary due to variations in E but leaf photosynthesis may still be high and carbon gain may not be affected (Sinclair et al. 1984; Mokotedi 2010). Regardless WUE i limitations, it is a physiological parameter that can be easily measured. Our results suggest that WUE i is not suitable by itself to select drought tolerant genotypes, but may provide evidence for discarding drought sensitive genotypes. In addition it could provide valuable information to select for freezing tolerance.
References
Almeida MH, Chaves MM, Silva JC (1994) Cold acclimation in eucalypt hybrids. Tree Physiol 14:921–932
Almeida AC, Landsberg JJ, Sands PJ (2004a) Parameterisation of 3-PG model for fast-growing Eucalyptus grandis plantations. For Ecol Manag 193:179–195
Almeida AC, Landsberg JJ, Sands PJ, Ambrogi MS, Fonseca S, Barddal SM, Bertolucci FL (2004b) Needs and opportunities for using a process-based productivity model as a practical tool in Eucalyptus plantations. For Ecol Manag 193:167–177
Almeida AC, Soares JV, Landsberg JJ, Rezende GD (2007) Growth and water balance of Eucalyptus grandis hybrid plantations in Brazil during a rotation for pulp production. For Ecol Manag 251:10–21
Ares A, Fownes JH (2000) Productivity, nutrient and water-use efficiency of Eucalyptus saligna and Toona ciliata in Hawaii. For Ecol Manag 139:227–236
Baesso RCE, Ribeiro A, Silva MP (2010) The impact of climatic changes on Eucalyptus productivity in Northern Espirito Santo and Southern Bahia. Cienc Florest 20:335–344
Bison O, Ramalho MAP, Rezende GDSP, Aguiar AM, Van der Resende MD (2007) Combining ability of elite clones of Eucalyptus grandis and Eucalyptus urophylla with Eucalyptus globulus. Genet Mol Biol 30:417–422
Boyden S, Binkley D, Stape JL (2008) Competition among Eucalyptus trees depends on genetic variation and resource supply. Ecology 89:2850–2859
Bravo LA, Gallardo J, Navarrete A, Olave N, Martínez J, Alberdi M, Close TJ, Corcuera LJ (2003) Cryoprotective activity of a cold-induced dehydrin purified from barley. Physiol Plant 118:262–269
Bravo LA, Saavedra-Mella FA, Vera F, Guerra A, Cavieres LA, Ivanov AG, Huner NPA, Corcuera LJ (2007) Effect of cold acclimation on the photosynthetic performance of two ecotypes of Colobanthus quitensis (Kunth) Bartl. J Exp Bot 58:3581–3590
Castillo RDP, Contreras D, Baeza J, Otto M, Agurto C, Freer J (2010) Classification of genotypes of Eucalyptus globulus under cold conditions using their free amino acids content on leaves and regularized discriminant analysis (RDA). J Chil Chem Soc 55:11–18
Cernusak LA, Aranda J, Marshall JD, Winter K (2007) Large variation in whole-plant water-use efficiency among tropical tree species. New Phytol 173:294–305
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30(3):239–264
Close TJ (1997) Dehydrins: a commonality in the response of plants to dehydration and low temperature. Physiol Plant 100:291–296
Close DC, Beadle CL, Brown PH, Holz GK (2000) Cold-induced photoinhibition affects establishment of Eucalyptus nitens (Deane and Maiden) Maiden and Eucalyptus globulus Labill. Trees Struct Funct 15:32–41
Coopman RE, Jara JC, Bravo LA, Sáez KL, Mella GR, Escobar R (2008) Changes in morpho-physiological attributes of Eucalyptus globulus plants in response to different drought hardening treatments. Electron J Biotechnol 11:30–39
Coopman RE, Jara JC, Escobar R, Corcuera LJ, Bravo LA (2010) Genotypic variation in morphology and freezing resistance of Eucalyptus globulus seedlings subjected to drought hardening in nursery. Electron J Biotechnol 13:5–6
Costa e Silva F, Shvaleva A, Broetto F, Ortuño MF, Rodrigues ML, Almeida MH, Chaves MM, Pereira JS (2009) Acclimation to short-term low temperatures in two Eucalyptus globulus clones with contrasting drought resistance. Tree Physiol 29:77–86
Cromer RN, Cameron DM, Rance SJ, Ryan PA, Brown M (1993) Response to nutrients in Eucalyptus grandis. 1. Biomass accumulation. For Ecol Manag 62:211–230
Dwivedi P, Alavalapati JRR (2009) Stakeholders’ perceptions on forest biomass-based bioenergy development in the southern US. Energ Policy 37:1999–2007
Fernandez M, Marcos C, Tapias R, Ruiz F, Lopez G (2007) Nursery fertilisation affects the frost-tolerance and plant quality of Eucalyptus globulus Labill. cuttings. Ann For Sci 64:865–873
Flexas J, Barón M, Bota J, Ducruet J-M, Gallé A, Galmés J, Jiménez M, Pou A, Ribas-Carbó M, Sajnani C, Tomàs M, Medrano H (2009) Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris). J Exp Bot 60:2361–2377
Flores FJ, Allen LH (2004) Efectos del clima y capacidad de almacenamiento de agua del suelo en la productividad de rodales de pino radiata en Chile: Un analisis utilizando el modelo 3-PG. Bosque 25:11–24
Gindaba J, Rozanov A, Negash L (2005) Photosynthetic gas exchange, growth and biomass allocation of two Eucalyptus and three indigenous tree species of Ethiopia under moisture deficit. For Ecol Manag 205:127–138
Gurovich LA, Holmberg J, Lyon A (1996) Eucalyptus globulus (Labill.) growth and water use under differential irrigation regimes. Ciencia e Investigacion Agraria 23:61–79
Kratsch HA, Wise RR (2000) The ultrastructure of chilling stress. Plant Cell Environ 23:337–350
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47:583–621
Levene H (1960) Robust tests for equality of variances. In: Olkin I, Hotelling H (eds) Stanford University Press, pp 278–292
Li C (2000) Population differences in water-use efficiency of Eucalyptus microtheca seedlings under different watering regimes. Physiol Plant 108:134–139
Loveys BR, Egerton JJG, Ball MC (2006) Higher daytime leaf temperatures contribute to lower freeze tolerance under elevated CO2. Plant Cell Environ 29:1077–1086
Loveys BR, Egerton JJG, Bruhn D, Ball MC (2010) Disturbance is required for CO2-dependent promotion of woody plant growth in grasslands. Funct Plant Biol 37:555–565
Martin B, Tauer CG, Lin RK (1999) Carbon isotope discrimination as a tool to improve water-use efficiency in tomato. Crop Sci 39:1775–1783
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Mokotedi MEO (2010) Physiological responses of Eucalyptus nitens × nitens under experimentally imposed water stress. South For 72:63–68
Mokotedi MEO, Watt MP, Pammenter NW (2009) Analysis of differences in field performance of vegetatively and seed-propagated Eucalyptus varieties I: survival and leaf gas exchange. South For 71:267–271
Munne-Bosch S, Jubany-Mari T, Alegre L (2001) Drought-induced senescence is characterized by a loss of antioxidant defences in chloroplasts. Plant Cell Environ 24:1319–1327
Nishida I, Murata N (1996) Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipids. Annu Rev Plant Physiol Plant Mol Biol 47:541–568
Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer CH (2002) Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann Bot 89 Spec No: 841–850
Osorio J, Pereira JS (1994) Genotypic differences in water use efficiency and 13C discrimination in Eucalyptus globulus. Tree Physiol 14:871–882
Pepper DA, McMurtrie RE, Medlyn BE, Keith H, Eamus D (2008) Mechanisms linking plant productivity and water status for a temperate Eucalyptus forest flux site: analysis over wet and dry years with a simple model. Funct Plant Biol 35:493–508
Piper FI, Corcuera LJ, Alberdi M, Lusk C (2007) Differential photosynthetic and survival responses to soil drought in two evergreen Nothofagus species. Ann For Sci 64:447–452
Pook EW, Gill AM, Moore PHR (1997) Long-term variation of litter fall, canopy leaf area and flowering in a Eucalyptus maculata forest on the south coast of New South Wales. Aust J Bot 45:737–755
Raison RJ, Khanna PK, Benson ML, Myers BJ, McMurtrie RE, Lang ARG (1992) Dynamics of Pinus radiata foliage in relation to water and nitrogen stress: II. Needle loss and temporal changes in total foliage mass. For Ecol Manag 52:159–178
Scheffé H (1959) The analysis of variance. Wiley, New York
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611
Shvaleva AL, Silva FCE, Breia E, Jouve J, Hausman JF, Almeida MH, Maroco JP, Rodrigues ML, Pereira JS, Chaves MM (2006) Metabolic responses to water deficit in two Eucalyptus globulus clones with contrasting drought sensitivity. Tree Physiol 26:239–248
Sinclair TR, Tanner CB, Bennett JM (1984) Water-use efficiency in crop production. Bioscience 34:36–40
Stape JL, Goncalves JLM, Goncalves AN (2001) Relationships between nursery practices and field performance for Eucalyptus plantations in Brazil—a historical overview and its increasing importance. New For 22:19–41
Stape JL, Binkley D, Ryan MG (2008) Production and carbon allocation in a clonal Eucalyptus plantation with water and nutrient manipulations. For Ecol Manag 255:920–930
Stape JL, Binkley D, Ryan MG, Fonseca S, Loos RA, Takahashi EN, Silva CR, Silva SR, Hakamada RE, Ferreira JMD, Lima AMN, Gava JL, Leite FP, Andrade HB, Alves JM, Silva GGC, Azevedo MR (2010) The Brazil Eucalyptus Potential Productivity Project: Influence of water, nutrients and stand uniformity on wood production. For Ecol Manag 259:1684–1694
Taiz L, Zeiger E (1998) Plant physiology, 2nd edn. Sinauer Associates, Sunderland
Valentini R, Mugnozza GS, Giordano E, Kuzminsky E (1990) Influence of cold hardening on water relations of three Eucalyptus species. Tree Physiol 6:1–10
Vellini ALTT, Figueiredo de Paula N, Da Costa PL, Pavani LC, Valencise CA, Escarpinati EA, De Paula RC (2008) Respostas fisiológicas de diferentes clones de eucalipto sob diferentes regimes de irrigação. Revista Árvore 32:651–663
Warren CR, Adams MA (2006) Internal conductance does not scale with photosynthetic capacity: implications for carbon isotope discrimination and the economics of water and nitrogen use in photosynthesis. Plant Cell Environ 29:192–201
White DA, Beadle CL, Worledge D (1996) Leaf water relations of Eucalyptus globulus ssp globulus and E.nitens: Seasonal, drought and species effects. Tree Physiol 16:469–476
White D, Beadle C, Worledge D, Honeysett J, Cherry M (1998) The influence of drought on the relationship between leaf and conducting sapwood area in Eucalyptus globulus and Eucalyptus nitens. Trees Struct Funct 12:406–414
White DA, Crombie DS, Kinal J, Battaglia M, McGrath JF, Mendharn DS, Walker SN (2009) Managing productivity and drought risk in Eucalyptus globulus plantations in south-western Australia. For Ecol Manag 259:33–44
Williams RJ, Myers BA, Muller WJ, Duff GA, Eamus D (1997) Leaf phenology of woody species in a North Australian tropical savanna. Ecology 78:2542–2558
Yang B, Pallardy SG, Meyers TP, Gu LH, Hanson PJ, Wullschleger SD, Heuer M, Hosman KP, Riggs JS, Sluss DW (2010) Environmental controls on water use efficiency during severe drought in an Ozark Forest in Missouri, USA. Glob Change Biol 16:2252–2271
Acknowledgments
This research was funded and supported by FONDECYT project No. 1085093, Forestal Mininco S.A., the Faculty of Forest Sciences and the Faculty of Natural and Oceanographic Sciences at the Universidad de Concepción. All are gratefully acknowledged for his contributions and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Navarrete-Campos, D., Bravo, L.A., Rubilar, R.A. et al. Drought effects on water use efficiency, freezing tolerance and survival of Eucalyptus globulus and Eucalyptus globulus × nitens cuttings. New Forests 44, 119–134 (2013). https://doi.org/10.1007/s11056-012-9305-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-012-9305-0