Abstract
Water stress is considered one of the main environmental factors that limit survival and productivity of Eucalyptus plantations. Identifying genotypes traits that provide evidence of tolerance to water stress may allow sustained productivity and secure better resilience of forest plantations under climate change in Mediterranean environments. Our study analyzed morphological, physiological, and carbon (C) balance responses of ten Eucalyptus genotypes under contrasting water regimes under nursery conditions. One-year-old cuttings of Eucalyptus nitens (En1 and En2), E. smithii (Es), E. badjensis (Eb), E. nitens x globulus (Eng1, Eng2, Eng3, and Eng4), E. globulus (Eg), and E. camaldulensis x globulus (Ecg) were evaluated under progressive drought from well-watered (soil water potential close to 0 MPa) to severe water stress (soil water potential close to − 2.5 MPa) conditions. Absolute growth rate (root collar diameter, height, shoot: root biomass ratio), net photosynthesis, stomatal conductance, transpiration, intrinsic water use efficiency (iWUE), predawn water potential (Ψpd) and C balance (flux and partitioning) were evaluated. As expected, water stress significantly reduced growth and physiological activity for all genotypes. Of all evaluated parameters iWUE and Ψpd were the key grouping physiological and growth response variables among genotypes. Genotypes En1, Eng3, and Eng4 were considered tolerant genotypes, with the smallest physiological change and larger morphological growth. Genotypes En2, Es, Eng1 and Eng2 were moderately tolerant showing intermediate responses compared to other groups. In contrast, Eg, Eb, and Ecg were considered highly sensitive, with major changes in morphology and physiological variables over time. Our results suggest that nursery stage physiological evaluation of genotypes may allow selection for sites with water resource availability risks and may allow to reduce mortality and early establishment productivity losses under drier climate change scenarios.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eucalyptus is one of the most planted hardwood genus worldwide with more than 25 million hectares (Martins et al. 2022). The wide natural distribution of Eucalyptus species under a broad range of contrasting environmental conditions (e.g., soil, temperature, and precipitation) have supported the selection of valuable species planted for production of wood, paper, bioenergy, charcoal, and biomaterials for forest industry (Hubbard et al. 2010; Zhang et al. 2022). In Chile, commercial plantations of Eucalyptus species have become an important source of fiber and timber resources with more than 850,000 ha (INFOR 2020) and intensive genetic and silvicultural management programs that have reduced rotation cycles, increased yields and reduced pests susceptibility (Gonçalves et al. 2013; Rubilar et al. 2018). In the last decade, Eucalyptus globulus (selected for its high pulp yield and adaptability to dry sites), E. nitens (selected for its high growth in cold environments) and their hybrid (E. nitens x globulus) are the dominant species being planted in commercial reforestation (Silva et al. 2017).
Despite advances in intensive forest management, climatic variability still strongly influences Eucalyptus productivity (Forrester et al. 2010; Whitehead and Beadle 2004). Water scarcity is considered the main factor limiting trees survival and growth (El- Sharkawy 2014; Martins et al. 2022). In fact, climate simulation models have projected an increase of 1 to 4 °C in the global mean temperatures, with a reduction in rainfall patterns that will lead to water deficits and large scale regional droughts in many regions planted with Eucalyptus (Bahuguna and Jagadish 2015; Booth 2013; Elli et al. 2020; Fagundes et al. 2021).
In order to reduce plantations risks, several authors have recommended an acclimation process through changes in early silvicultural management and genetic selection (Corrêa et al. 2017; Pita-Barbosa et al. 2023; Saadaoui et al. 2017). Evidence has shown that, some taxa/genotypes have greater tolerance to water stress conditions from early ages by adjusting morphological, physiological, biochemical, and hormonal response mechanisms (Mueller et al. 2020). Identifying these water stress tolerance physiological or morphological traits in young trees may help to reduce uncertainty of Eucalyptus survival and productivity in many regions where climate change has increased the frequency or intensity of drought periods (Saadaoui et al. 2017). Water stress tolerance has been linked to morphological adaptations. For example, Susiluoto & Berninger (2007), found that the root:shoot ratio of juvenile trees of E. microtheca, was the key trait that differentiated sensitive to tolerant clones to drought (tolerant clones showed greater survival by increasing roots at the expense of shoot growth). Similarly, Corrêa et al. (2017), comparing 13 commercially planted Eucalyptus genotypes, evaluated 34 morphological and nutritional variables and obtained a tolerance ranking explained by 70% only based on morphological traits that included diameter, height, and leaf area. Physiological traits, such as the ability to regulate stomatal and mesophyll conductivity (gs and gm, respectively) (Chaves et al. 2009; Flexas et al. 2008; Warren et al. 2011), control of water loss by transpiration (Flexas et al. 2008), and photosynthetic adaptation, that allows maintaining metabolic processes and in some cases growth (Erice et al. 2010; Ma et al. 2014), have become relevant to determine tolerance thresholds of genotypes to water stress environmental restrictions. To illustrate, Aspinwall et al. (2016) found that photosynthesis and respiration were key traits for survival and acclimation of E. tereticornis genotypes under different stress conditions. Similarly, Nóia Júnior et al. (2020) differentiated tolerant clones of Eucalyptus grandis x Eucalyptus urophylla through changes in C balance, a decrease in stomatal conductance, size, and the number of stomata and variation in water use efficiency. Muller et al. (2020) classified the response of ten juvenile genotypes (E. urophylla and E. grandis x urophylla) to water deficit conditions based on changes in net photosynthesis, transpiration, and root growth. Finally, Duan et al. (2013) suggested that water stress adaptability is driven by carbon (C) allocation, with significant reductions in aboveground (leaves>branch>stem) compared to belowground production.
Maintaining forest-sustained production and plantation sustainability in the face of climate change requires an early selection of taxa/genotypes with morphological and physiological tolerance to water stress traits (Hodecker et al. 2018; Pita-Barbosa et al. 2023). Therefore, our study objective was to evaluate morphological, physiological, and C balance responses of ten Eucalyptus genotypes under contrasting water regimes to understand better the potential response of these selected materials under challenging climate change scenarios.
Materials and methods
Plant material and experimental design
The study evaluated 10 selected genotypes of Eucalyptus nitens (En1 and En2), E. nitens x globulus (Eng1, Eng2, Eng3, and Eng4), E. globulus (Eg), E. badjensis (Eb), E. camaldulensis x globulus (Ecg) and E. smithii (Es). Taxa and genotype selection was based on mid-rotation survival, productivity and growth response until eight years of age of the selected materials growing under a large-scale experiment named EucaHydro evaluating their performance under contrasting water stress treatments (details in Rubilar et al. (2020). The plants were produced at Forestal Mininco SpA Forest nursery (Yumbel, Bio-Bio, Chile; 37° 8′0.01″ S, 72° 27′34.70″ W); and at 11 months old, 420 plants (42 per genotype) were selected considering plants of similar height and root collar diameter showing no signs of mechanical damage or disease (Table 1).
Selected plants were transported to the nursery of the University of Conception, Concepción, Bio-Bio, Chile (36° 50′3.35″ S, 73° 1′55.74″ W), transferred to 7.5 L plastic pots filled with homogenized (< 2 mm) pine bark compost, and acclimated during 90 days before initiation of the experiment by maintaining soil water potential (Ψsoil) close to field capacity that was monitored daily using SMP 229-L psychrometers (Campbell Scientific, USA). In order to remove potential nutrient limitations and maintain standard plant nutrition, fertilization was applied every 15 days with a standard growth media solution of Phostrogen ® (NPK 13–10-27, S, Mg, Ca, Fe, B, Mn, Cu, Mo, Bayer Garden, UK) at 1 g L−1. From this point, the experiment was carried out under natural light, temperature, and relative humidity conditions that were monitored using an automated Vantage Pro 2 weather station (Davis Instruments, USA) placed at the nursery site.
The experiment started in February 2021 (summer) for 22 days. Ambient temperature during this period ranged from 10.9 to 24.2 °C (Fig. 1a), vapor pressure deficit (VPD) ranged from 0.24 to 1.72 kPa (Fig. 1a), relative humidity ranged from 25.1 to 93.6% (Fig. 1b); ambient light was homogeneous for all treatments, with an average daily exposure of 12.3 h with maximum photosynthetically active radiation (PAR) ranging from 1020 to 1520 µmol m−2 s−1 (Fig. 1b). A single rainfall event of 0.75 mm was recorded, but a polypropylene insulation plastic film was used to cover plants to avoid undesired input of water in the experiment during the event.
Plants of each genotype were randomly split in two water regime treatments (21 plants per genotype). Water regimes considered: (i) well-watered (WW) treatment, where soil water potential (Ψsoil) was maintained near 0 MPa (control treatment); and (ii) water-stressed (WS) treatment, in which no irrigation was applied until Ψsoil reached −2.5 MPa (considered a severe plant water deficit). Ψsoil was estimated indirectly using volumetric moisture content (VWC) values estimated using a TDR sensor (CS-615 Campbell Scientific Inc.). A volumetric moisture content relationship with Ψsoil was adjusted by fitting a generalized cubic polynomial equation (Eq. 1, R2 = 0.94, p-value˂0.001) after taking repeated soil samples at a range of Ψsoil values following the methodology described by Silva et al. (2014a) using a Richards pressure plate model 1500 (Soilmoisture Equipment Corp.).
where Ψsoil is soil water potential in kPa; and VWC is volumetric moisture content in percentage.
Morphological variables
Plant height (H) and root collar diameter (RCD) were measured at the beginning and end of the experiment (n = 6 plants per genotype x water regime). Also, shoot (SB) and root (RB) dry biomass was determined using destructive sampling (n = 4 plants per genotype x water regime). Stem, leaves, branches, and root biomass components were weighed green, and dried at 65 °C for 48 h to estimate dry weight. Measured biomass dry weights, H, and RCD, were used to develop allometric equations for each genotype and water regime (supplementary information S1) to obtain biomass dry weight estimates for all plants at each measurement instance. Morphological variables were used to estimate the absolute growth rate using the equation reported by Pommerening and Muszta (2015) (Eq. 2).
where VAGR is the absolute growth rate of morphological variable V (RCD in mm each 10 days−1, H in mm day−1, and dry biomass in g day−1); Vi is the morphological measurement at time i Vi+1 at time i, t and ti+1 are starting and final times of measurement (day).
Physiological variables
Individual plant physiological assessments considered net photosynthetic assimilation rate (AN), stomatal conductance (gs), transpiration (E), intrinsic water-use efficiency was estimated as the ratio of AN/gs (iWUE), and predawn leaf water potential (Ψpd). Measurements were taken at the beginning of the experiment when Ψsoil was close to 0 MPa, and when plants reached − 2.5 MPa, on six leaves of six independent plants for each genotype and water regime. AN, E and gs were measured using an infrared gas analyzer (IRGA) model LI-6400XT (LI-COR, Inc. USA) under a photosynthetic photon flux density (PPFD) of 1500 µmol m−2 s−1, a leaf temperature of 20 °C, a constant flow rate of 500 µmol s−1, a CO2 concentration of 400 µmol mol−1 CO2, and maintaining a relative humidity range of 40 to 70%. Measurements were performed between 10:00 to 12:00 (UTC − 3:00 Chile summer time) under sunny weather conditions. Ψpd was evaluated between 4:00 to 6:00 (UTC − 3:00 Chile summer time) using a Scholander pressure pump model 1505D (PMS Instruments Co.) by sampling tree plants per genotype for each water regime.
Carbon balance
C flux (expressed on g C m−2 month−1) and C partition (% of each biomass components) were estimated for the study period using the mass balance approach proposed by Ryan (1991), Giardina and Ryan (2002) and Giardina et al. (2003). Gross primary production (GPP) was determined as the sum of aboveground net primary production (ANPP), aboveground autotrophic respiration (Rag) and total belowground C flux (TBCF). ANPP was calculated as the sum of foliage net primary production (FNPP) and wood net primary production (WNPP); both variables were estimated using biomass allometric equations developed and determining C concentration (n = 9 for each biomass component of each genotype and water regime) using a CHONS elemental analyzer (SERCON. Co.) (supplementary information S2). In the case of Rag, it was calculated using Eq. 3 and considering a CUE (carbon use efficiency) value of 0.53 as suggested from previous studies of Eucalyptus spp. plantations (Campoe et al. 2012; Epron et al. 2012; Stape et al. 2008).
TBCF was estimated using Eq. 4, where Soil CO2 efflux (FS) was measured using a gas analyzer system model LI-850 (Lincoln, NE, USA) on days where genotypes reached each target Ψsoil (n = 4 plants per genotype x water regime). Measurements were conducted on daytime (6:00 to 20:00) and nighttime hours (21:00 to 24:00) to evaluate diurnal genotype belowground respiration (n = 4 trees per genotype x water regimes). FE was not considered of significance given that different of other studies there was no C loss by erosion or leaching in the substrate of the pots at these nursery conditions (Campoe et al. 2020; Rodrigues et al. 2021; Ryan et al. 2010). Also, FA was discarded, given that litterfall was not significant (no liter fall during the duration of the study). Similarly, changes in substrate-soil C (ΔCS) and the organic horizon (ΔCL) were considered negligible in our study given the short period of the study (22 days). Finally, ΔCR was estimated as the change in coarse root biomass C content using biomass allometric equations and C concentration.
Statistical analysis
The experiment considered a complete factorial randomized design with genotype (10 genotypes) and irrigation (control vs suppressed irrigation regimes) as main factors and individual plants as replicates. Morphological (RCD, H, SB, RB, total dry mass TDM), physiological (AN, gs, E, iWUE and Ψpd), and C balance (GPP, ANPP, Rag and TBCF) data were analyzed using a two-way ANOVA mixed model (Eq. 5). ANOVA analyses considered genotype (Gi) and genotype x water regime interaction (Iij) as random and water regime (τj) as a fixed effect. Differences among treatment means were determined using a Tukey test. Principal Component Analysis (PCA) and a dendrogram analysis using Ward’s linkage with Bray–Curtis dissimilitude index were used to identify variables that contributed more significantly to explain experimental variation and were used to define similar genotype groups response for each measured variable.
All data were analyzed with R version 4.2.1 (Team, 2021) and analyses were considered statistically significant with a level of p<0.05.
Results
Morphological and physiological responses
Differences in all morphological variables were found among genotypes, water regimes, and their interaction (Table 2). The RCD, H, and SB absolute growth rate were significantly reduced for all genotypes under water stress conditions compared to well-watered conditions (Table 3). Interestingly, RCD, showing almost no growth for several genotypes, was the most affected variable under water stress compared to H, SB, and RB. The RCD absolute growth rate of Eng1, Eng2, Eb, Ecg, and Es did not show any changes under WS regime; in contrast, En1, En2, Eng3, and Eng4 showed an average increment of 0.85 mm × 10 day−1. In the case of En1 and Eng3 genotypes, both reported the lowest reduction in absolute growth rate under water stress conditions with average values of 7.5% for RCD, 46.2% for H, and 29.0% for SB. Contrastingly, Eg, Eb, and Ecg genotypes obtained the most significant reduction in H (average 78.2%) and SB (average 65.5%). An interesting result was observed for the absolute growth rate of RB comparing both water regimes that under water stress showed an average increment of 23.1% for En1, En2, Eng1, Eng3, and Eb, while for Eng2, Eng4, Eb, Ecg, and Es showed an average reduction of 43.0%. Finally, for the root-to-shoot ratio (R:S) differences were observed between WW and WS water regimes where only Ecg reported a decrease of 33.1% underwater stress, compared to the rest of the genotypes that showed average increases from 3.0 to 69.2%.
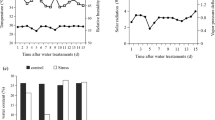
Regarding all evaluated physiological variables, only gs did not show differences for the effect of genotype and genotype x water regime interaction (Table 2). In the case of AN, genotypes under WW showed average values ranging from 13.2 to 19.6 µmol CO2 m−2 s−1; contrastingly, under water stress conditions, AN presented values ranging from 0.01 to 3.10 µmol CO2 m−2 s−1 (Fig. 2a). Genotypes Eng, Eg, and Eb showed the largest AN reductions under water stress (average 95.4%), followed by En2, Eng1, Eng2, Eng4, and Es with an average reduction of 91.2% and En1 and Eng3 showing the smallest reduction (84.0%). In the case of gs an average reduction of 95.1% was observed for all genotypes under WS (Fig. 2b). For E (Fig. 2c), genotypes under WW showed values ranging from 2.51 to 4.42 mmol H2O m−2 s−1, and 0.73 to 2.02 mmol H2O m−2 s−1 under WS. Of all evaluated genotypes, Eb, Ecg, and Es showed the lowest declines in E (average 37.0%). In the case of Ψpd, plants showed a drop ranging from − 1.12 to –3.02 (MPa) under water stress (Fig. 2d), where Eng1, Eb, and Ecg presented the largest reductions, and En1 and En2 the smallest change in Ψpd. Finally, all genotypes under water stress reported higher iWUE values (Fig. 2e), where En1, Eng3, and Eng4 showed the highest increase with an average of 65.20% followed by En2 and Es with an average of 35.42%. Contrastingly, Eng1, Eng2, Eg, Eb, and Ecg showed the smallest change in iWUE with an average of 15.21%.
Changes in net photosynthesis rate (AN), stomatal conductance (gs), transpiration rate (E), predawn water potential (Ψpd), and intrinsic water use efficiency (iWUE) for all the Eucalyptus genotypes evaluated under well-watered (WW) and water stress (WS) regimes. Points and bars represent the mean ± standard error of individual genotypes, and the corresponding blue and red lines are the general average value of all genotypes under WW and WS, respectively
Carbon flux and partitioning
Anova analyses of C flux and partitioning showed significant effects of genotype, water regime and the genotype x water regime interaction (Table 2). Under WW regime, C flux (Fig. 3a) ranged from 200 to 1170 g C m−2 month−1; where En 2, Eng 1, and Eng2 genotypes showed the highest C flow (average 1140 g C m−2 month−1) and Eb showed the lowest flow with 460 g C m−2 month−1. Considering C partitioning (Fig. 3b) under WW regime values ranged from 32 to 46% for Rag, less than 25% for TBCF, and from 20 to 36% for WNPP. In contrast, under WS regime C flux showed significant reductions ranging from 60 to 235 g C m−2 month−1; where En1, En2, and Eb genotypes in average reduced C flux by less than 78%, while Ecg and Eng2 showed the largest falls with an average of 96%. Genotypes under water stress conditions revealed three types of response: (i) En1, Eng1 and Es showed a significant reduction in TBCF; (ii) En2 and Eng4 maintained TBCF and ANPP partition; and (iii) Eng2, Eng3, Eg, Eb end Ecg raised TBCF (Table 4).
Carbon flux and partition for all Eucalyptus genotypes evaluated under well-watered (WW) and water stress (WS) regimes. Measurements include wood net primary production (WNPP), foliage net primary production (FNPP), aboveground autotrophic respiration (Rag), and total belowground carbon flux (TBCF). Capital letters compare different genotypes under WW regime, and lower letters compare different genotypes under WS regime using the Tukey test (p < 0.05). * denotes an statistical significance (p < 0.05) difference between means of each genotype under different water regimes
Genotypes dissimilitude
When analyzing differences among genotypes, the principal component analysis test (PCA) explained 90.65% of the variability (Fig. 4a). The first PCA component was strongly associated with iWUE (59.10%), and Ψpd was associated with the second component explaining 28.96% of the variability. The analysis suggested three genotype groups (Fig. 4b) which considered: (i) Ecg, Eg, and Eb that included stress-sensitive or less tolerant genotypes that showed larger or stronger morphological and/or physiological changes under water stress; (ii) Es, En2, Eng1, and Eng2 genotypes that showed moderate responses or were moderately tolerant to water stress, and showed the smaller physiological changes but allowing an increase in biomass; (iii) En1 Eng3 and Eng4 showed the most tolerant pattern, showing smaller physiological responses with the highest iWUE values maintaining plant growth under stress.
Principal components biplot analysis (PC1 and PC2) showing the contribution of morphological and physiological variables differentiating genotypes groups and the hierarchical clustering using Ward’s linkage with Bray–Curtis dissimilarity index for all Eucalyptus genotypes under the water stress (WS) regime
Discussion
Identification of water stress tolerant genotypes by functional traits
Is well known that water stress-tolerant genotypes may survive during long periods of water restriction by maintaining minimal growth (Eilmann and Rigling 2012; Pita-Barbosa et al. 2023). However, adaptation to shortages in soil water availability has been strongly related to plasticity and is expressed through morphological, physiological, and biochemical mechanisms (Chen et al. 2020; Corrêa et al. 2017). On this regard, morphological traits have been usually been considered as a baseline for comparison and pre-selection of genotypes susceptible to water stress (Gonçalves et al. 2017; Lucani et al. 2018). Silva et al. (2014b) found that stress-tolerant E. urograndis hybrids showed a higher survival rate by increasing their root:shoot ratio but maintaining height growth. Similarly, Chaves et al. (2003) concluded that drought-sensitive genotypes are characterized by showing an abrupt change in growth with significant increases in belowground biomass and reduction in leaf area as a protection mechanism to prevent dehydration. Our results showed similar morphological responses for stress tolerant genotypes that showed a reduction in the absolute growth rate, maintained growth and showed a notorious increase in the root: shoot ratio.
Physiological traits in our experiment also showed interesting responses among genotypes under water stress where differences in Ψpd and iWUE were the key physiological variables that explained experimental variation and final grouping of genotypes of similar response to water stress. Previous experiments have shown that soil/substrate water stress produces a reduction in photosynthetic rate followed by stomatal closure as a mechanism to avoid water loss through transpiration (Mueller et al. 2020; Saadaoui et al. 2017), but the magnitude and timing of this response has been shown to depend on taxa and/or genotype for Eucalyptus (Chaves et al. 2003; Coopman et al. 2008b; Mokotedi 2010). The Ψpd is considered an indicator of the tree water status of the soil prospected by roots (Silva et al. 2014a); it has been strongly correlated with the relative transpiration rate (Madhibha et al. 2013). Previous studies by Hakamada et al. (2017) highlighted that Eucalyptus genotypes with greater susceptibility to water stress showed lower Ψpd values as soil water limitation negatively affect growth and physiological activity. Our results are similar to those reported by Maseda and Fernández (2016) and Muller et al. (2020) for Eucalyptus genotypes at early stages of development where a strong correlation between mortality and Ψpd has been observed. This strong correlation has also been suggested to differentiate Eucalyptus genotypes response to water stress (Coopman et al. 2008b; Silva et al. 2017), where stress-tolerant genotypes show high stomatal closure sensitivity to changes affecting plant water. Similarly, iWUE has been considered as an indicator associated with resistance and growth of genotypes under water stress (Pita-Barbosa et al. 2023). Florencio et al. (2022) reported that tolerant genotypes showed higher iWUE under water stress conditions by enhancing photosynthetic assimilation metabolism that, in turns, allows to reduce the effect of stress by maintaining above- and below-ground plant growth. Consequently, stress tolerance of different genotypes is reflected in the net plant C balance (Booth 2013). Several authors have suggested that water stress reduces aboveground C by drastically reducing FNPP and eventually leaf fall as a mechanism of plant adaptation to drought. In the case of TBCF, changes in this flux may be observed for genetic materials that increase root biomass, a response that has been usually associated with the strategy of plant survival by increasing its physical capability to capture soil/substrate water resource (Amrutha et al. 2019; Coopman et al. 2008a; Duan et al. 2013; Maseda and Fernández 2016). Interestingly, our C flux and partition results at nursery stage are similar to those reported by Campoe et al. (2020) investigating Eucalyptus plantations response to a gradient of tropical and semitropical sites, where ANPP decreased considerably for drought-susceptible genotypes.
Implications for Eucalyptus plantations under climate change scenarios
Our results may require a long-term validation; however, may be considered a potential first step procedure for selecting genotypes at young ages that may adapt better to water stress conditions. It is estimated that given the current climate change scenario, 80% of the areas dedicated to Eucalyptus plantations will present moderate to severe water stress in the forthcoming decades (Martins et al. 2022). Therefore, is critical to develop strategies for predicting the match of highly improved genotypes to sites with increasing risk of prolonged droughts under climate change (Gonçalves et al. 2017). Previous studies have suggested that genotype selection should focus on productivity and consider phenotypic plasticity under water stress conditions and focus on efficiency in water use to create productive mosaics in which planted genotypes and silvicultural management also guarantee ecological and social water sustainability needs (Chaves et al. 2003; de Toledo Picoli et al. 2021; Saadaoui et al. 2017).
Early selection of genotypes may provide a strategy to reduce uncertainty in Eucalyptus’s survival and early productivity (Nóia Júnior et al. 2020). The first 24 months of development after establishment of new plantations is when individuals are more susceptible to water stress due to high competition for site space and resources (e.g., nutrients and light), and the root systems are not consolidated in depth so that drought events could affect survival and long-term productivity (Chaves et al. 2003; Elli et al. 2020). However, uncertainties exist in selecting genotypes at nursery or young ages (Mueller et al. 2020; Pita-Barbosa et al. 2023), as genotypes may show different long-term responses at different soil environments if unexpected genetic x environment interactions exist (de Toledo Picoli et al. 2021; McDowell et al. 2008), and also when intraspecific competition, before canopy closure, may enhance genetic x environment x silvicultural interactions (Boreham and Pallett 2009). Therefore, selecting genotypes at early stages may reduce some uncertainty in establishing Eucalyptus plantations under increasing drought risk climate change scenarios (Beltrame et al. 2012; Matusick et al. 2016). However, long-term experiments linking early genotype selection, based on physiological or morphological traits and the long-term maintenance of such adaptation mechanisms, are required to validate their tolerance to water stress and impacts on forest productivity.
Conclusions
Our results showed large differential response effects on morphological, physiological, and C balance of ten Eucalyptus genotypes under water stress. Reductions water availability showed contrasting effects on C flux and partition, but most genotypes reduced photosynthesis rates, transpiration, and stomatal conductivity in contrast to increasing iWUE. Interestingly, the magnitude of stress response in growth matched physiological adaptation mechanisms allowing to identify tolerant, intermediate, and sensitive genotypes groups characterized by their response pattern in iWUE and Ψpd. Identifying physiological traits that differentiate Eucalyptus genotypes at nursery or early stages of development may provide opportunities to maintain site productivity of commercial plantations growing at sites with frequent and/or longer drought events associated with climate change.
References
Amrutha S, Parveen ABM, Muthupandi M, Sivakumar V, Nautiyal R, Dasgupta MG (2019) Variation in morpho-physiological, biochemical and molecular responses of two Eucalyptus species under short-term water stress. Acta Bot Croat 78:125–134
Aspinwall MJ, Drake JE, Campany C, Vårhammar A, Ghannoum O, Tissue DT, Reich PB, Tjoelker MG (2016) Convergent acclimation of leaf photosynthesis and respiration to prevailing ambient temperatures under current and warmer climates in Eucalyptus tereticornis. New Phytol 212:354–367
Bahuguna RN, Jagadish KS (2015) Temperature regulation of plant phenological development. Environ Exp Bot 111:83–90
Beltrame R, Bisognin DA, Mattos BD, Cargnelutti Filho A, Haselein CR, Gatto DA, Santos GAd (2012) Silvicultural performance and early selection of clones from Eucalyptus hybrids. Pesq Agrop Brasileira 47:791–796
Booth TH (2013) Eucalypt plantations and climate change. For Ecol Manage 301:28–34
Boreham G, Pallett R (2009) The influence of tree improvement and cultural practices on the productivity of Eucalyptus plantations in temperate South Africa. South for: a J for Sci 71:85–93
Campoe OC, Stape JL, Laclau J-P, Marsden C, Nouvellon Y (2012) Stand-level patterns of carbon fluxes and partitioning in a Eucalyptus grandis plantation across a gradient of productivity, in Sao Paulo State, Brazil. Tree Physiol 32:696–706
Campoe OC, Alvares CA, Carneiro RL, Binkley D, Ryan MG, Hubbard RM, Stahl J, Moreira G, Moraes LF, Stape JL (2020) Climate and genotype influences on carbon fluxes and partitioning in Eucalyptus plantations. For Ecol Manage 475:118445
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239–264
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Chen X, Zhao P, Ouyang L, Zhu L, Ni G, Schäfer KV (2020) Whole-plant water hydraulic integrity to predict drought-induced Eucalyptus urophylla mortality under drought stress. For Ecol Manage 468:118179
Coopman RE, Jara JC, Bravo LA, Sáez KL, Mella GR, Escobar R (2008a) Changes in morpho-physiological attributes of Eucalyptus globulus plants in response to different drought hardening treatments. Electron J Biotechnol 11(2):30–39
Coopman RE, Jara JC, Bravo LA, Sáez KL, Mella GR, Escobar R (2008b) Changes in morpho-physiological attributes of Eucalyptus globulus plants in response to different drought hardening treatments. Electron J Biotechnol 11:30–39
Corrêa TR, de Toledo Picoli EA, de Souza GA, Conde SA, Silva NM, Lopes-Mattos KLB, de Resende MDV, Zauza EAV, Oda S (2017) Phenotypic markers in early selection for tolerance to dieback in Eucalyptus. Ind Crops Prod 107:130–138
de Toledo Picoli EA, de Resende MDV, Oda S (2021) Come hell or high water: breeding the profile of Eucalyptus tolerance to abiotic stress focusing water deficit. Plant Growth Stress Physiol. https://doi.org/10.1007/978-3-030-78420-1_5
Duan H, Amthor JS, Duursma RA, O’Grady AP, Choat B, Tissue DT (2013) Carbon dynamics of eucalypt seedlings exposed to progressive drought in elevated [CO2] and elevated temperature. Tree Physiol 33:779–792
Eilmann B, Rigling A (2012) Tree-growth analyses to estimate tree species drought tolerance. Tree Physiol 32:178–187
El- Sharkawy M (2014) Global warming: causes and impacts on agroecisistems productivity and food security with emphasis on cassava competitive advantage in the tropics/subtropics A. R.–1 Institute of experimental botany, Ed.) Photosynthetica. Int J Photosynth Res 52(2):161–178
Elli EF, Sentelhas PC, Bender FD (2020) Impacts and uncertainties of climate change projections on Eucalyptus plantations productivity across Brazil. For Ecol Manage 474:118365
Epron D, Nouvellon Y, Ryan MG (2012) Introduction to the invited issue on carbon allocation of trees and forests. Tree Physiol 32:639–643
Erice G, Louahlia S, Irigoyen JJ, Sanchez-Diaz M, Avice JC (2010) Biomass partitioning, morphology and water status of four alfalfa genotypes submitted to progressive drought and subsequent recovery. J Plant Physiol 167:114–120
Fagundes FFA, da Silva Reis FY, Martins FB (2021) A model for predicting the initial development of two native forest species under current and future climates. Environ Exp Bot 192:104662
Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell Environ 31:602–621
Florêncio GWL, Martins FB, Fagundes FFA (2022) Climate change on Eucalyptus plantations and adaptive measures for sustainable forestry development across Brazil. Ind Crops Prod 188:115538
Forrester D, Collopy J, Morris J (2010) Transpiration along an age series of Eucalyptus globulus plantations in southeastern Australia. For Ecol Manage 259(1754):1760
Giardina CP, Ryan MG (2002) Total belowground carbon allocation in a fast-growing Eucalyptus plantation estimated using a carbon balance approach. Ecosyst 5:487–499
Giardina CP, Ryan MG, Binkley D, Fownes JH (2003) Primary production and carbon allocation in relation to nutrient supply in a tropical experimental forest. Glob Change Biol 9:1438–1450
Gonçalves JLDM, Alvares CA, Higa AR, Silva LD, Alfenas AC, Stahl J, Ferraz SFDB, Lima WDP, Brancalion PHS, Hubner A, Bouillet JPD, Laclau JP, Nouvellon Y, Epron D (2013) Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For Ecol Manage 301:6–27
Gonçalves JL, Alvares CA, Rocha JH, Brandani CB, Hakamada R (2017) Eucalypt plantation management in regions with water stress. South for: J for Sci 79:169–183
Hakamada R, Hubbard RM, Ferraz S, Stape JL, Lemos C (2017) Biomass production and potential water stress increase with planting density in four highly productive clonal Eucalyptus genotypes. South for: J for Sci 79:251–257
Hodecker BER, Pita-Barbosa A, de Barros NF, Merchant A (2018) Water availability preceding long-term drought defines the tolerance of Eucalyptus to water restriction. New for 49:173–195
Hubbard RM, Stape J, Ryan MG, Almeida AC, Rojas J (2010) Effects of irrigation on water use and water use efficiency in two fast growing Eucalyptus plantations. For Ecol Manage 259:1714–1721
INFOR (2020) Anuario Forestal 2020. Chilean Statistical Yearbook Of Forestry 2020. Boletín Estadístico/Statistical Bulletin N° 174.
Lucani CJ, Brodribb TJ, Jordan G, Mitchell PJ (2018) Intraspecific variation in drought susceptibility in Eucalyptus globulus is linked to differences in leaf vulnerability. Funct Plant Biol 46:286–293
Ma F, Xu TT, Ji MF, Zhao CM (2014) Differential drought tolerance in tree populations from contrasting elevations. AoB PLANTS 6
Madhibha T, Murepa R, Musokonyi C, Gapare W (2013) Genetic parameter estimates for interspecific Eucalyptus hybrids and implications for hybrid breeding strategy. New for 44:63–84
Martins FB, Benassi RB, Torres RR, de Brito Neto FA (2022) Impacts of 1.5 C and 2 C global warming on Eucalyptus plantations in South America. Sci Total Environ 825:153820
Maseda PH, Fernández RJ (2016) Growth potential limits drought morphological plasticity in seedlings from six Eucalyptus provenances. Tree Physiol 36:243–251
Matusick G, Ruthrof KX, Fontaine JB, Hardy GESJ (2016) Eucalyptus forest shows low structural resistance and resilience to climate change-type drought. J Veg Sci 27:493–503
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
Mokotedi ME (2010) Physiological responses of Eucalyptus nitens× nitens under experimentally imposed water stress. South for 72:63–68
Mueller C, Hodecker BER, De Barros NF, & Merchant A (2020) A physiological approach for preselection of eucalyptus clones resistant to drought. iFor Biogeosci For, 13(1): 16.
Nóia Júnior RdS, Amaral GC, Pezzopane JEM, Fonseca MDS, Câmara da Silva AP, Xavier TMT (2020) Ecophysiological acclimatization to cyclic water stress in Eucalyptus. J for Res 31:797–806
Pita-Barbosa A, Oliveira LA, de Barros NF, Hodecker BER, Oliveira FS, Araújo WL, Martins SC (2023) Developing a Roadmap to Define a Potential Ideotype for Drought Tolerance in Eucalyptus. For Sci 69:101–114
Pommerening A, Muszta A (2015) Methods of modelling relative growth rate. For Ecosyst 2:5
Rodrigues GG, Silva LD, Nouvellon Y (2021) Production and carbon allocation in clonal Eucalyptus plantations under different planting spacings. For Ecol Manage 493:119249
Rubilar R, Allen H, Fox T, Cook R, Albaugh T, Campoe O (2018) Advances in silviculture of intensively managed plantations. Curr for Rep 4:1–12
Rubilar R, Hubbard R, Emhart V, Mardones O, Quiroga JJ, Medina A, Valenzuela H, Espinoza J, Burgos Y, Bozo D (2020) Climate and water availability impacts on early growth and growth efficiency of Eucalyptus genotypes: the importance of GxE interactions. For Ecol Manage 458:117763
Ryan MG (1991) A simple method for estimating gross carbon budgets for vegetation in forest ecosystems. Tree Physiol 9:255–266
Ryan MG, Stape JL, Binkley D, Fonseca S, Loos RA, Takahashi EN, Silva CR, Silva SR, Hakamada RE, Ferreira JM, Lima AMN, Gava JL, Leite FP, Andrade HB, Alves JM, Silva GGC (2010) Factors controlling Eucalyptus productivity: how water availability and stand structure alter production and carbon allocation. For Ecol Manage 259:1695–1703
Saadaoui E, Yahia KB, Dhahri S, Jamaa MLB, Khouja ML (2017) An overview of adaptative responses to drought stress in spp. Forest Stud 67:86–96
Silva BM, Silva ÉAd, Oliveira GCd, Ferreira MM, Serafim ME (2014a) Plant-available soil water capacity: estimation methods and implications. Rev Bras Ciênc Solo 38:464–475
Silva CD, Nascimento JS, Scarpinati EA, Paula RC (2014b) Classification of Eucalyptus urograndis hybrids under different water availability based on biometric traits. For Syst 23:209–215
Silva M, Rubilar R, Espinoza J, Yánez M, Emhart V, Quiroga JJ (2017) Respuesta en parámetros de intercambio gaseoso y supervivencia en plantas jóvenes de genotipos comerciales de Eucalyptus spp sometidas a déficit hídrico. Bosque (valdivia) 38:79–87
Stape JL, Binkley D, Ryan MG (2008) Production and carbon allocation in a clonal Eucalyptus plantation with water and nutrient manipulations. For Ecol Manage 255:920–930
Susiluoto S, Berninger F (2007) Interactions between morphological and physiological drought responses in Eucalyptus microtheca. Silva Fennica 41:221
Team RC (2021) R: a language and environment for statistical computing. Sci Rep 11:12957
Warren CR, Aranda I, Cano FJ (2011) Responses to water stress of gas exchange and metabolites in Eucalyptus and Acacia spp. Plant Cell Environ 34:1609–1629
Whitehead D, Beadle CL (2004) Physiological regulation of productivity and water use in Eucalyptus: a review. For Ecol Manage 193:113–140
Zhang D, Li J, Huang Y, Gao S, Zhang J (2022) Root-soil facilitation in mixed Eucalyptus grandis plantations including nitrogen-fixing species. For Ecol Manage 516:12021
Acknowledgements
Smurfit Kappa Colombia facilitated the MSc. scholarship for the first author. In addition, the Forestry Productivity Cooperative (FPC) and the Faculty of Forest Sciences of the University of Concepción (UdeC) provided staff, infrastructure, and acquired equipment for experiment development. Finally, Forestal Mininco SpA provided the Eucalyptus genotypes evaluated in this study.
Funding
This research was funded by the Chilean National Commission for Scientific and Technological Research FONDECYT Project Grant 1190835, ANID BASAL FB210015 (CENAMAD), the Forest Productivity Cooperative and Smurfit Kappa Colombia.
Author information
Authors and Affiliations
Contributions
LG participated in the study design, planning, data analysis and was the main responsible for experimental set-up, measurements, and manuscript writing. RR developed the core research project, design and planning of the experiment, guided data analyses, and revised the manuscript. JCV provided support on manuscript writing,analysis and revision of the manuscript. VE, and AM provided experimental material, funding for trial installation and genotypes information used in the experiment. LB, provided support on experimental measurements and DB participated in initial set-up and development of the experiment.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors give their informed consent to this publication and its content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
García, L.Y., Rubilar, R., Valverde, J.C. et al. Morphological, physiological and carbon balance response of Eucalyptus genotypes under water stress. New Forests 55, 441–457 (2024). https://doi.org/10.1007/s11056-023-09985-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-023-09985-7