Abstract
The exponential growth of nanotechnology has focused on therapeutic pursuits, notably for brain disorders. Nanocarriers have submicron particle sizes, typically around 500 nm. Over the past few years, interest in nanostructured lipid carriers as alternative pharmaceutical delivery systems has increased on both the scientific and commercial fronts. It belongs to a more recent incarnation of lipid nanoparticulate systems with a solid matrix and superior room temperature stability. More recently, they have also been used in gene therapy, cancer treatment, and brain targeting. In this review, the uses and significance of nanostructured lipid carriers (NLC) in combating brain diseases have been thoroughly covered, with examples from several recent research papers. A summary of NLCs’ present patent status has been provided to highlight their promising potential. Related patents and research reports associated with this topic are gathered and availed. The paper thoroughly summarizes NLCs and their application in different routes of administration. Drug administration directly to the brain via nose through NLCs is mainly focused. Nose-to-brain delivery has more benefits compared to the other routes. NLCs provide significant advantages such as targeted delivery, less toxicity, higher drug loading capacity due to imperfect structure, etc. However, the particle size of NLCs for intranasal drug delivery should be < 200 nm. Also, NLC is combined with a medical device to achieve the add-on benefits for delivering drugs effectively. According to the comprehensive review of literature and patents like CN110013471B, IN-MUM-2015-00667A, IN202021023420A, and AU2021104270A4, drug-loaded NLCs in nose-to-brain delivery show promising results. NLCs can increase bioavailability and reduce dosing frequency, indirectly reducing dose-related side effects. Intervening NLCs directly on the targeted site displays site specificity and many more benefits than conventional dosage forms. Thus, using NLCs in nose-to-brain drug delivery is invigorating.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
An overview of nanotechnology and nanocarriers
The still-evolving science of nanomedicine includes nanodelivery as a significant component. Nanomedicine is a multidisciplinary field incorporating elements of nanotechnology, chemistry, biochemistry, and pharmaceutical sciences [1]. The study of creating, characterizing, and using objects with at least one dimension on the “nano” scale is known as nanotechnology. In comparison to conventional medication delivery techniques, nanodistribution systems have some advantages. For example, they might be able to deliver medicines that target various body tissues with excellent specificity and treat various ailments. Since they should, in principle, prevent or significantly reduce drug interactions with non-target tissues, targeted therapy using nanodelivery systems could also reduce the side effects of many medications. As a direct result of fewer undesirable drug interactions, the amount of medication needed to treat the illness would need to be reduced, ultimately resulting in a cost reduction overall [2].
Nanocarriers are colloidal drug delivery devices with submicron particle sizes, typically around 500 nm. In the last few decades, nanocarriers have received much attention owing to their tremendous potential for drug delivery [3]. Since nanocarriers have a high surface-to-volume ratio, a medication’s basic properties and bioactivity can be altered. The use of nanocarriers in drug delivery systems has been shown to improve pharmacokinetics and biodistribution, decrease toxicities, boost solubility, and stability, provide controlled release, and deliver therapeutic molecules to targeted sites [4]. Nanocarriers’ physiochemical qualities (functional groups, PEGylation or other coatings, surface charge, inclusion of targeted molecules) as well as their sizes (large or small), shapes (spherical, rod, or cube), and surface features can also be altered. Nanocarriers are used in drug delivery to effectively cure a disease with little adverse effects, as shown in Fig. 1 [5].
Although not always, many different forms of nanoparticles (NPs) utilized as drug carriers typically comprised polymers or lipids. The lipid NPs outperform the polymeric NPs when the two are compared because polymeric NPs have several drawbacks, including cytotoxicity and the absence of effective mass production techniques. Solid lipid nanoparticles (SLNs), the first generation of lipid-based nanoparticles, were characterized in the early 1990s [6]. Due to polymorphism, drug-carrying SLNs were constrained by poor drug loading efficacy and a high probability of drug expulsion from the formulation during storage. As a next-generation SLN, nanostructured lipid carriers (NLCs) were developed in the late 1990s to overcome SLN formulation challenges [7]. Müller et al. created NLCs by combining solid lipids (SL) with liquid lipids (LL), which create an amorphous solid matrix at body and room temperatures. Since LL considerably improved the formulation’s characteristics compared to SLNs, incorporating LL into the matrix was the first and most crucial step. LL aided amorphous lattice formation with significant flaws in its solid crystalline matrix, enabling a higher drug load to be added, as opposed to SLNs, which form a solid crystalline matrix that restricts the quantity of drug loaded and causing its expulsion due to their spatial capacity [8]. According to numerous scientific studies, the NLC overcomes the SLN’s drawbacks by preventing drug expulsion during storage and enhancing stability and loading efficiency [9] [10].
Types of NLCs
NLC can be divided into three types based on the variations in lipid and oil volume fractions and the diverse manufacturing techniques: the amorphous type, the imperfect type, and the multiple oil-in-solid-fat-in-water (O/F/W) types [7]. In comparison to SLN, Fig. 2 shows the many forms of NLC.
Improper-type NLC includes mixing spatially dissimilar lipids, such as glycerides, which are made up of many fatty acids and introduce flaws in the crystal order. By combining a combination of different glycerides with differing saturation and carbon chain length levels, the amount of drug load can be additionally enhanced by expanding defects.
Specific SL, such as isopropyl myristate or hydroxyoctacosanyl hydroxy stearate, create an amorphous matrix lacking structure. The NLC thus exists in an amorphous state as opposed to an organized state, preventing drug ejection brought on by modification during storage [11]. Numerous liquid oil compartments of various sizes spread that throughout the solid matrix are present in multiple O/F/W-type NLCs. Drug loading is augmented because these nanosized compartments have better drug solubility. The release is also prolonged because a solid lipid matrix surrounds the compartments.
Nanostructured lipid carriers (NLCs)
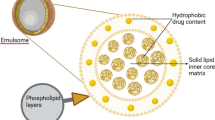
Due to the drug moieties’ improved solubility in the solid lipid, the combination of liquid and solid lipids makes it simple to encapsulate a variety of hydrophobic medicines. However, hydrophilic drug loading into NLC can be tricky and best accomplished by conjugating the functional groups of a matrix of lipids and drugs. Notably, NLC retained a solid state throughout the body [11]. This happens because the mixture of LL and SL tends to lower the core substance’s melting temperature without changing its physical attributes. NLCs are more stable than other nanocarriers and have lower toxicity while allowing for regulated or prolonged medication release [12]. They can also encapsulate both hydrophilic and lipophilic drug substances. Figure 3 shows a comparison between SLN and NLC.
Glycerol monostearate (GMS), Precirol® ATO5, stearic acid, mono-stearin, stearyl alcohol, cetyl palmitate, Witepsol®, and Gelucire® are examples of solid lipids that are frequently utilized in NLC formulation. Examples of liquid lipids include almond oil, oleic acid (OA), olive oil, sesame oil, soyabean oil, peanut oil, phosphatidylcholine, maize oil, soy lecithin, vitamin E, caprylic triglycerides, glyceryl caprylate/caprate, isopropyl myristate, etc. Poloxamer 188(P188), Polysorbate 80, Polysorbate 20, PVA21, Cremophor® RH, polyoxyethylene esters of 12-hydroxystearic acid, Cremophor® EL, Tego Care 450, Pluronic F68, Span® 85, and numerous other surfactants are also used to prepare NLC. Some of the LL, SL, and surfactants are shown in Figs. 4, 5, and 6, respectively.
Potentials of NLCs in drug delivery [13]
Figure 7 shows the potential of NLCs in drug delivery. Owing to the lipid matrix’s constitution, the concentration, and the irritative and sensitizing actions of the surfactants, there might be some cytotoxic effects. Gene delivery technologies and applications for protein- and peptide-based medications still have untapped potential. One must look for scale-up strategies, improper methods, and lipid stability. In the end, most study researchers are unable to extensively replicate the results because they are entrenched at the laboratory scale or in the research evidence throughout the preclinical or clinical stages. The application of NLC for the delivery of bio-actives like peptides, genes, proteins, ribonucleic acid (RNA), and deoxyribonucleic acid (DNA) should also be given serious consideration [13].
An emphasis on fabrication techniques of NLC
Numerous methods can be employed to produce NLCs. The high-pressure homogenization technique is the most frequently employed that combines high temperature and high pressure. Other is the homogenization approach using low temperatures and high pressure. Table 1 shows the processes that include solvent dispersion, high-temperature emulsion evaporation–low-temperature curing, ultrasonic emulsion evaporation, microtube methods, film-ultrasonic method, microemulsion, supercritical fluid (SCF), emulsion, membrane contactor, and microchannel [14]. Some of the preparation methods are explained below.
Melt emulsification method
SL is melted at 10 °C above its melting point, followed by adding LL. Then drug is added to the molten lipid phase. At the same temperature, the aqueous surfactant solution is also heated. The aqueous phase is drop-wise added into the lipid phase with constant stirring on a magnetic stirrer at a specific rpm. Then the mixture is sonicated and allowed to cool down at room temperature.
High-pressure homogenization (HPH)
High pressure (100–2000 bar) is used, which causes shear stress and the breakup of micro- to nanosized particles. Different cycles (10,000 rpm, 800 bar with 10–12 cycles) are carried out depending on the required particle size. Both the aqueous and lipid phases must homogenize at the same temperature to produce nanoparticles. NLC can be prepared using a hot and cold high-pressure homogenization process.
Solvent emulsification-evaporation technique
Using a high-speed homogenizer, a water-immiscible organic solvent dissolves hydrophobic drugs and lipophilic materials. Furthermore, an organic solvent is evaporated at room temperature by either gentle heating or mechanical stirring.
Solvent diffusion
Using ethanol and methanol, two water-miscible solvents, to dissolve the medication and lipid in a single solvent or a solvent mixture. At the same temperature and with mechanical stirring, the organic phase is introduced to the aqueous phase that already has stabilizers, surfactants, and other excipients. The mixture is then cooled to room temperature to allow the organic phase to evaporate.
Solvent injection
Through fine needle injection, lipids are first dissolved in a water-miscible solvent and then introduced to the aqueous phase. Avoiding highly complex mixtures like high-pressure homogenizers and probe sonicators is the main benefit of adopting this mixture [15].
Landscaping of NLCs in drug delivery
We have presented the comprehensive work done with NLCs’ drug delivery via different routes. Recently, NLCs have been used to deliver drugs to particular areas utilizing various administration methods. For instance, topical administration of NLCs is frequently used in dermatological and cosmetic treatments. Fungal infections are incredibly prevalent in the current situation [16] [17]. Over a billion people yearly receive diagnoses for severe systemic or localized fungal infections. Despite their effectiveness in treating fungal infections, antifungal medications are linked to severe side effects such as liver damage, estrogen levels, and allergic reactions [18]. In the skin, there will be effective drug targeting and increased skin absorption by the NLCs. Drug absorption in the human skin enhances by incorporating NLCs. NLCs can be prepared for major tropical diseases like gout, sunburn, rheumatoid arthritis, and inflammation-related skin diseases like atopic dermatitis and psoriasis. This gives rapid onset and intermediate action [19].
Tables 2 and 3 show the use of NLC in topical and oral drug delivery, respectively. Figures 8 and 9 show the graphical representation of Tables 2 and 3, respectively. Because of the poor water solubility and unfavorable physicochemical and pharmacokinetic characteristics of researchers’ identified chemical entities, over 40% of them used in the pharmaceutical sector have limited oral bioavailability. Therefore, producing a conventional formulation that can reduce the threats bound with lipophilic medications is challenging [40]. Researchers have tried many strategies to increase bioavailability, including synthesis of the pro drug, formation of salt, drug nanosizing, and drug encapsulation in nanocarriers such as liposomes, polymeric micelles, NPs, emulsions, etc. Drug delivery techniques incorporating lipids have provided a glimmer of hope for their positive impacts on the absorption of encapsulated pharmaceuticals throughout the past few decades [41].
Further, owing to their extended absorption in the gastrointestinal tract, oral treatment of NLCs for inflammatory bowel illnesses is the subject of substantial research. Regarding chemotherapy, anti-cancer medications like paclitaxel and doxorubicin have been encapsulated in NLCs to be administered via the pulmonary route [42]. As discussed above, it offers significant advantages compared to the other conventional formulations.
Brain
Figure 10 illustrates the complex anatomy of the brain. About 2% of body weight belongs to the brain, which also needs 25% of the total glucose and oxygen supply and 20% of the overall cardiac blood supply [61]. The defensive system’s three main layers are arachnoid, blood-cerebrospinal fluid, and blood–brain barriers (BBB). The brain is unique in that it has a highly complex network of neurons that communicate with each other via neurotransmitters and neuromodulators, as well as the presence of synaptic potential in such networks and a significant number of capillaries in the range of 100 billion. The intricate BBB effectively shields each of these components. The brain’s ability to transmit signals to other body parts relies on the BBB’s ongoing exchange of ions and other chemicals.
Brain and related disorders
The prevalence of neurodegenerative illnesses, defined by a continuous diminution in neuronal networks and functions, is highest in the age, although the actual reasons are unknown [62]. Central nervous system (CNS) ailments, such as brain tumors and neurological disorders, continue to rank among the leading causes of disability and fatality around the globe. Treatments for CNS illnesses are lacking despite an alarming rise in occurrence over the past few years. Limited medication accessibility to the intended brain location is one of the factors contributing to the high failure rates in CNS drug development. A drug must cross the BBB in a sufficient concentration and interact with a target in the brain to have a pharmacological effect.
The burden of neurological illnesses has significantly expanded during the last 25 years on a global scale. The Lancet Neurology released the 2015 Global Burden of Disease (GBD) survey report, which states that neurological diseases are the second highest cause of mortality internationally and the primary cause of disability-adjusted life years (DALYs) worldwide.
Only the early phases of a disease’s progression are shown to be responsive to currently available treatments. Most treatments only have symptomatic effects, meaning they treat the disease’s symptoms and do not address its underlying causes. As a result, they are ineffective at curing the disease. The primary reasons for this are the brain’s intricate structure and, more significantly, the BBB’s protective barrier, which prevents most drugs from entering the brain [63]. Therefore, researchers are developing novel approaches, nanocarrier systems, and drug delivery systems that precisely deliver active substances to the brain by bridging the BBB or any alternate route, such as intranasal drug delivery systems [11].
Blood brain barrier (BBB)
Drug distribution to the brain is greatly aided by the BBB, which divides the blood from the brain interstitial fluid (extracellular fluid). The cause is a vast network of blood capillaries, which permit the movement of substances from the blood to the brain parenchyma with an average surface area of 100 to 200 cm2 per gram of adult human brain. Although the BBB’s selectivity is essential for preserving brain homeostasis, it presents a significant obstacle to the delivery of medicines into tumor areas. Passive diffusion via the extracellular matrix is significantly hampered by tight junctions that form between endothelial cells [64].
Active moiety/nanocarrier transportation from the nose to the brain
According to the current study, the nose-to-brain channel offers a viable method for the non-invasive delivery of therapeutic agents or nanocarriers into the brain without crossing the BBB. The olfactory pathway, trigeminal nerve pathway, and systemic pathway are three transport mechanisms involved, shown in Fig. 11.
Nose-to-brain drug delivery
An intriguing method for delivering medicine directly to the brain via the nose is the “nose-to-brain” drug delivery device. The olfactory area is directly connected to the CNS due to the olfactory receptors in neurons and their axons, which end in the olfactory bulb. The olfactory region is the sole area of the body where the CNS interacts with the surrounding environment. The active molecules can immediately enter the brain by the trigeminal and olfactory nerve pathways from the olfactory area, which is a crucial location. When medicine or formulation is delivered, it comes into contact with mucosa and travels straight to the brain, avoiding the BBB. This results in good bioavailability, a lower dose, and fewer side effects. Although nasal medication delivery generates research interest, it has certain drawbacks, such as a small dosage range (25–200 µL), mucociliary clearance, and nasal enzymatic barriers. These discourage scientists from creating cutting-edge drug delivery technology to overcome these constraints. The underlying benefit of the nose to brain medication delivery is that this is a quick, secure, non-invasive, and practical drug delivery approach [65]. It prevents the breakdown of medications in the digestive tract, especially peptide medicines [66].
Additionally, it prevents the gut wall and hepatic first-pass metabolism of medicines, resulting in increased bioavailability. Bypassing the BBB provides CNS-targeted drug delivery, lowering systemic exposure to treatments and their accompanying systemic adverse effects. No modifications are necessary for the therapeutic agent using a nose-to-brain medication delivery platform. Rapid drug absorption and speedy commencement of an action through the highly vascularized and permeable nasal mucosa are essential advantages of nose-to-brain transfer [67]. This improves patient compliance and provides a different method of parenteral delivery, particularly for medications containing proteins and peptides or even stem cells. It has good bioavailability for medicines with low molecular weight [68].
NLCs in nose-to-brain drug delivery
NLC is a combination of SL, LL, and surfactant. It can be prepared by the methods enlisted in Table 1. Some of the principles can be considered to develop NLCs for efficient brain drug delivery further, as shown in Fig. 12. Limiting the influences of protein corona/biological molecule corona/nutrient molecule corona is crucial for nose-to-brain drug delivery, as these coronas can alter the behavior and fate of nanocarriers and affect their therapeutic efficacy. It can be done through surface modification, shealth coatings, surface charge optimization, preconditioning strategies, engineered surface ligands, corona modulation agents, biological fluid mimicking media, in vitro and in vivo screenings, etc. By employing these strategies, it is possible to minimize the influences of the protein corona, biological molecule corona, or nutrient molecule corona and enhance the effectiveness of nose-to-brain drug delivery. Table 4 and Fig. 13 display the research done in NLCs for nose-to-brain drug delivery.
Modish stratagem of NLCs in drug delivery
Further, to develop better NLCs, we can also do PEGylation or surface modification on NLCs to enhance the drug-loaded carrier’s effectiveness [92] [93]. Combination devices, or those that include both functional prosthetic implants and drug-releasing components, are versatile, developing clinical technology that has the potential to enhance the functionality of implant devices across a variety of classes. Formulation techniques are insufficient to utilize this route to deliver human drugs. To target formulation deposition in the nasal passage’s olfactory region and circumvent its architecture limitations, novel technologies are currently being developed. The Vianase™ was used to administer insulin intranasally in one of the few experiments on brain delivery via the nose in humans. Kurve Technology® created the Vianase™ electronic atomizer, comprising a nebulizer coupled to a vortex chamber. The vortex chamber, where the nebulized drug particles move, maintains this flow after the particles have left the device. Increased transit to the brain encourages deposition to the olfactory area [94]. The necessity for additional administration routes for new medications and to address various targets is driving the growth of the nasal drug delivery industry. A non-invasive drug delivery method for both localized and systemic effects is aerosolization. The formulation will likely reach the olfactory area and achieve direct brain targeting if deeper nasal penetration beyond the nasal valve can be accomplished [95]. Table 5 shows the comprehensive work done in NLCs with the combination of the medical device to deliver drugs at different target sites.
The medium- and long-term fate of nanoparticles is likely to depend on several factors, including tissue distribution, clearance from the body, potential toxicity, metabolism, biodegradation, and environmental factors, including their stability, associations with other environmental elements, and exposure circumstances. It is still emerging, and continuous studies are geared at better comprehending their behavior and possible threats and creating techniques to lessen negative impacts.
Patent search
The patent search was carried out using the licensed version of the PatSeer® PRO database using search strings shown in Table 6. Researchers have thoroughly worked on the synthesis and solubility of the NLCs. Here we have presented formulation-based patents, including formulations with enhanced drug solubility and loading capacity, improved encapsulation efficiency, lowered dose frequency, in vitro studies, etc., as shown in Table 7. This type of literature suggests that these formulations have achieved targeted delivery of drugs and thus lessened side effects related to drug and dose.
Conclusion
With better drug incorporation and enhanced bioavailability, NLCs are chemically and physically stable systems. In recent years, improvements have been significantly accelerated by the industry’s growing interest in lipid carrier systems. Currently, the industry offers more than 30 commercial NLC formulations with ingredients for drugs and cosmetics. NLCs can increase bioavailability and reduce dosing frequency, indirectly reducing dose-related side effects. Intervening NLCs directly on the targeted site displays site specificity and many more benefits than conventional dosage forms. Thus, the use of NLCs in nose-to-brain drug delivery is invigorating. NLCs provide advantageous aerosolization properties and practical stability for pulmonary use. Additionally, they can get past local defenses and gather in the lung. NLCs can enter the brain by furnishing the surface of the formidable barrier enclosing it, and they can then pass the BBB by receptor-mediated transcytosis. Given the recent increase in patent filings, NLCs should have the same opportunity for accurate clinical translation and pharmaceutical marketing in all cases. Success in this field can be imagined if the pharmaceutical business takes academic study to design this carrier system for diverse therapeutic and cosmetic agents.
Abbreviations
- NLCs :
-
Nanostructured lipid carriers
- NPs :
-
Nanoparticles
- SLNs :
-
Solid lipid nanoparticles
- PNPs :
-
Polymeric nanoparticles
- SL :
-
Solid lipid
- LL :
-
Liquid lipids
- GMS :
-
Glyceryl monostearate
- OA :
-
Oleic acid
- P188 :
-
Poloxamer 188
- RNA :
-
Ribonucleic acid
- DNA :
-
Deoxyribonucleic acid
- SCF :
-
Supercritical fluid
- PS :
-
Particle size
- ZP :
-
Zeta potential
- PDI :
-
Polydispersity index
- %EE :
-
% Entrapment efficiency
- P407 :
-
Poloxamer 407
- AUC :
-
Area under curve
- BBB :
-
Blood-brain barrier
- CNS :
-
Central nervous system
- GBD :
-
Global burden of disease
- DALYs :
-
Disability-adjusted life years
- TFM :
-
Teriflunomide
- TNLCGHG :
-
Teriflunomide loaded nanolipid carrier–carbopol-gellan gum in situ gel
- TNLC :
-
Teriflunomide loaded nanolipid carrier
- 99m TC :
-
Technetium
- DTE :
-
Drug targeting efficiency
- DTP :
-
Drug transport percentage
- ALM :
-
Almotriptan maleate
- IN-ALM-NLC :
-
Intranasal almotriptan maleate NLC
- TFM-MNLC :
-
TFM mucoadhesive NLC
- OLZ :
-
Olanzapine
- OLZ-MNLC (P + H) :
-
OLZ mucoadhesive NLC (Poloxamer 407 and HPMC K4M)
- OLZ-MNLC (C) :
-
OLZ mucoadhesive NLC (Carbopol 974P)
- IN-NLC :
-
Intranasal NLC
- IV-NLC :
-
Intravenous NLC
- DLX :
-
Duloxetine
- ARM :
-
Artemether
- ARM-SOL :
-
Artemether solution
- FPF :
-
Fine particle fraction
- FPD :
-
Fine particle dose
- MMAD :
-
Mass median aerodynamic diameter
- DPI :
-
Dry powder inhaler
- MNLC :
-
Montelukast NLC
- CIP :
-
Ciprofloxacin hydrochloride
- NAC :
-
N-acetyl cysteine
References
Liu Y, Wen H, Wu X, Wu M, Liu L, Wang J, … Dan M (2021) The bio-persistence of reversible inflammatory, histological changes and metabolic profile alterations in rat livers after silver/gold nanorod administration. Nanomaterials 11(10). https://doi.org/10.3390/nano11102656
Ayub A, Wettig S (2022) An overview of nanotechnologies for drug delivery to the brain. Pharmaceutics. https://doi.org/10.3390/pharmaceutics14020224
Gaté L, Disdier C, Cosnier F, Gagnaire F, Devoy J, Saba, W, … Mabondzo A (2017) Biopersistence and translocation to extrapulmonary organs of titanium dioxide nanoparticles after subacute inhalation exposure to aerosol in adult and elderly rats. Toxicol Lett 265:61–69. https://doi.org/10.1016/j.toxlet.2016.11.009
Patel AA, Patel RJ, Mishra P (2021) Nanosuspension for oral delivery of tadalafil: pharmacodynamic and pharmacokinetic studies. J Drug Deliv Sci Technol. 61:102203. https://doi.org/10.1016/j.jddst.2020.102203
Din FU, Aman W, Ullah I, Qureshi OS, Mustapha O, Shafique S, Zeb A (2017) Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomedicine. https://doi.org/10.2147/IJN.S146315
Prajapati JB, Katariya H, Patel R (2018) Peyer’e patch targeting of Isradipine loaded solid lipid nanoparticles: it’s cellular uptake study. J Drug Deliv Sci Technol 43:318–326. https://doi.org/10.1016/j.jddst.2017.10.017
Khosa A, Reddi S, Saha RN (2018) Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2018.04.055
Haider M, Abdin SM, Kamal L, Orive G (2020) Nanostructured lipid carriers for delivery of chemotherapeutics: a review. Pharmaceutics. https://doi.org/10.3390/pharmaceutics12030288
Patel RJ, Patel ZP (2013) Formulation optimization and evaluation of nanostructured lipid carriers containing valsartan. Int J Pharm Sci Nanotechnol 6(2):2077–2086. https://doi.org/10.37285/ijpsn.2013.6.2.10
Das S, Ng WK, Tan RBH (2012) Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur J Pharm Sci 47(1):139–151. https://doi.org/10.1016/j.ejps.2012.05.010
Agrawal M, Saraf S, Saraf S, Dubey SK, Puri A, Patel RJ, … Alexander A (2020) Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. J Control Release. https://doi.org/10.1016/j.jconrel.2020.02.020
Agrawal M, Saraf S, Pradhan M, Patel RJ, Singhvi G, Ajazuddin Alexander A (2021) Design and optimization of curcumin loaded nano lipid carrier system using Box-Behnken design. Biomed Pharmacother 141. https://doi.org/10.1016/j.biopha.2021.111919
Chauhan I, Yasir M, Verma M, Singh AP (2020) Nanostructured lipid carriers: a groundbreaking approach for transdermal drug delivery. Adv Pharm Bull. https://doi.org/10.34172/apb.2020.021
Li Q, Cai T, Huang Y, Xia X, Cole SPC, Cai Y (2017) A review of the structure, preparation, and application of NLCs, PNPs, and PLNs. Nanomaterials. https://doi.org/10.3390/nano7060122
Tekade AR, Mittha PS, Pisal CS (2022) Nanostructured lipid carriers for nose to brain delivery targeting CNS: diversified role of liquid lipids for synergistic action. Adv Pharm Bull. https://doi.org/10.34172/apb.2022.078
Patel R, Yadav BK, Patel G (2022) Progresses in nano-enabled platforms for the treatment of vaginal disorders. Recent Pat Nanotechnol 17(3):208–227. https://doi.org/10.2174/1872210516666220628150447
Patel R, Shah R, Patel A, Hadiya K, Parmar J, Patel G (2023) Off-Label use of Raloxifene hydrochloride in uterine fibroids: a novel insert-based formulation approach and IN-VIVO preclinical evaluation. J Drug Deliv Sci Technol 84:104552. https://doi.org/10.1016/j.jddst.2023.104552
Gaba B, Fazil M, Khan S, Ali A, Baboota S, Ali J (2015) Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull Fac Pharmacy, Cairo Univ 53(2):147–159. https://doi.org/10.1016/j.bfopcu.2015.10.001
Chandana KV, Gupta NV, Kanna S (2019) Nanostructured lipid carriers: the frontiers in drug delivery. Asian J Pharm Clin Res 8–12. https://doi.org/10.22159/ajpcr.2019.v12i7.33595
Samprasit W, Vasarach C, Opanasopit P, Sriamornsak P, Chamsai B (2022) Topical nanostructured lipid carriers of alpha-mangostin and resveratrol for synergistic antioxidant activity. Pharm Nanotechnol 10. https://doi.org/10.2174/2211738510666220426112508
Mahmood A, Rapalli VK, Gorantla S, Waghule T, Singhvi G (2022) Dermatokinetic assessment of luliconazole-loaded nanostructured lipid carriers (NLCs) for topical delivery: QbD-driven design, optimization, and in vitro and ex vivo evaluations. Drug Deliv Transl Res 12(5):1118–1135. https://doi.org/10.1007/s13346-021-00986-7
Oliveira PM, Alencar-Silva T, Pires FQ, Cunha-Filho M, Gratieri T, Carvalho JL, Gelfuso GM (2022) Nanostructured lipid carriers loaded with an association of minoxidil and latanoprost for targeted topical therapy of alopecia. Eur J Pharm Biopharm 172:78–88. https://doi.org/10.1016/j.ejpb.2022.02.003
Wu B, Li Y, Li YY, Shi ZH, Bian XH, Xia Q (2022) Nanostructured-lipid carriers-chitosan hydrogel beads carrier system for loading of resveratrol: a new method of topical application. J Biomater Appl 36(8):1444–1457. https://doi.org/10.1177/08853282211053923
Sharma N, Kumar S, Joshi G, Choudhary D (2021) Formulation and characterization of febuxostat loaded nanostructured lipid carriers (NLCs) - gel for topical treatment of gout. Recent Pat Nanotechnol 16(3):250–258. https://doi.org/10.2174/1872210515666210415114118
Rajpoot K, Prajapati SK, Malaiya A, Jain R, Jain A (2022) Meropenem-loaded nanostructured lipid carriers for skin and soft tissue infection caused by Staphylococcus aureus: formulation, design, and evaluation. AAPS PharmSciTech 23(7):241. https://doi.org/10.1208/s12249-022-02381-y
Ahmad Nasrollahi S, Koohestani F, Naeimifar A, Samadi A, Vatanara A, Firooz A (2021). Preparation and evaluation of adapalene nanostructured lipid carriers for targeted drug delivery in acne. Dermatol Ther 34(2). https://doi.org/10.1111/dth.14777
Patil A, Tuencar V, Gadad A, Dandagi P, Masareddy R (2021) Nanostructured lipid carrier-incorporated gel for efficient topical delivery of fluconazole. Ther Deliv 12(8):565–574. https://doi.org/10.4155/tde-2021-0029
Pereira MN, Tolentino S, Pires FQ, Anjos JLV, Alonso A, Gratieri T, … Gelfuso GM (2021) Nanostructured lipid carriers for hair follicle-targeted delivery of clindamycin and rifampicin to hidradenitis suppurativa treatment. Colloids Surf B Biointerfaces 197. https://doi.org/10.1016/j.colsurfb.2020.111448
Rapalli VK, Sharma S, Roy A, Singhvi G (2021) Design and dermatokinetic evaluation of Apremilast loaded nanostructured lipid carriers embedded gel for topical delivery: a potential approach for improved permeation and prolong skin deposition. Colloids Surf B Biointerfaces 206. https://doi.org/10.1016/j.colsurfb.2021.111945
Cheon SH, Park SY, Sung J-H, Lee JG, Choi S-H, Jang J-W, … Jang D-J (2021) Preparation and evaluation of nanostructured lipid carrier for topical delivery of velutin: synthetic tyrosinase inhibitor. J Nanosci Nanotechnol 21(7):4093–4097. https://doi.org/10.1166/jnn.2021.19173
Youshia J, Kamel AO, El Shamy A, Mansour S (2021) Gamma sterilization and in vivo evaluation of cationic nanostructured lipid carriers as potential ocular delivery systems for antiglaucoma drugs. Eur J Pharm Sci 163. https://doi.org/10.1016/j.ejps.2021.105887
Shaaban M, Nasr M, Tawfik AA, Fadel M, Sammour O (2021) Bergamot oil as an integral component of nanostructured lipid carriers and a photosensitizer for photodynamic treatment of vitiligo: characterization and clinical experimentation. Expert Opin Drug Deliv 18(1):139–150. https://doi.org/10.1080/17425247.2021.1844180
Khezri K, Saeedi M, Morteza-Semnani K, Akbari J, Hedayatizadeh-Omran A (2021) A promising and effective platform for delivering hydrophilic depigmenting agents in the treatment of cutaneous hyperpigmentation: kojic acid nanostructured lipid carrier. Artif Cells, Nanomed Biotechnol 49(1):38–47. https://doi.org/10.1080/21691401.2020.1865993
Rapalli VK, Kaul V, Waghule T, Gorantla S, Sharma S, Roy A, … Singhvi G (2020) Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur J Pharm Sci 152. https://doi.org/10.1016/j.ejps.2020.105438
Madan JR, Khobaragade S, Dua K, Awasthi R (2020) Formulation, optimization, and in vitro evaluation of nanostructured lipid carriers for topical delivery of Apremilast. Dermatol Ther 33(3). https://doi.org/10.1111/dth.13370
Ammar HO, Ghorab MM, Mostafa DM, Abd El-Alim SH, Kassem AA, Salah S, Shalaby ES (2020) Development of folic acid–loaded nanostructured lipid carriers for topical delivery: preparation, characterisation and ex vivo investigation. J Microencapsul 37(5):366–383. https://doi.org/10.1080/02652048.2020.1761904
Geng Q, Zhao Y, Wang L, Xu L, Chen X, Han J (2020) Development and evaluation of astaxanthin as nanostructure lipid carriers in topical delivery. AAPS PharmSciTech 21(8). https://doi.org/10.1208/s12249-020-01822-w
Na YG, Huh HW, Kim MK, Byeon JJ, Han MG, Lee HK, Cho CW (2020) Development and evaluation of a film-forming system hybridized with econazole-loaded nanostructured lipid carriers for enhanced antifungal activity against dermatophytes. Acta Biomater 101:507–518. https://doi.org/10.1016/j.actbio.2019.10.024
Kesharwani P, Jain A, Srivastava AK, Keshari MK (2020) Systematic development and characterization of curcumin-loaded nanogel for topical application. Drug Dev Ind Pharm 1443–1457. https://doi.org/10.1080/03639045.2020.1793998
Patel P, Patel M (2020) Nanostructured lipid carriers- a versatile carrier for oral delivery of lipophilic drugs. Recent Pat Nanotechnol 15(2):154–164. https://doi.org/10.2174/1872210514666200909154959
Poonia N, Kharb R, Lather V, Pandita D (2016) Nanostructured lipid carriers: versatile oral delivery vehicle. Futur Sci OA. https://doi.org/10.4155/fsoa-2016-0030
Subramaniam B, Siddik ZH, Nagoor NH (2020) Optimization of nanostructured lipid carriers: understanding the types, designs, and parameters in the process of formulations. J Nanoparticle Res. https://doi.org/10.1007/s11051-020-04848-0
Alam T, Ansari MA, Baboota S, Ali J (2022) Nanostructured lipid carriers of isradipine for effective management of hypertension and isoproterenol induced myocardial infarction. Drug Deliv Transl Res 12(3):577–588. https://doi.org/10.1007/s13346-021-00958-x
Kataria D, Zafar A, Ali J, Khatoon K, Khan S, Imam SS, … Ali A (2022) Formulation of lipid-based nanocarriers of lacidipine for improvement of oral delivery: Box-Behnken design optimization, in vitro, ex vivo, and preclinical assessment. Assay Drug Dev Technol 20(1):5–21. https://doi.org/10.1089/adt.2021.084
Zewail M, EL-Deeb NM, Mousa MR, Abbas H (2022) Hyaluronic acid coated teriflunomide (A771726) loaded lipid carriers for the oral management of rheumatoid arthritis. Int J Pharm 623:121939. https://doi.org/10.1016/j.ijpharm.2022.121939
Gurumukhi VC, Bari SB (2022) Quality by design (QbD)–based fabrication of atazanavir-loaded nanostructured lipid carriers for lymph targeting: bioavailability enhancement using chylomicron flow block model and toxicity studies. Drug Deliv Transl Res 12(5):1230–1252. https://doi.org/10.1007/s13346-021-01014-4
Sartaj A, Annu AM, Biswas L, Yar MS, Mir SR, ... Ali J (2022) Combinatorial delivery of ribociclib and green tea extract mediated nanostructured lipid carrier for oral delivery for the treatment of breast cancer synchronising in silico, in vitro, and in vivo studies. J Drug Target.https://doi.org/10.1080/1061186X.2022.2104292
Jitta SR, Bhaskaran NA, Salwa, Kumar L (2022) Anti-oxidant containing nanostructured lipid carriers of ritonavir: development, optimization, and in vitro and in vivo evaluations. AAPS PharmSciTech 23(4). https://doi.org/10.1208/s12249-022-02240-w
Sharma T, Katare OP, Jain A, Jain S, Chaudhari D, Borges B, Singh B (2021) QbD-steered development of biotin-conjugated nanostructured lipid carriers for oral delivery of chrysin: role of surface modification for improving biopharmaceutical performance. Colloids Surf B Biointerfaces 197. https://doi.org/10.1016/j.colsurfb.2020.111429
Sharma M, Chaudhary D (2021) Exploration of bromelain laden nanostructured lipid carriers: an oral platform for bromelain delivery in rheumatoid arthritis management. Int J Pharm 594. https://doi.org/10.1016/j.ijpharm.2020.120176
Saghafi Z, Mohammadi M, Mahboobian MM, Derakhshandeh K (2021) Preparation, characterization, and in vivo evaluation of perphenazine-loaded nanostructured lipid carriers for oral bioavailability improvement. Drug Dev Ind Pharm 47(3):509–520. https://doi.org/10.1080/03639045.2021.1892745
Elkomy MH, Elmowafy M, Shalaby K, Azmy AF, Ahmad N, Zafar A, Eid HM (2021) Development and machine-learning optimization of mucoadhesive nanostructured lipid carriers loaded with fluconazole for treatment of oral candidiasis. Drug Dev Ind Pharm 47(2):246–258. https://doi.org/10.1080/03639045.2020.1871005
Agarwal S, HariKumar SL, Negi P, Upadhyay N, Garg R (2020) Quetiapine fumarate loaded nanostructured lipid carrier for enhancing oral bioavailability: design, development and pharmacokinetic assessment. Curr Drug Deliv 18(2):184–198. https://doi.org/10.2174/1567201817999200728135119
Patel P, Patel M (2021). Enhanced oral bioavailability of nintedanib esylate with nanostructured lipid carriers by lymphatic targeting: in vitro, cell line and in vivo evaluation. Eur J Pharm Sci 159. https://doi.org/10.1016/j.ejps.2021.105715
Khan AS, ud Din F, Ali Z, Bibi M, Zahid F, Zeb A, … Khan GM (2021) Development, in vitro and in vivo evaluation of miltefosine loaded nanostructured lipid carriers for the treatment of cutaneous Leishmaniasis. Int J Pharm 593. https://doi.org/10.1016/j.ijpharm.2020.120109
Zhu Y, Liang X, Lu C, Kong Y, Tang X, Zhang Y, … He H (2020) Nanostructured lipid carriers as oral delivery systems for improving oral bioavailability of nintedanib by promoting intestinal absorption. Int J Pharm 586. https://doi.org/10.1016/j.ijpharm.2020.119569
Soni NK, Sonali LJ, Singh A, Mangla B, Neupane YR, Kohli K (2020). Nanostructured lipid carrier potentiated oral delivery of raloxifene for breast cancer treatment. Nanotechnology 31(47). https://doi.org/10.1088/1361-6528/abaf81
Pyo YC, Tran P, Kim DH, Park JS (2020) Chitosan-coated nanostructured lipid carriers of fenofibrate with enhanced oral bioavailability and efficacy. Colloids Surf B Biointerfaces 196. https://doi.org/10.1016/j.colsurfb.2020.111331
Dong Z, Iqbal S, Zhao Z (2020). Preparation of ergosterol-loaded nanostructured lipid carriers for enhancing oral bioavailability and antidiabetic nephropathy effects. AAPS PharmSciTech 21(2). https://doi.org/10.1208/s12249-019-1597-3
Tirumalesh C, Suram D, Dudhipala N, Banala N (2020) Enhanced pharmacokinetic activity of zotepine via nanostructured lipid carrier system in Wistar rats for oral application. Pharm Nanotechnol 8(2):148–160. https://doi.org/10.2174/2211738508666200225113359
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. https://doi.org/10.1038/nrn3114
Fonseca LC, Lopes JA, Vieira J, Viegas C, Oliveira CS, Hartmann RP, Fonte P (2021) Intranasal drug delivery for treatment of Alzheimer’s disease. Drug Deliv Transl Res 11(2):411–425. https://doi.org/10.1007/s13346-021-00940-7
Patel RJ, Parikh RH (2020) Intranasal delivery of topiramate nanoemulsion: pharmacodynamic, pharmacokinetic and brain uptake studies. Int J Pharm 585. https://doi.org/10.1016/j.ijpharm.2020.119486
Reddy S, Tatiparti K, Sau S, Iyer AK (2021) Recent advances in nano delivery systems for blood-brain barrier (BBB) penetration and targeting of brain tumors. Drug Discov Today. https://doi.org/10.1016/j.drudis.2021.04.008
Parikh RH, Patel RJ (2017) Nanoemulsions for intranasal delivery of riluzole to improve brain bioavailability: formulation development and pharmacokinetic studies. Curr Drug Deliv 13(7):1130–1143. https://doi.org/10.2174/1567201813666151202195729
Agrawal M, Pradhan M, Singhvi G, Patel R, Ajazuddin, Alexander A (2022) Thermoresponsive in situ gel of curcumin loaded solid lipid nanoparticle: design, optimization and in vitro characterization. J Drug Deliv Sci Technol 71. https://doi.org/10.1016/j.jddst.2022.103376
Patel AA, Patel RJ, Patel SR (2017) Nanomedicine for intranasal delivery to improve brain uptake. Curr Drug Deliv 14. https://doi.org/10.2174/1567201814666171013150534
Pardeshi CV, Belgamwar VS (2013) Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: an excellent platform for brain targeting. Expert Opin Drug Deliv. https://doi.org/10.1517/17425247.2013.790887
Cunha S, Swedrowska M, Bellahnid Y, Xu Z, Sousa Lobo JM, Forbes B, Silva AC (2022) Thermosensitive in situ hydrogels of rivastigmine-loaded lipid-based nanosystems for nose-to-brain delivery: characterisation, biocompatibility, and drug deposition studies. Int J Pharm 620:121720. https://doi.org/10.1016/j.ijpharm.2022.121720
Mathure D, Ranpise H, Awasthi R, Pawar A (2022) Formulation and characterization of nanostructured lipid carriers of rizatriptan benzoate-loaded in situ nasal gel for brain targeting. Assay Drug Dev Technol 20(5):211–224. https://doi.org/10.1089/adt.2022.044
Markova E, Taneska L, Kostovska M, Shalabalija D, Mihailova L, Glavas Dodov M, … Simonoska Crcarevska M (2022) Design and evaluation of nanostructured lipid carriers loaded with Salvia officinalis extract for Alzheimer’s disease treatment. J Biomed Mater Res-Part B Appl Biomater 110(6):1368–1390. https://doi.org/10.1002/jbm.b.35006
Gadhave D, Rasal N, Sonawane R, Sekar M, Kokare C (2021) Nose-to-brain delivery of teriflunomide-loaded lipid-based carbopol-gellan gum nanogel for glioma: pharmacological and in vitro cytotoxicity studies. Int J Biol Macromol 167:906–920. https://doi.org/10.1016/j.ijbiomac.2020.11.047
Kaur S, Nautiyal U, Chawla PA, Chawla V (2021) Nanostructured lipid carriers for intranasal administration of olanzapine in the management of schizophrenia. Curr Mol Pharmacol 14(3):439–447. https://doi.org/10.2174/1874467214666210120160016
Gautam D, Singh S, Maurya P, Singh M, Kushwaha S, Saraf SA (2021) Appraisal of nano-lipidic astaxanthin cum thermoreversible gel and its efficacy in haloperidol induced Parkinsonism. Curr Drug Deliv 18(10):1550–1562. https://doi.org/10.2174/1567201818666210510173524
Masjedi M, Azadi A, Heidari R, Mohammadi-Samani S (2020) Nose-to-brain delivery of sumatriptan-loaded nanostructured lipid carriers: preparation, optimization, characterization and pharmacokinetic evaluation. J Pharm Pharmacol 72(10):1341–1351. https://doi.org/10.1111/jphp.13316
Salem LH, El-Feky GS, Fahmy RH, El Gazayerly ON, Abdelbary A (2020) Coated lipidic nanoparticles as a new strategy for enhancing nose-to-brain delivery of a hydrophilic drug molecule. J Pharm Sci 109(7):2237–2251. https://doi.org/10.1016/j.xphs.2020.04.007
Du W, Li H, Tian B, Sai S, Gao Y, Lan T, … Ding C (2019) Development of nose-to-brain delivery of ketoconazole by nanostructured lipid carriers against cryptococcal meningoencephalitis in mice. Colloids Surf B Biointerfaces 183. https://doi.org/10.1016/j.colsurfb.2019.110446
Gadhave DG, Kokare CR (2019) Nanostructured lipid carriers engineered for intranasal delivery of teriflunomide in multiple sclerosis: optimization and in vivo studies. Drug Dev Ind Pharm 45(5):839–851. https://doi.org/10.1080/03639045.2019.1576724
Gadhave DG, Tagalpallewar AA, Kokare CR (2019) Agranulocytosis-protective olanzapine-loaded nanostructured lipid carriers engineered for CNS delivery: optimization and hematological toxicity studies. AAPS PharmSciTech 20(1). https://doi.org/10.1208/s12249-018-1213-y
Mishra N, Sharma S, Deshmukh R, Kumar A, Sharma R (2018) Development and characterization of nasal delivery of selegiline hydrochloride loaded nanolipid carriers for the management of Parkinson’s disease. Cent Nerv Syst Agents Med Chem 19(1):46–56. https://doi.org/10.2174/1871524919666181126124846
Jojo GM, Kuppusamy G, De A, Karri VVSNR (2019) Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design. Drug Dev Ind Pharm 45(7):1061–1072. https://doi.org/10.1080/03639045.2019.1593439
Wavikar P, Pai R, Vavia P (2017) Nose to brain delivery of rivastigmine by in situ gelling cationic nanostructured lipid carriers: enhanced brain distribution and pharmacodynamics. J Pharm Sci 106(12):3613–3622. https://doi.org/10.1016/j.xphs.2017.08.024
Gartziandia O, Herrán E, Ruiz-Ortega JA, Miguelez C, Igartua M, Lafuente JV, … Hernández RM (2016) Intranasal administration of chitosan-coated nanostructured lipid carriers loaded with GDNF improves behavioral and histological recovery in a partial lesion model of Parkinson’s disease. J Biomed Nanotechnol 12(12):2220–2230. https://doi.org/10.1166/jbn.2016.2313
Wavikar PR, Vavia PR (2015) Rivastigmine-loaded in situ gelling nanostructured lipid carriers for nose to brain delivery. J Liposome Res 25(2):141–149. https://doi.org/10.3109/08982104.2014.954129
Gartziandia O, Herran E, Pedraz JL, Carro E, Igartua M, Hernandez RM (2015) Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf B Biointerfaces 134:304–313. https://doi.org/10.1016/j.colsurfb.2015.06.054
Devkar TB, Tekade AR, Khandelwal KR (2014) Surface engineered nanostructured lipid carriers for efficient nose to brain delivery of ondansetron HCl using Delonix regia gum as a natural mucoadhesive polymer. Colloids Surf B Biointerfaces 122:143–150. https://doi.org/10.1016/j.colsurfb.2014.06.037
Alam MI, Baboota S, Ahuja A, Ali M, Ali J, Sahni JK, Bhatnagar A (2014) Pharmacoscintigraphic evaluation of potential of lipid nanocarriers for nose-to-brain delivery of antidepressant drug. Int J Pharm 470(1–2):99–106. https://doi.org/10.1016/j.ijpharm.2014.05.004
Gabal YM, Kamel AO, Sammour OA, Elshafeey AH (2014) Effect of surface charge on the brain delivery of nanostructured lipid carriers in situ gels via the nasal route. Int J Pharm 473(1–2):442–457. https://doi.org/10.1016/j.ijpharm.2014.07.025
Jain K, Sood S, Gowthamarajan K (2015) Optimization of artemether-loaded NLC for intranasal delivery using central composite design. Drug Deliv 22(7):940–954. https://doi.org/10.3109/10717544.2014.885999
Alam MI, Baboota S, Ahuja A, Ali M, Ali J, Sahni JK (2013) Intranasal infusion of nanostructured lipid carriers (NLC) containing CNS acting drug and estimation in brain and blood. Drug Deliv 20(6):247–251. https://doi.org/10.3109/10717544.2013.822945
Alam T, Pandit J, Vohora D, Aqil M, Ali A, Sultana Y (2015) Optimization of nanostructured lipid carriers of lamotrigine for brain delivery: in vitro characterization and in vivo efficacy in epilepsy. Expert Opin Drug Deliv 12(2):181–194. https://doi.org/10.1517/17425247.2014.945416
Amendola V, Guadagnini A, Agnoli S, Badocco D, Pastore P, Fracasso G, … Marzola P (2021). Polymer-coated silver-iron nanoparticles as efficient and biodegradable MRI contrast agents. J Colloid Interface Sci 596:332–341. https://doi.org/10.1016/j.jcis.2021.03.096
Miao Z, Chen S, Xu CY, Ma Y, Qian H, Xu Y, … Zha Z (2019). PEGylated rhenium nanoclusters: a degradable metal photothermal nanoagent for cancer therapy. Chem Sci 10(21):5435–5443. https://doi.org/10.1039/c9sc00729f
Khan I, Hussein S, Houacine C, Khan Sadozai S, Islam Y, Bnyan R, … Yousaf S (2021) Fabrication, characterization and optimization of nanostructured lipid carrier formulations using Beclomethasone dipropionate for pulmonary drug delivery via medical nebulizers. Int J Pharm 598. https://doi.org/10.1016/j.ijpharm.2021.120376
Maaz A, Blagbrough IS, De Bank PA (2021) In vitro evaluation of nasal aerosol depositions: an insight for direct nose to brain drug delivery. Pharmaceutics 13(7):1079. https://doi.org/10.3390/pharmaceutics13071079
Almurshedi AS, Aljunaidel HA, Alquadeib B, Aldosari BN, Alfagih IM, Almarshidy SS, … Mohamoud AZ (2021) Development of inhalable nanostructured lipid carriers for ciprofloxacin for noncystic fibrosis bronchiectasis treatment. Int J Nanomed 16:2405–2417. https://doi.org/10.2147/IJN.S286896
Patil-Gadhe A, Pokharkar V (2016) Pulmonary targeting potential of rosuvastatin loaded nanostructured lipid carrier: optimization by factorial design. Int J Pharm 501(1–2):199–210. https://doi.org/10.1016/j.ijpharm.2016.01.080
Patil-Gadhe A, Kyadarkunte A, Patole M, Pokharkar V (2014) Montelukast-loaded nanostructured lipid carriers: part II Pulmonary drug delivery and in vitro-in vivo aerosol performance. Eur J Pharm Biopharm 88(1):169–177. https://doi.org/10.1016/j.ejpb.2014.07.007
Murthy RSR, Khattar H, Singh S (2012) Formulation and characterization of nano lipid carrier dry powder inhaler containing ciprofloxacin hydrochloride and N-acetyl cysteine. Int J Drug Deliv 4(3). https://doi.org/10.5138/ijdd.v4i3.647
Pardeike J, Weber S, Haber T, Wagner J, Zarfl HP, Plank H, Zimmer A (2011) Development of an itraconazole-loaded nanostructured lipid carrier (NLC) formulation for pulmonary application. Int J Pharm. https://doi.org/10.1016/j.ijpharm.2011.07.040
CN110013471B - Nanostructure lipid carrier for synergistic treatment of glioma and its preparation method and application- Google Patents. (n.d.). Retrieved October 12, 2022, from https://patents.google.com/patent/CN110013471B/zh
Belgamwar VS, Pardeshi CV, (2015) Novel nanocarrier composition for brain targeting (Application No.: 667/MUM/2015) Intellectual Property India.
AU2021104270A4 - Desvenlafaxine succinate loaded nanostructured lipid carrier (nlc) for brain targeting via nasal route - Google Patents. (n.d.). Retrieved October 12, 2022, from https://patents.google.com/patent/AU2021104270A4/en?oq=AU2021104270A4
Dharamsi A, Patel D, Prajapati B, Patel R, (2021) Novel pharmaceutical formulation of ziprasidone hydrochloride with improved bioavailability (Application ID: 202021023420) Intellectual Property India.
Acknowledgements
The authors are thankful to Ramanbhai Patel College of Pharmacy, Charotar University of Science and Technology (CHARUSAT), for extended facilities to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, S., Patel, A.A., Prajapati, B.G. et al. Multifaceted nanolipidic carriers: a modish stratagem accentuating nose-to-brain drug delivery. J Nanopart Res 25, 150 (2023). https://doi.org/10.1007/s11051-023-05804-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05804-4