Abstract
Silver nanoparticles (AgNPs) have been widely employed due to their antimicrobial properties; however, several studies sustain that AgNPs can induce brain damage, like the blood–brain barrier (BBB) disruption. Among the BBB defense mechanisms, the metallothioneins (MTs), a collection of proteins that regulate intracellular levels of zinc (Zn), play an important role. The goal of this work was to investigate whether the brain damage caused by an intraperitoneal administration of AgNPs (15 mg/ g body weight) at the level of the BBB permeability disruption, damage of the brain tissue, and systemic inflammation could be prevented by 24 h of previous treatment with Zn (27 mg/kg body weight). Evans blue (EB) extravasation, modification of claudin-5 expression, alterations on MTs, N-cadherin expression, and systemic inflammation were evaluated. Our results show that AgNPs induce BBB damage by increasing EB extravasation and decreasing claudin-5 expression, associated with overexpression of MTs, effects that were related with systemic inflammation, evidenced by the increase of granulocytes. Zn pretreatment partially prevented the BBB permeability from the damage induced by AgNPs, whereas the MTs expression and granulocytes count exhibited a reversal effect, suggesting that the effect of Zn could be related with the BBB regulation process. The rat brain histological analysis confirmed that pretreatment with Zn prevented at least in part the toxic effect of AgNPs. This work provides relevant information about the role of Zn as a protectant against the noxious effects of AgNPs upon the rat brain physiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is defined as a discipline dedicated to the study and development of systems at nanometer scale (1–100 nm), named nanomaterials (NMs), (British Standards Institution 2007; Liu et al. 2013). Silver nanoparticles (AgNPs) are NMs with quite a lot of applications mostly due to their antimicrobial properties. Among the application areas of AgNPs are optical, electrical, and biological, which are constantly increased and incorporated into society through a wide range of products (Chen and Schluesener 2008; Gonzalez et al. 2016). It is estimated that about 30% of nanotechnology-based consumer products contain AgNPs (Liu et al. 2017). However, it has also been addressed that AgNPs possess a wide diversity of biological effects (AshaRani et al. 2009) which vary depending on the NPs size, shape, concentration, cellular target, and exposure route (Gliga a et al. 2014; Rosas-Hernandez et al. 2015); all of them being parameters that could confer beneficial, protective, or harmful effects to living organisms.
The AgNPs can be distributed to the whole organism through the cardiovascular system (Oberdörster et al. 2005), eventually reaching the central nervous system (CNS). In this system, neurons, together with highly specialized cerebral capillaries and pericytes, constitute the blood–brain barrier (BBB), defined as the frontier that separates the brain tissue from the circulating substances in the vascular system (Zlokovic 2008; Luther et al. 2011). The BBB function is to regulate the permeability through the exchange of substances from the blood to the brain and vice versa; this process is mediated by a protein complex, named tight junctions (TJ) located between the BBB capillary endothelial cells. TJ are formed by a series of integral proteins located on the capillary endothelial cell membrane like claudin-5, occludine, and adhesion molecules associated to cytoplasmic proteins like ZO1, linked to actin (Wen et al. 2014). The molecules responsible for the induction and maintenance of BBB properties are poorly known; however, several studies suggest that N-cadherin (transmembrane glycoproteins which mediate cell–cell contact in a calcium-dependent manner) expression by brain cells represents an initial and transient signal, which may be involved in the commitment of blood vessels to regulate BBB properties (Gerhardt 1999). Hence, despite that BBB operates as an effective defense mechanism of the CNS (Tsukita and Furuse 1999), several exogenous and endogenous factors or conditions could compromise its functionality and consequently, the brain integrity. For instance, the systemic inflammation characterized by the alteration of blood cell components like granulocytes is caused by several agents that in turn increase the permeability of the microvasculature and promotes the release of blood components into the extravascular tissues (Huber et al. 2001).
Several studies have shown that AgNPs can compromise the BBB functionality, depending on the particle size, dose, and route of administration. In this regard, it is known that smaller nanoparticles (< 10 nm) tend to be more toxic (Sharma and Ali 2006; Sharma et al. 2009a; Trickler et al. 2010). If BBB integrity is eventually compromised, the body may use alternative and adaptive mechanisms in order to restore the damage or protect against its development and progression. Zinc (Zn) is an essential metal responsible for several endogenous functions that per se has been described as an antioxidant and anti-inflammatory agent (Kim et al. 2015). The protective effects of Zn are also associated with the metallothionein (MT) expression (Ruttkay-Nedecky et al. 2013), which are a series of low molecular weight proteins enriched with cysteine residues (Ioachim et al. 2000; Coyle et al. 2002), playing an important role as antioxidant, detoxifying heavy metals, due to the ability of cysteine residues to sequestrate metal ions and oxygen free radicals in a large number of cell types, including brain cells (Luther et al. 2011, 2012).
To date, there are multiple and controversial scientific evidences related to the AgNP brain toxicity, most of them upon the BBB alteration (Sharma et al. 2009a, b; Trickler et al. 2010); however, few studies related to the repair or reversion mechanisms associated to toxicity promoted by AgNPs are reported. In this context, we decided to evaluate the possible protective role of Zn on the brain damage induced by AgNPs, using as source of Zn, the ZnCl2 as referred by other authors (Franciscato et al. 2011; Baiomy et al. 2015). We evaluated whether the pre-administration of Zn could exert a protective effect against the deleterious actions induced by AgNPs on BBB permeability, alteration of the MTs and N-cadherin expression, damage of the brain tissue, and their association to the systemic inflammation.
Considering this background, the aim of this work was to investigate whether the damage induced by AgNPs (< 10 nm) upon the brain physiology and integrity is prevented by a Zn pretreatment.
Methods
Chemicals
Evans blue (EB) dye, zinc chloride (ZnCl2), horseradish peroxidase (HRP) secondary antibody, impregnation with silver nitrate, and other chemical reagents were purchased from Sigma-Aldrich Inc. (St. Louis, MO). AgNO3 and N,N-dimethylformamide (DMF) was purchased from Fermont Laboratories (Monterrey, Mexico), eosin was purchased from Hycel Reactivos Quimicos (Zapopan, Mexico), saline solution was obtained from Pisa laboratories (Guadalajara, Mexico), antibodies against claudin-5 were acquired from Abcam (Cambridge, MA), MT antibodies were obtained from Santa Cruz Biotechnology, and anti-CD325 (N-cadherin) antibody was obtained from BioLegend (San Diego, CA).
Synthesis of AgNPs
AgNPs were synthesized previously described by (Espinosa-Cristobal et al. 2013). AgNPs with spherical shape were synthesized from a 0.35-M AgNO3 solution placed in a 250-mL reaction vessel. Under magnetic stirring, 10 mL of deionized water containing gallic acid (0.1 g) was added to 100 mL of Ag+ solution. After the addition of gallic acid, the pH of the solution was immediately adjusted to 11 with 1.0 M NaOH. Afterwards, the solution was heated for 30 min at 80 °C, and the final concentration of AgNPs used for in vivo treatments was 3500 μg/mL.
AgNPs characterization
AgNPs were characterized using a dynamic light scattering (DLS) assay to determine hydrodynamic particle size, using a DLS Malvern Zetasizer Nano ZS (Instruments Worcestershire, UK), operating with a He–Ne laser at a wavelength of 633 nm and a detection angle of 90°. All samples were analyzed for 60 s at 25 °C. To confirm the shape and particle size, each sample was diluted with deionized water, and 50 μL of each suspension was placed on a copper grid for transmission electron microscopy (TEM); the AgNP size by this technique was calculated with the average of 400 particles, using the ImageJ software. All samples were analyzed using a JEOL JEM-1230 microscope at an accelerating voltage of 100 kV.
Animals and treatments
To evaluate the effect of AgNPs on brain damage, adult male Wistar rats (250–300 g) were divided into four groups (n = 11 animals per group). The first group received an intraperitoneal (IP) administration of saline solution; the second group (as Zn exposure control) received an IP dose of ZnCl2 (27 mg/kg body weight, accordingly to (Franciscato et al. 2011); the third group (damage control by AgNPs) received an IP dose of AgNPs (15 mg/kg body weight) for 24 h; and to evaluate the possible protective role of zinc, the fourth group received an IP dose of ZnCl2 (27 mg/kg body weight) and 24 h later an IP dose of AgNPs (15 mg/kg body weight). From each group, eight animals were employed to evaluate the BBB permeability and the protein expression by western blot.
In addition, three animals per group were destined for the histological and blood component analysis. All experiments were performed in accordance with the National Institutes of Health guide for care and use of laboratory animals and approved by the Animal Care and Use Committee of the Faculty of Chemistry of the University of San Luis Potosi (CEID2017109R1).
Evans blue extravasation
In order to analyze the BBB disruption, the Evans blue (EB) technique was used following the method by (Sharma and Ali 2006). After the animals were anesthetized and the blood samples were collected, a solution of EB dye (45 mg/kg) was injected into the jugular vein and allowed to circulate in the bloodstream for 2 h. Later on, the animals were euthanized and transcardially perfused with 150 mL of phosphate-buffered solution (PBS) pH 3.0. The brains were removed and separated into right and left hemispheres to minimize the number of animals used, since there was no difference in the appearance between the two hemispheres. The right hemisphere was immersed in 1 mL DMF and incubated for 18 h at 80 °C and then centrifuged (13,200 rpm, 1 h, 4 °C); the supernatant was collected and the absorbance was measured at 620 nm. Values were interpolated in a standard curve of EB in DMF, and data were expressed as micrograms of EB per gram of brain tissue.
Western blot analysis
Modifications in claudin-5 and MT protein expression were analyzed using the left hemisphere of each rat brain. Hemisphere samples were collected and homogenized in a radioimmunoprecipitation buffer, and the protein concentration was determined by the bicinchoninic acid (BCA) method. Fifty micrograms of protein sample were loaded into sodium dodecyl sulfate-polyacrylamide gels, then run for 60 min at 200 mV, and transferred into polyvinylidene fluoride membranes for 30 min at 100 mV. Membranes were blocked with 5% bovine serum albumin and then incubated overnight at 4 °C in primary antibody (1:200). Membranes were washed and incubated with secondary antibody (1:5000) during 2 h and revealed with an HRP kit. Images were digitalized and analyzed using the ImageJ software and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Brain histology
An additional group of male Wistar rat (250–300 g) (n = 3) were used for the histological analysis. After treated, the animals were sacrificed by an overdose injection of sodium pentobarbital and the brain tissue immediately were prefixed by cardiac perfusion using 10% paraformaldehyde and subsequently preserved in 30% sucrose. Tissue samples were dehydrated graded ethanol, embedded in paraffin, and cut at 3-μm thickness. For histological examination, cortex sections were stained with the alkaline acid contrast technique hematoxylin-eosin, the silver impregnation technique Cajal, and differential interference contrast (Nomarski). The images were obtained in a Leica TCS SP8 inverted confocal laser-scanning microscope.
Immunohistochemistry for N-cadherin
The paraffin-embedded sections of brain (n = 3) were analyzed for the presence of N-cadherin by immunohistochemistry, according to Soler a et al. (1997). Paraffin sections were dewaxed, rehydrated, and blocked with 1% BSA. Then, cells were permeated in 1% PBS-Triton X-100 for 7 min at 27 °C and washed with PBS. Sections were incubated overnight at 4 °C with mouse monoclonal anti-N-cadherin (1:500 BioLegend) and then developed following the conventional technique (Soler a et al. 1997). The images were obtained in a Leica TCS SP8 inverted confocal laser-scanning microscope.
Blood component analysis
As one of the markers of systemic inflammation, the quantification of the cellular blood components was carried out. Five hundred microliters of blood samples from each group were collected from jugular vein and stored in BD Microtainer® tubes with ethylenediaminetetraacetic acid (K2EDTA) as an anticoagulant. The following blood components were analyzed: white blood cells (WBC), monocytes (MID), granulocytes (GRA), lymphocytes (LY), red blood cells (RBC), and platelet (PLT), using a hematology autoanalyzer (Abott Diagnostics Cell-Dyn 1700).
Statistical analysis
Data were collected from triplicates of eight independent experiments. A normal distribution by Kolmogorov–Smirnov’s test was applied. One-way analysis of variance (ANOVA) followed by Tukey’s test were used to detect differences among treatments. The Kruskal–Wallis nonparametric test followed by post hoc Bonferroni’s test, with Mann–Whitney’s U correction test, were all used for comparison between two or more groups of non-normal data, using the GraphPad 5 software. Values were considered of statistical significance at p < 0.05.
Results
AgNPs characterization
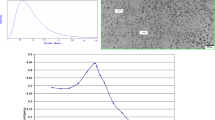
TEM and DLS analysis revealed that AgNPs have spherical and pseudospherical shapes (Fig. 1a), displaying a slight narrow size distribution with a mean particle size of 9.0 ± 2.0 nm for the hydrodynamic diameter (Fig. 1b). The diameter obtained by TEM was 3.0 ± 1.5 nm. The particles’ zeta potential was − 30.0 ± 10 mV, confirming its stability, since particles with positive zeta potential values above + 30 mV or negative values below − 30 mV are considered stable (Meléndrez et al. 2010). The values of this characterization are shown in Fig. 1c.
AgNPs treatment increased BBB permeability and was partially reversed by a pre-administration of Zn
We found that the experimental group treated with AgNPs during 24 h increased the content of EB in the brain tissue (> 2.0 μg/g of brain tissue), in comparison to the control (< 0.5 μg/g of brain tissue), as a consequence of the increased BBB permeability. In contrast, the group treated with Zn alone did not show differences compared to the control, whereas a pre-administration of Zn partially blocked (1.7 μg/g of brain tissue) the effects induced by AgNPs on the BBB permeability (Fig. 2).
AgNPs treatment increased BBB permeability and was partially reversed by a pre-administration of Zn. Male Wistar rats were exposed to different treatments; EB dye extravasation was taken as an index of BBB permeability. The results are expressed as mean ± SEM (n = 8 animals per group). *p < 0.05, **p < 0.01 vs control
AgNPs treatment decreased the claudin-5 expression
Since an increase in BBB permeability has been related with a decrease in TJ proteins (Rosas-Hernandez et al. 2013, 2015), we analyzed the expression of claudin-5 as a classic marker for BBB permeability (Tsukita and Furuse 1999). We found that AgNPs increased the BBB permeability through a modified expression of claudin-5 compared to the control. Moreover, the pre-treatment with Zn and further administration of AgNPs was not different to the AgNP treatment (Fig. 3).
AgNPs treatment decreased the expression of claudin-5. Male Wistar rats were exposed to different treatments. The expression of claudin-5 was detected at 23 kDa (a). Densitometric analysis of the western blots was performed using the ImageJ software (b). The results are expressed as mean ± SEM (n = 8 animals per group). *p < 0.05 vs control
AgNPs treatment increased MT levels in brain tissue, and a pre-administration of Zn restored these levels
To assess the role of MTs in the BBB affected by AgNPs, their protein expression was measured in the brains of rats exposed to these treatments. We observed that the group of rats exposed to Zn alone showed an increase of MTs in comparison to the control (p < 0.05), and the group of rats exposed to AgNPs also increased the MTs expression (p < 0.01), whereas the pre-treatment with Zn plus the administration of AgNPs restored the expression levels of MTs to those observed with the group of Zn treatment (not different to the control values; Fig. 4).
AgNPs treatment increased MT levels in brain tissue, while a pre-administration of Zn re-established these levels. Male Wistar rats were exposed to different treatments. The expression of MTs polymerized (FL-61 sc 11377) was detected at 17 kDa (a). Densitometric analysis of the western blots was performed using the ImageJ software (b). The results are expressed as mean ± SEM (n = 8 animals per group). *p < 0.05 vs control
AgNPs increased the number of granulocytes in blood tissue, while the pre-administration of Zn prevented this effect
In order to evaluate the systemic damage induced by AgNPs and its association with the damage to the BBB permeability, we determined, through the blood analyses, the count of the following cellular elements: WBC, MID, GRA, LY, RBC, and PLT. The AgNP treatment induced a significant increase in the GRA values (3.0 k/μL) in comparison to the control (< 1.00 k/μL) (Fig. 5c), whereas no changes were observed in the rest of leukocytes (Fig. 5); neither RBC nor PLT were modified in the presence of this treatment (Fig. 6), a result that agrees with previous reports (Laloy et al. 2014). Similarly, those cell lines were not affected by Zn or Zn + AgNP treatments (Figs. 5 and 6).
AgNPs treatment increased granulocyte levels blood, while a pre-administration of Zn re-established these levels. Male Wistar rats were exposed to different treatments. Leukocytes (a), lymphocytes (b), granulocytes (c), and MID (d) were counted in peripheral blood. The results are expressed as mean ± SEM (n = 8 animals per group). *p < 0.05 vs control
The AgNPs treatment induced degeneration of the nervous tissue, and this effect was partially prevented by a pre-administration of Zn
We found, through the hematoxylin–eosin (H&E) and Nomarski techniques, that the experimental group treated with AgNPs exhibited considerable damage at the level of the cortex tissue, causing vasogenic edema on the blood vessels of the cerebral cortex compared with the control and Zn treatments (H&E staining and differential interference contrast microscopy) (Fig. 7a, b). The Cajal staining showed tissue destruction in the cortex (Fig. 8), and a lymphocytic infiltrate is inferred. Comparatively, the Zn pre-treatment group showed a reduction of tissue damage than that observed with the single administration of AgNPs (Figs. 7 and 8).
AgNPs treatment induced degeneration of the nervous tissue and was partially prevented by a pre-administration of Zn. Male Wistar rats were exposed to different treatments. Histological changes in the brain were observed using H&E staining (panel A) and differential interference contrast (Nomarski) (panel B)
The Zn pre-administration increased the N-cadherin levels in brain cortex
Due to the preventive effects induced by the pre-treatment with Zn on the BBB permeability, we decided to evaluate the degree of expression of N-cadherin, observing that the expression of this protein was increased in the brain cortex in rats exposed to AgNPs after Zn exposure, compared with the control and the Zn and AgNP treatments (Fig. 9).
Discussion
In the present work, we investigated whether a pre-treatment with Zn could prevent the brain damage induced by an IP administration of AgNPs, firstly upon the BBB permeability, and the possible mechanisms involved. The size of AgNPs employed was below 10 nm; this information is relevant because it is known that smaller NPs exert higher cytotoxic effects (Kim et al. 2012; Eckhardt et al. 2013).
Herein, we found that an IP administration of < 10 nm AgNPs (15 mg/kg) induced an increase of the BBB permeability, decreased the protein expression of claudin-5, increased MT expression, and promoted nervous tissue degeneration after 24 h of exposure. In addition, at the systemic level, we observed that AgNPs increased the granulocytes levels, but no other blood components compared to the control. This inflammatory effect could, in turn, influence the CNS physiology, including the BBB integrity (Hawkins 2005).
In this sense, in vivo studies revealed the toxic effect of AgNPs upon the BBB. Sharma et al. (2009a, 2009b) administered 60 nm AgNPs by different exposure routes to mice and rats by IP (50 mg/kg), intravenous (IV) (30 mg/kg), intracarotid (IC) (2.5 mg/kg), and intracerebroventricular (ICV) (20 μg/10 μL), showing that after 4 h of exposure, AgNPs promoted a discrete increase in BBB permeability when the route of exposure was IP; however, when the administration was IV, IC, and ICV, the AgNPs induced an important BBB permeability increasing from 4 h to 24 h. Xu et al. (2015) examined the effect of AgNPs in rats after an intragastric administration during a 2-week exposure scheme; rats were treated with AgNPs at low and high doses (1 and 10 mg/kg, body weight, respectively). Both dosages triggered neuron shrinkage, extravascular lymphocytes, and a significant increase of IL-4. These authors suggested that AgNPs promoted neuronal effects associated with an inflammatory process. In this context, our data are in agreement with some of these studies (Sharma et al. 2009b, a; Xu et al. 2015), showing that AgNPs are capable to induce damage at the BBB level, according to the size, dose, and route of administration. In the present work, we used a smaller size (< 10 nm) via IP route, based on the reports by Sharma et al., who mentioned that this route produced less damage in comparison to others (Sharma et al. 2009a, 2009b). In addition, with recent data obtained by our research group, we have found that these AgNPs, at the dose and route used in this study, are capable of being stored in brain tissue (data in process). Taking into account this evidence, we developed a model of altered BBB by AgNPs (15 mg/kg), but using a dose at which the neuronal and systemic effects could be reverted in a total or partial manner with Zn, as it has been described for this metal (Kim et al. 2015).
Previously, we reported that 7.8 nm AgNPs exert differential effects in function of different brain cell types. The exposure of AgNPs at different concentrations on astrocyte primary cultures and C6 glioma cell line showed that these NPs were more toxic to the C6 line (the malignant astrocytes) than astrocytes (Salazar-García et al. 2015), suggesting the presence of cell-specific mechanisms of action regulating the effects and interactions activated by AgNPs. Among the possible mechanisms involved, Luther et al. (2011) observed that primary cultured astrocytes survived after an exposure of 55 nm AgNPs, and this effect comprised an overexpression of MTs as a potential mechanism for cell survival. Based on this evidence, we decided to evaluate the role of MTs induced by Zn upon the BBB permeability damage induced by AgNPs.
In order to evaluate the protective role of Zn, 24 h before the administration of AgNPs, group 4 received IP dose of ZnCl2 as (27 mg/kg). In parallel, group 2 (Zn control) received only the IP dose of ZnCl2 (27 mg/kg). We observed that Zn did not modify the BBB permeability, neither also the expression of the claudin-5 protein. However, the pre-treatment with Zn and the subsequent administration of AgNPs showed a partial prevention upon the increase of the BBB permeability in comparison to the control as well as upon the tissue damage. In addition, this treatment did not restore the claudin-5 expression. However, when we evaluated the expression of N-cadherin in the brain tissue, we observed an increase in the expression of this protein in the group exposed to AgNPs with previous exposure to Zn. The function of the N-cadherin is not yet very clear in the BBB; however, it has been reported that it participates in early stages of the formation of this barrier (Gerhardt 1999). In addition, several studies suggest that N-cadherin plays indispensable role in the cellular responses to brain injury through the induction of reactive astrogliosis and the consequent neuroprotection dependent on calcium signaling (Kanemaru et al. 2013). Therefore, our results suggest that the increase of N-cadherin might be due to a first step of re-establishment of the BBB function, while TJ are restored. This would explain why the Zn pre-treatment apparently produced an increased damage at the BBB level with a higher expression of N-cadherin, but with the levels of claudin-5 still diminished.
Conversely, when we evaluated, at the systemic level the cellular elements of the blood in the presence or absence of AgNPs and/or Zn, we found that experimental group 3 (AgNPs) increased around threefold the granulocyte levels in comparison to the control. The granulocyte alterations have been previously reported, specifically in human neutrophils exposed to AgNPs, suggesting that these NPs are associated to a granulocyte-mediated inflammatory process (Liz et al. 2015; Poirier et al. 2016).
Moreover, we observed that the groups of rats exposed to AgNPs with the Zn pre-administration, the GRA levels were not increased with respect to the control, suggesting that at the systemic level, Zn exerts a protective role as an anti-inflammatory agent. This finding agrees with the reported by Franciscato et al. (2011), who evaluated in vivo the toxic effects of mercury and the effectiveness of Zn in the prevention of biochemical changes induced by the first. This protective effect was achieved at the same dose of Zn (27 mg/kg) that we used in the present work. The ability of Zn to reduce inflammation has been reported by Kim et al. (2012), showing that this element can act as an intracellular reactive oxygen species (ROS) scavenger through the modulation of the cyclooxygenase-2 (COX-2) expression, reducing the generation of the pro-inflammatory prostaglandin.
In our study, we observed that the Zn pre-administration partially prevented the damage induced by the AgNPs on BBB permeability, as well as on the brain cortex tissue, and was capable also to attenuate the MT overexpression enhanced by AgNPs, suggesting that AgNPs induced MTs as an adaptive mechanism to the injury in progress as result from the accumulation of various harmful effects such as oxidative stress and inflammation activated by AgNPs (Alessandrini et al. 2017). These effects suggest that the protective effect induced by Zn could be related in part to its anti-inflammatory properties (Kim et al. 2012; Garla et al. 2017) that was evidenced and associated with the decrease in the granulocyte levels.
The anti-inflammatory properties of Zn have been the most evidenced as the most relevant protective mechanism, Mbiydzenyuy et al. (2018) showed that the pre-treatment of Zn intravenously administered (30 mg/kg) attenuated the brain damage in an experimental model of Parkinsonism by preventing lipid peroxidation and preserving the levels of reduced glutathione and the antioxidant enzymes in the midbrain. In this line, 0.02% Zn in the drinking water prevented behavioral and cholinergic perturbations induced by arsenic exposure (Kumar and Reddy 2018). Oral administration of 15 ppm of Zn was also able to ameliorate alterations in neurogenic markers (nestin, Ki67, doublecortin (DCX), and 5-bromo-2′-deoxyuridine) which decreased in obese mice (Nam et al. 2017). Based on the above information, we suggest that Zn could directly protect not only against BBB disrupted permeability induced by AgNP administration but also through the amelioration of oxidative stress measured as the reduced-to-oxidized glutathione ratio, which has been well demonstrated after AgNPs exposure in the brain (Skalska et al. 2016).
In summary, we provide evidence in the brain about the role of Zn on the protective actions against the deleterious effects of AgNPs when administered via IP. Further studies are needed to determine the precise mechanisms of action of Zn in the preventive events against the brain damage induced by AgNPs.
Abbreviations
- BCA:
-
Bicinchoninic acid
- BBB:
-
Blood–brain barrier
- CNS:
-
Central nervous system
- DLS:
-
Dynamic light scattering
- EB:
-
Evans blue
- IC:
-
Intracarotid
- ICV:
-
Intracerebroventricular
- IP:
-
Intraperitoneal
- IV:
-
Intravenous
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GRA:
-
Granulocytes
- LY:
-
Lymphocytes
- MTs:
-
Metallothioneins
- MID:
-
Monocytes
- DMF:
-
N,N-Dimethylformamide
- NMs:
-
Nanomaterials
- PLT:
-
Platelets
- RBC:
-
Red blood cells
- AgNPs:
-
Silver nanoparticles
- AgNO3 :
-
Silver nitrate
- TJ:
-
Tight junctions
- TEM:
-
Transmission electron microscopy
- WBC:
-
White blood cells
- Zn:
-
Zinc
- ZnCl2 :
-
Zinc chloride
References
Alessandrini F, Vennemann A, Gschwendtner S et al (2017) Pro-inflammatory versus immunomodulatory effects of silver nanoparticles in the lung: the critical role of dose, size and surface modification. Nanomaterials. https://doi.org/10.3390/nano7100300
AshaRani P, Hande MP, Valiyaveettil S (2009) Anti-proliferative activity of silver nanoparticles. BMC Cell Biol 10:65. https://doi.org/10.1186/1471-2121-10-65
Baiomy AA, Attia HF, Soliman MM, Makrum O (2015) Protective effect of ginger and zinc chloride mixture on the liver and kidney alterations induced by malathion toxicity. Int J Immunopathol Pharmacol 28:122–128. https://doi.org/10.1177/0394632015572083
British Standards Institution (2007) Terminology for nanomaterials. Publicly Available Specif 16. doi: 9780580613210
Chen X, Schluesener HJ (2008) Nanosilver: a nanoproduct in medical application. Toxicol Lett 176:1–12. https://doi.org/10.1016/j.toxlet.2007.10.004
Coyle P, Philcox JC, Carey LC, Rofe AM (2002) Metallothionein: the multipurpose protein. Cell Mol Life Sci 59:627–647. https://doi.org/10.1007/s00018-002-8454-2
Eckhardt S, Brunetto PS, Gagnon J, Priebe M, Giese B, Fromm KM (2013) Nanobio silver: its interactions with peptides and bacteria, and its uses in medicine. Chem Rev 113:4708–4754
Espinosa-Cristobal LF, Martinez-Castañon GA, Loyola-Rodriguez JP, Patiño-Marin N, Reyes-Macías JF, Vargas-Morales JM, Ruiz F (2013) Toxicity, distribution, and accumulation of silver nanoparticles in Wistar rats. J Nanopart Res 15. https://doi.org/10.1007/s11051-013-1702-6
Franciscato C, Moraes-Silva L, Duarte FA, Oliveira CS, Ineu RP, Flores EMM, Dressler VL, Peixoto NC, Pereira ME (2011) Delayed biochemical changes induced by mercury intoxication are prevented by zinc pre-exposure. Ecotoxicol Environ Saf 74:480–486. https://doi.org/10.1016/j.ecoenv.2010.11.011
Garla R, Kango P, Gill NK, Garg ML (2017) Induction of metallothionein in rat liver by zinc exposure: a dose and time dependent study. Protein J 36:433–442. https://doi.org/10.1007/s10930-017-9737-7
Gerhardt H (1999) N-Cadherin expression in endothelial cells during early angiogenesis in the eye and brain of the chicken: relation to blood-retina and blood-brain barrier development. Eur J Neurosci 11:1191–1201. https://doi.org/10.1046/j.1460-9568.1999.00526.x
Gliga a R, Skoglund S, Wallinder IO, et al (2014) Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol 11:11. doi: https://doi.org/10.1186/1743-8977-11-11
Gonzalez C, Rosas-Hernandez H, Ramirez-Lee MA, Salazar-García S, Ali SF (2016) Role of silver nanoparticles (AgNPs) on the cardiovascular system. Arch Toxicol 90:493–511. https://doi.org/10.1007/s00204-014-1447-8
Hawkins BT (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57:173–185. https://doi.org/10.1124/pr.57.2.4
Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP (2001) Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Circ Physiol 280:H1241–H1248. https://doi.org/10.1152/ajpheart.2001.280.3.H1241
Ioachim EE, Kitsiou E, Carassavoglou C, Stefanaki S, Agnantis NJ (2000) Immunohistochemical localization of metallothionein in endometrial lesions. J Pathol 191:269–273. https://doi.org/10.1002/1096-9896(2000)9999:9999<::AID-PATH616>3.0.CO;2-Q
Kanemaru K, Kubota J, Sekiya H, Hirose K, Okubo Y, Iino M (2013) Calcium-dependent N-cadherin up-regulation mediates reactive astrogliosis and neuroprotection after brain injury. Proc Natl Acad Sci 110:11612–11617. https://doi.org/10.1073/pnas.1300378110
Kim TH, Kim M, Park HS, Shin US, Gong MS, Kim HW (2012) Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res - Part A 100(A):1033–1043. https://doi.org/10.1002/jbm.a.34053
Kim J, Kim S, Jeon S, Hui Z, Kim Y, Im Y, Lim W, Kim C, Choi H, Kim O (2015) Anti-inflammatory effects of zinc in PMA-treated human gingival fibroblast cells. Med Oral Patol Oral Cir Bucal:e180–e187. https://doi.org/10.4317/medoral.19896
Kumar MR, Reddy GR (2018) Influence of age on arsenic-induced behavioral and cholinergic perturbations: amelioration with zinc and α-tocopherol. Hum Exp Toxicol 37:295–308. https://doi.org/10.1177/0960327117698540
Laloy J, Minet V, Alpan L, Mullier F, Beken S, Toussaint O, Lucas S, Dogné JM (2014) Impact of silver nanoparticles on haemolysis, platelet function and coagulation. Nanobiomedicine 1:4. https://doi.org/10.5772/59346
Liu P, Huang Z, Chen Z, Xu R, Wu H, Zang F, Wang C, Gu N (2013) Silver nanoparticles: a novel radiation sensitizer for glioma? Nanoscale 5:11829–11836. https://doi.org/10.1039/c3nr01351k
Liu W, Worms IAM, Herlin-Boime N, Truffier-Boutry D, Michaud-Soret I, Mintz E, Vidaud C, Rollin-Genetet F (2017) Interaction of silver nanoparticles with metallothionein and ceruloplasmin: impact on metal substitution by Ag(i), corona formation and enzymatic activity. Nanoscale 9:6581–6594. https://doi.org/10.1039/c7nr01075c
Liz R, Simard JC, Leonardi LBA, Girard D (2015) Silver nanoparticles rapidly induce atypical human neutrophil cell death by a process involving inflammatory caspases and reactive oxygen species and induce neutrophil extracellular traps release upon cell adhesion. Int Immunopharmacol 28:616–625. https://doi.org/10.1016/j.intimp.2015.06.030
Luther EM, Koehler Y, Diendorf J, Epple M, Dringen R (2011) Accumulation of silver nanoparticles by cultured primary brain astrocytes. Nanotechnology 22. https://doi.org/10.1088/0957-4484/22/37/375101
Luther EM, Schmidt MM, Diendorf J, Epple M, Dringen R (2012) Upregulation of metallothioneins after exposure of cultured primary astrocytes to silver nanoparticles. Neurochem Res 37:1639–1648. https://doi.org/10.1007/s11064-012-0767-4
Mbiydzenyuy NE, Ninsiima HI, Valladares MB, Pieme CA (2018) Zinc and linoleic pre-treatment attenuates biochemical and histological changes in the midbrain of rats with rotenone-induced Parkinsonism. BMC Neuroscience 19:29. https://doi.org/10.1186/s12868-018-0429-9
Meléndrez MF, Cárdenas G, Arbiol J (2010) Synthesis and characterization of gallium colloidal nanoparticles. J Colloid Interface Sci 346:279–287. https://doi.org/10.1016/j.jcis.2009.11.069
Nam SM, Kim JW, Kwon HJ, Yoo DY, Jung HY, Kim DW, Hwang IK, Seong JK, Yoon YS (2017) Differential effects of low- and high-dose zinc supplementation on synaptic plasticity and neurogenesis in the hippocampus of control and high-fat diet-fed mice. Neurochem Res 42:3149–3159. https://doi.org/10.1007/s11064-017-2353-2
Oberdörster G, Oberdörster E, Oberdörster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–839. https://doi.org/10.1289/ehp.7339
Poirier M, Simard JC, Girard D (2016) Silver nanoparticles of 70 nm and 20 nm affect differently the biology of human neutrophils. J Immunotoxicol 13:375–385. https://doi.org/10.3109/1547691X.2015.1106622
Rosas-Hernandez H, Cuevas E, Lantz S, Hamilton W, Ramirez-Lee M, Ali S, Gonzalez C (2013) Prolactin and blood-brain barrier permeability. Curr Neurovasc Res 10:278–286. https://doi.org/10.2174/15672026113109990025
Rosas-Hernandez H, Ramirez M, Ramirez-Lee MA, Ali SF, Gonzalez C (2015) Inhibition of prolactin with bromocriptine for 28days increases blood-brain barrier permeability in the rat. Neuroscience 301:61–70. https://doi.org/10.1016/j.neuroscience.2015.05.066
Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R (2013) The role of metallothionein in oxidative stress. Int J Mol Sci 14:6044–6066. https://doi.org/10.3390/ijms14036044
Salazar-García S, Silva-Ramírez AS, Ramirez-Lee MA, Rosas-Hernandez H, Rangel-López E, Castillo CG, Santamaría A, Martinez-Castañon GA, Gonzalez C (2015) Comparative effects on rat primary astrocytes and C6 rat glioma cells cultures after 24-h exposure to silver nanoparticles (AgNPs). J Nanopart Res 17:1–13. https://doi.org/10.1007/s11051-015-3257-1
Sharma HS, Ali SF (2006) Alterations in blood-brain barrier function by morphine and methamphetamine. Ann N Y Acad Sci 1074:198–224. https://doi.org/10.1196/annals.1369.020
Sharma HS, Ali SF, Hussain SM, Schlager JJ, Sharma A (2009a) Influence of engineered nanoparticles from metals on the blood-brain barrier permeability, cerebral blood flow, brain edema and neurotoxicity. An experimental study in the rat and mice using biochemical and morphological approaches. J Nanosci Nanotechnol 9:5055–5072. https://doi.org/10.1166/jnn.2009.GR09
Sharma HS, Ali SF, Tian ZR, Hussain SM, Schlager JJ, Sjöquist PO, Sharma A, Muresanu DF (2009b) Chronic treatment with nanoparticles exacerbate hyperthermia induced blood-brain barrier breakdown, cognitive dysfunction and brain pathology in the rat. Neuroprotective effects of nanowired-antioxidant compound H-290/51. J Nanosci Nanotechnol 9:5073–5090. https://doi.org/10.1166/jnn.2009.GR10
Skalska J, Dąbrowska-Bouta B, Strużyńska L (2016) Oxidative stress in rat brain but not in liver following oral administration of a low dose of nanoparticulate silver. Food Chem Toxicol 97:307–315. https://doi.org/10.1016/j.fct.2016.09.026
Soler a P, Harner GD, Knudsen KA et al (1997) Expression of P-cadherin identifies prostate-specific-antigen-negative cells in epithelial tissues of male sexual accessory organs and in prostatic carcinomas. Implications for prostate cancer biology. Am J Pathol doi 25:3433–3437. https://doi.org/10.1002/adma.201300292
Trickler WJ, Lantz SM, Murdock RC, Schrand AM, Robinson BL, Newport GD, Schlager JJ, Oldenburg SJ, Paule MG, Slikker W Jr, Hussain SM, Ali SF (2010) Silver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol Sci 118:160–170. https://doi.org/10.1093/toxsci/kfq244
Tsukita S, Furuse M (1999) Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol 9:268–273. https://doi.org/10.1016/S0962-8924(99)01578-0
Wen J, Qian S, Yang Q et al (2014) Overexpression of netrin-1 increases the expression of tight junction-associated proteins, claudin-5, occludin, and ZO-1, following traumatic brain injury in rats. Exp Ther Med 8:881–886. https://doi.org/10.3892/etm.2014.1818
Xu L, Shao A, Zhao Y, Wang Z, Zhang C, Sun Y, Deng J, Chou LL (2015) Neurotoxicity of silver nanoparticles in rat brain after Intragastric exposure. J Nanosci Nanotechnol 15:4215–4223. https://doi.org/10.1166/jnn.2015.9612
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201. https://doi.org/10.1016/j.neuron.2008.01.003
Acknowledgments
The authors thank to Francisco Javier Torres de la Rosa, José Fernando García de la Cruz, and Edgar Rangel Lopez for their technical assistance. This work was supported by the grant C16-PIFI-09-08.08 and the National Council of Science and Technology Project 268769. Samuel Salazar was a recipient of a scholarship from CONACyT (342918).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salazar-García, S., Delgado-Buenrostro, N.L., Rodríguez-Escamilla, J.C. et al. Zinc protects the rat brain from damage induced by 24 h exposure to silver nanoparticles. J Nanopart Res 21, 172 (2019). https://doi.org/10.1007/s11051-019-4616-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-019-4616-0