Abstract
Cyanoacrylate nanoparticles have been usually prepared by anionic polymerization initiated by hydroxyl ions derived from dissociation of water. In the current research, amine groups on the surface of gliadin aggregates were utilized as initiator for the polymerization of ethyl cyanoacrylate (ECA). Gliadin, a protein found in the endosperms of wheat (Triticum aestivum L.), is not soluble in water, but dissolves in aqueous ethanol in the form of aggregates. As a result of the reaction with ECA monomers, gliadin molecules are chemically bound to poly(ethyl cyanoacrylate) (PECA) chains. The nanoparticles thus produced are made up of block copolymers that are consisted of hydrophilic moiety (gliadin) and hydrophobic moiety (PECA). The suspension containing these nanoparticles showed an excellent coating capability on the surface of hydrophobic materials such as glass or plastics. A simple spray coating changed the wetting property of the material instantly and dramatically. Since both protein and poly(alkyl cyanoacrylate) are degradable polymers, the developed nanoparticles are degradable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(alkyl cyanoacrylate) (PACA) nanoparticles have been studied in great detail with a view to their use as controlled release drug delivery materials during the last three decades (Irache et al. 2011; Vauthier et al. 2003). PACA’s are biodegradable and can be used for the production of nanoparticles via emulsion polymerization. Currently, preparation methods of PACA nanoparticles are well understood, whereby nanoparticles with well-defined properties can be produced. A majority of PACA nanoparticles are obtained through anionic polymerization of the corresponding monomer (Nicolas and Couvreur 2009; Bertholon et al. 2006). In most cases, hydroxyl ions in water have been utilized as initiator for the production of nanoparticles in previous research. Amine groups are also a good initiator for the polymerization of alky cyanoacrylates. In this report, amine groups of a wheat protein, gliadin, were utilized as initiator of the polymerization of alkyl cyanoacrylates. Since the initiator itself is a polymer, amphiphilic copolymers were prepared as a result of polymerization reaction. In other words, the reaction products are copolymers consisting of two types of homopolymers, protein and PACA. Since proteins are highly hydrophilic, it works as the hydrophilic end in the resultant amphiphile molecule because of its charges on amino acids, while polymerized alkylcyanoacrylate works as the hydrophobic end because there is no charge on its chain. In our previous research, an animal protein, bovine serum albumin (BSA) was employed for the production of nanoparticles (Kim et al. 2013). Since the physicochemical properties of BSA are well documented, it served as a model protein for understanding the structure of the developed nanoparticles. Because of the high cost of BSA, however, the commercialization of the developed nanoparticles was not feasible. Therefore, a wheat protein (gliadin) was chosen because of its ease of preparation and low production cost. The optimum reaction condition for gliadin is much different from that of BSA because their aggregation behaviors are significantly different from each other.
Gliadin belongs to the characteristic class of proteins known as prolamins, which occur specifically in cereals (Shukla and Cheryan 2001). It is one of the main fractions of gluten found in the endosperms of wheat. Traditionally, 70 % (v/v) aqueous ethanol has been used as a solvent for gliadin (Jackson et al. 1983; Robertson et al. 2004). Most prolamins are enriched in glutamine, proline, and hydrophobic amino acids; they are insoluble in water or buffered salt solutions, but soluble in solutions containing alcohols. Gliadins show aggregate-forming behavior in aqueous ethanol solution. They are mainly monomeric proteins with molecular weights around 28,000–55,000 and can be classified according to their different primary structures into the α/β, γ, and ω type. Each gluten protein type consists or two or three different structural domains; one of them contains unique repetitive sequences rich in glutamine and proline. Non-covalent bonds such as hydrogen bonds, ionic bonds, and hydrophobic bonds are important for the aggregation of gliadins (Wieser 2007).
The produced protein-based nanoparticle possesses peculiar properties. It readily adheres to hydrophobic surfaces and improves the wetting property instantly and dramatically. This report investigates the optimum condition for the production of nanoparticles, and examines their physical properties. Although any alkyl cyanoacrylates can be used as a monomer for the production of PACA nanoparticles, ethyl cyanoacrylate (ECA) was chosen because of its low cost and availability.
Experimental section
Reagents
Ethyl cyanoacrylate (ECA) monomer (E–Z Bond, viscosity; 5 cps) was purchased from K&R International (Laguna Niguel, CA). ECA contained ~0.5 % of hydroquinone as a free radical inhibitor that prevents the monomer from undergoing free radical-induced repolymerization on storage. Gliadin was a gift from MGP Ingredients, Inc (Atchison, KS). Ethanol and Hydrochloric acid were reagent grade.
Purification of gliadin
Gliadin was purified by the procedure used by Hussain and Lukow (1997) with minor modification to obtain α-gliadin. Gliadin powder was mixed with 5 times larger volume of 70 % (v/v) aqueous ethanol and stirred overnight. Then, the solution was kept overnight without stirring, the supernatant was collected, and ethanol was evaporated by putting the solution in a hood for a week. The sedimented precipitate was discarded, and the residual solution was freeze dried. The obtained solid was crushed to a fine powder in a mold.
Preparation of nanoparticle suspensions
Particles were prepared by polymerization of ethyl cyanoacrylate on to the surface of gliadin aggregates. 20 mg of gliadin was dissolved in 10 g of x wt% (62 ≤ x ≤ 80) aqueous ethanol solution that was premixed with 40 μL of 4 N HCl. Then, y μL (10 ≤ y ≤ 200) of ECA was slowly added during constant stirring with a magnetic stirrer at 500 rpm. Reaction time was set to 40 min to 2 h depending on the added amount of ECA (see "Results and Discussion" for details). As the reaction proceeded, turbidity was developed indicating nanoparticles were formed in the reaction medium. The reaction product was centrifuged at 10000×g for 20 min. The produced nanoparticle suspension (supernatant) was collected and stored at room temperature for the characterization of nanoparticles. The PECA polymers initiated by the hydroxyl ions without stabilizing agent form large aggregates because of the intermolecular forces acting between their polymer chains. On the other hand, PECA initiated by protein molecules form amphiphiles, and do not form aggregates unless PECA polymer chains are too large. Therefore, these two types of polymers can be separated by centrifuge.
Reaction time
Reaction time was decided by monitoring the heat of reaction in a custom-built solution calorimeter (Fig. 1). Polymerization reaction was performed in Dewar #1, and the temperature variation was monitored with a thermistor connected to a Wheatstone bridge. Dewar #2 is for the reference. In this configuration, the same amount of heat generated in both Dewar jars at the same time does not contribute to the output signal. In other words, the Wheatstone bridge circuit cancels out the output as long as the stirring speeds are the same for both Dewar jars. It means that only the heat generated (or absorbed) in Dewar #1 (reactor) contributes to the output signal. The working principle of this design is similar to that of double-beam spectrophotometers. This configuration was necessary because the reaction condition for the polymerization of alkyl cyanoacrylate requires vigorous stirring. The output of the bridge circuit was digitized and recorded on a computer as a function of time. For the conversion of the output voltage to the heat of reaction, the calorimeter was calibrated by applying a calculated amount of heat with an electric heater immersed in Dewar #1.
For the estimation of reaction time, reaction was performed in 150 g of 68 % aqueous ethanol which is 15 times larger scale than the usual sample preparation. The amounts of hydrochloric acid, gliadin, and ECA were all scaled up 15 times.
Particle size measurement
Dynamic light scattering (DLS) experiments were carried out with the dispersions using a particle size analyzer equipped with a 658 nm diode laser and an avalanche photodiode detector (Model 90 Plus, Brookhaven Instruments Corporation, Holtsville, NY, USA). For the size measurement of gliadin aggregates, 20 mg of gliadin was dissolved in 10 g of x wt% (62 ≤ x ≤ 80) aqueous ethanol solution that was premixed with 40 μL of 4 N HCl. Nanoparticle samples (prepared by the procedure in the previous section) were diluted twenty times with the same solvent, and measurements were performed without filtration. All measurements were done at a 90° detection angle at 20.0 °C. For each sample, ten DLS measurements were conducted and each run lasted 20 s. All measurements were processed using the software supplied by the manufacturer (9kpsdw, v.5.31). Data from ten measurements were averaged to obtain the size of nanoparticles. Viscosity of acidified aqueous ethanol was measured with Schott ViscoSystem AVS 360 viscometer (Mainz, Germany) using Ubbelohde viscometer tube.
Dynamic contact angle (DCA) measurement
Dynamic contact angle analysis was performed using a DCA 315 (Thermo Cahn Instruments, Madison, WI, USA) by the Wilhelmy plate method to determine the effect of nanoparticle coating on surface wettability (Lander et al. 1993). Samples for DCA analysis were prepared by dipping plates made of the material to be tested into the nanoparticle suspension and rinsing with a stream of distilled water a few seconds. The prepared plate was consecutively immersed in and removed from distilled water at a speed of 60 mm/min. Curves relating the interfacial tension to the immersion depth were plotted and used to calculate the receding contact angle. A representative contact angle was calculated for each formulation using the mean and standard deviation of five independent measurements. DCAs were determined from a simple force balance equation, Fm = mg + pγL cos(θ) − Fb where Fm is the measured force, mg is weight of the plate, p is the perimeter of the meniscus formed at the interface, γL is the surface tension, pγL cos(θ) is the surface tension force acting on the meniscus, and Fb is the buoyance force (Davies et al. 1996). When the plate is right at the surface of liquid, Fb equals zero. The surface tension γL for all samples was calculated from receding contact angles (θ) in distilled water. The receding contact angles (θ) were calculated from surface tension γL of distilled water at room temperature. Two materials used as plate were cover glass (30 × 24 × 0.17 mm, Fisher Scientific, Pittsburgh, PA) and poly(ethylene terephthalate) (PET) sheet prepared from a water bottle (30.4 × 25 × 0.13 mm, Aquafina, Dallas, TX).
Thermogravimetric analysis
For the composition analysis of produced nanoparticles, thermogravimetric analysis (TGA) was performed using a TGA 2050 (TA Instruments, New Castle, DE). Freeze-dried samples (~3 mg) were heated in a platinum TGA pan in an air atmosphere. Each sample was heated from room temperature to 180 °C at 20 °C/min, and then held at 180 °C for 100 min. For data analysis, results were viewed as weight % vs. time.
Surface morphology of adsorbed nanoparticles
The surface morphology of adsorbed nanoparticles on the surface of glass was observed with a scanning electron microscopy (SEM). Samples prepared from nanoparticles with 77 % PECA content were sprayed on cover slips (12 mm dia.), and washed with flowing water immediately. A Zeiss Ultra 60 Field Emission-Scanning Electron Microscope (FE-SEM, Carl Zeiss Inc., Thornwood, NY) at 5 kV operating voltage was used to acquire surface images of the coatings. All SEM samples were sputter coated with 3 nm of Au/PD (60/40 mass fraction %) prior to SEM imaging.
Results and discussion
Aggregate formation of gliadin in aqueous ethanol
Gliadin belongs to prolamin; therefore, it does not dissolve in water, but dissolves in 65 % (w/w) aqueous ethanol (i.e., 70 % (v/v) aqueous ethanol) (Bietz and Wall 1980). Even in aqueous ethanol solutions, it forms aggregates, and the size of aggregate varies depending on the content of ethanol in the solvent mixture. This aggregation behavior in acidified aqueous ethanol was investigated by DLS (Fig. 2). The procedure for sample preparation was specified in the Experimental section. As the percentage of ethanol increased from 62 to 69 % (w/w), the hydrodynamic diameter (Dh) of gliadin decreased and increased again with higher percentage of ethanol. It means that the size of gliadin aggregate is the smallest in 69 % aqueous ethanol. This behavior is similar to that of zein which shows minimum size at around 90 % (w/w) aqueous ethanol (Kim and Xu 2008). The reason for showing a minimum in the DLS data is that there is a structural inversion of aggregates (Kim et al. 2010). In other words, the surface of gliadin aggregates in lower than 69.5 % ethanol is hydrophilic, while that in higher than 69.5 % ethanol is hydrophobic. Since gliadin also showed a similar behavior (but at different ethanol contents), both zein and gliadin had been used as binder for the fabrication of polymer composites (Kim 2008, 2011). According to the DLS data, the hydrodynamic diameter of gliadin aggregates in acidified 69 % ethanol is around 32 nm. Knowing that the MW of α-gliadin ranges 28–35 kDa (Wieser 2007), while that of Bovine Serum Albumin is 67 kDa (Dh = 7.6 ± 0.4 nm) (Adel et al. 2008), it is estimated that each aggregate is an assembly of more than one hundred gliadin molecules.
When the ECA monomers are polymerized on the surface of these gliadin aggregates, reaction will occur on the surface of the gliadin aggregates. This means that the size of produced particles will get larger as more monomers are involved in the polymerization. Since the size of reaction product (i.e., nanoparticles) needs to be small to form stable suspensions, the size of gliadin aggregates before the reaction with ECA monomers has to be as small as possible. According to Fig. 2, 65–70 % ethanol fulfills this requirement. Since 69 % ethanol is too close to micellar inversion, 68 % ethanol was selected as a reaction medium for the polymerization of ECA.
Reaction scheme
In the case of conventional synthesis of nanoparticles from cyanoacrylates, nanoparticles are fabricated in an aqueous medium that is acidified with hydrochloric acid. Since cyanoacrylates are hydrophobic monomers, they are not soluble in water. Therefore, the reaction has been performed via emulsion polymerization, while the reactant mixture is vigorously stirred (Behan et al. 2001). In our reaction scheme, amine groups on the surface of protein (gliadin) molecules react with ECA. By choosing 68 % aqueous ethanol as a reaction medium, a two-phase reaction was avoided: aqueous ethanol is more hydrophobic than water, allowing both gliadin and ECA to dissolve in the same reaction medium without showing phase separation. Acidic condition was necessary to control the size of nanoparticles and reaction rate. It is known that the size distribution of produced nanoparticles depends on the pH of the reaction medium (Behan et al. 2001). Neutral water supplies too much hydroxyl ion which causes formation of large chunks of poly(ethyl cyanoacrylate) (PECA) aggregates instead of nanoparticles.
For the production of nanoparticles, anionic polymerization of alkyl cyanoacrylate has been commonly used with hydroxyl ions as an initiator (Vauthier et al. 2003). For this project, however, amine groups on the surface of gliadin molecules are used as initiator. As a result of this polymerization, copolymers are produced. Since gliadin is hydrophilic and PACA is hydrophobic, the resultant copolymer will behave as an amphiphile. In our reaction condition, ethyl cyanoacrylate (ECA) monomers will react with aggregated gliadin, and the produced nanoparticles will look like gliadin aggregate covered with PACA polymer chains (Fig. 3).
In order to make sure that the amine groups in protein molecules initiate the polymerization reaction of ECA, the three chemical groups in the protein molecules, i.e., amine, carboxyl, and hydroxyl groups, were examined in acidified aqueous ethanol. For this purpose, a compound that contains only one of the aforementioned chemical groups was added in the reaction medium instead of protein. Since our reaction medium contains plenty of ethanol, it is obvious that hydroxyl group is not the initiator. In order to examine carboxyl groups, oleic acid and acetic acid were tried, but the result was negative. On the other hand, when butyl amine was tried, it was found that the initiation of the reaction was extremely fast. The polymerization reaction was even initiated by the small amount of butyl amine vapor on the top of reaction mixture. In other words, ECA was polymerized in the air, before it is immersed in the solution and mixed with butyl amine. Therefore, there is no doubt that amine groups in protein molecules work as initiator for the polymerization of cyanoacrylate.
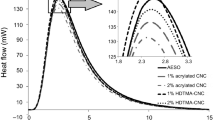
The size of nanoparticles depends on the amount of ECA monomers reacted with each protein molecule. Since the reaction rate depends on the concentration of reactants, the required reaction time was investigated by varying the amount of ECA per 20 mg of gliadin. It is expected that the reaction proceeds until all monomers are consumed for the polymerization reaction. Data from solution calorimeter show that this polymerization reaction is highly exothermic and the reaction time varies depending on the amount of added ECA monomer (Fig. 4). The termination of the reaction is indicated by arrows in the figure, and the termination time on each curve is used as a minimum required reaction time. The negative slope after the termination of reaction indicates the loss of heat to the surroundings. Because of the long reaction time, heat loss from the reaction mixture to surroundings was clearly recorded, although the reaction was performed in the Dewar jar.
Size of produced nanoparticles
Since the produced nanoparticles are the polymerization reaction product of ECA initiated by the amine groups on the surface of gliadin aggregates, gliadin molecules are in the core of the produced nanoparticles, while PECA chains are in the corona. Therefore, it is expected that the size of the produced nanoparticles would increase as more ECA monomers are added to the given amount of gliadin, and the additionally added ECA will increase the chain length of PACA in the corona. This prediction was supported by the data obtained by DLS experiment (Fig. 5). When more than 120 μL was added to 20 mg gliadin, an unstable nanoparticle suspension was obtained whereby Dh data from DLS were not reproducible.
Composition of nanoparticles
As was stated in the Introduction section, ECA can also be polymerized with hydroxyl ions as an initiator. Therefore, there should be a competition of the two initiators, amine groups on gliadin molecules and hydroxyl ions that were dissociated from water. For hydroxyl ion-initiated products, particles are easily precipitated if a stabilizer (e.g., dextran) is not used (Sommerfeld et al. 1997). On the other hand, amine-initiated nanoparticles form a stable suspension without a stabilizer. Therefore, these two types of reaction products can be separated by centrifugation. After the centrifugation, the precipitates, i.e., hydroxyl ion-initiated particles, are removed from the reaction product. It means that the weight fraction of PECA in the prepared nanoparticle is smaller than the weight fraction of ECA in the reactant. In order to understand the composition of nanoparticles, TGA was performed (Fig. 6).
The TGA thermogram was obtained by setting the temperature to 180 °C and monitoring weight loss of the nanoparticle samples. Since gliadin degrades at higher than 250 °C, while PECA begins to degrade at around 110 °C, the weight loss at 180 °C was supposed to be due to the degradation of PECA only. In actual situation, gliadin contains moisture that begins to evaporate as the sample is heated (Fig. 6). Therefore, the weight loss of the sample is due to both PECA and the moisture in gliadin. Figure 6 shows that more decrease in the weight of product is observed as higher percentage of ECA is included in the reactant.
If the polymerization reaction is initiated by protein only, there should be no precipitation, whereby ECA fraction in the reactant is the same as PECA fraction in the product. Because of the side reaction, i.e., hydroxyl ion-initiated polymerization, however, the PECA fraction in the product is always smaller than the ECA fraction in the reactant. This situation is presented in Fig. 7 by comparing the actual PECA fraction in the nanoparticle with the case that assumed all ECA monomers were consumed by protein-initiated polymerization. The difference in the PECA fraction corresponds to the polymerization of ECA initiated by hydroxyl ion.
Each gliadin molecule contains many amine groups that can be used as an initiator of the polymerization of ECA. Although it is not known how many of them are located on the surface of gliadin aggregates, it is reasonable to assume that more than one PECA chain is attached to each gliadin molecule. Therefore, produced nanoparticles can be described as an assembly of (PECA)n-gliadin star copolymers, where PECA chains are localized on the surface of each gliadin molecule (Gao and Matyjaszewski 2009).
Adhesion of nanoparticles
The produced nanoparticles readily adhere to hydrophobic surfaces. Simple spraying on the target surface followed by washing with flowing water induces adherence of nanoparticles. The reason for washing with flowing water is to remove trace of ethanol from the coated surface. The adhesion of nanoparticles (i.e., coating) takes place instantly and the coated surface turns hydrophilic. Any hydrophobic surfaces such as glass panes, plexiglases, stainless steel, porcelain, and polymer films that were made of polyethylene, polypropylene, polystyrene, or PET, etc., could be coated. A demonstration of this behavior on the window glass of a car is shown in Fig. 8. For direct comparison, only front window glass was coated with nanoparticle, and both windows were sprayed with water. In the case of uncoated glass, sprayed water formed droplets on the glass surface. On the other hand, sprayed water on the surface of nanoparticle-coated glass formed a thin water layer, and subsequently sprayed water did not form droplets on its surface. It means that the hydrophilic coating on window glasses greatly improves visibility on rainy days. If no more water is sprinkled on the surface of glass, the coated surface dries quickly, while uncoated surface dries very slowly. The accelerated drying of the coated surface should be caused by two reasons: (1) in the case of a thin water layer on the coated surface, the weight of water to be evaporated should be much less than that the water droplets on the uncoated one. (2) The surface area per gram of water is much larger in the case of a thin water layer.
For quantitative evaluation of the functionality of produced nanoparticles, contact angle was measured before and after the coating. Data from a glass plate and a polystyrene (PS) sheet are shown in Fig. 9. Compared with glass plates, PS is more hydrophobic material. Therefore, its contact angle is much larger than that of the glass plate. After coating with nanoparticles, an enormous decrease in contact angle was observed with both PS and glass plates.
The nanoparticles used in this experiment contain the same amount of gliadin. In other words, as the PECA fraction contained in nanoparticles increases from 65 to 82 %, the size of nanoparticles increases in the same order. According to the data shown in Fig. 9, the effect of adhesion of nanoparticles was not dependent on the size or percentage of PECA of nanoparticles. All the examined nanoparticles showed a good enough functionality as a coating material.
The morphology of nanoparticle-coated glass surface was observed with SEM (Fig. 10). SEM revealed that the nanoparticles were pretty much evenly spread over the glass surface. The size of nanoparticles is in the range of 30–80 nm. Since nanoparticles that contain 77 % PECA were prepared by reacting 20 mg gliadin with 80 μL ECA, the hydrodynamic diameter measured by DLS was supposed to be around 90 nm. Considering the harsh condition for sputter coating of samples, it is probable that gliadin molecules will be dehydrated and the PACA chains will not be extended as much as those in aqueous ethanol solution. Since the size of adsorbed particles is smaller than the wavelength of visible light and the coated surface was not fully covered, the SEM image explains why the visibility of the glasses is not affected by coating.
Degradation of nanoparticles
Since both protein and PACA are degradable polymers, the developed nanoparticles are degradable. The produced nanoparticles will not be used in vivo. Therefore, a reverse Knoevenagel reaction which releases formaldehyde is the most possible degradation pathway of cyanoacrylate moiety of nanoparticles (Fattal et al. 1997). In addition, heat is another possible degradation pathway according to the thermodiagram obtained by TGA. If any material was coated with the produced nanoparticles and exposed to higher than 120 °C, the cyanoacrylate part of the nanoparticle will degrade quickly. In the case of protein moiety, decomposition by microorganisms is the main degradation pathway in nature (Morihara and Oda 1992).
Conclusions
In this article, it is shown that surface modifying nanoparticles can be produced by utilizing amine groups on the surface of protein (gliadin) aggregates as initiator for the polymerization of ethyl cyanoacrylates. Unlike conventional reactions for the preparation of cyanoacrylate nanoparticles, acidified aqueous ethanol as a solvent offered a one-phase reaction. The produced nanoparticle has a strong adsorption characteristic that changes the wetting property of hydrophobic materials. Since the average diameter of adsorbed nanoparticles is much smaller than the wavelength of visible light, transparent materials such as glass or Plexiglas can be coated with the presented nanoparticles without deteriorating transparency. This characteristic is useful for improving visibility on rainy days because it suppresses water-droplet formation on the surface of nanoparticle-coated windows. Since hydrophilic coating leads to the formation of a thin water layer instead of water-droplet formation, the hydrophilic coating also accelerates drying. Self-cleaning by a rainfall is another advantage of hydrophilic coating.
References
Adel A, Nadia M, Mohamed O, Abdelhafidh G (2008) Study of thermally and chemically unfolded conformations of bovine serum albumin by means of dynamic light scattering. Mater Sci Eng C 28:594–600
Behan N, Birkinshaw C, Clarke N (2001) Poly n-butyl cyanoacrylate nanoparticles: a mechanistic study of polymerisation and particle formation. Biomaterials 22:1335–1344
Bertholon I, Ponchel G, Labarre D, Couvreur P, Vauthier C (2006) Bioadhesive properties of poly(alkylcyanoacrylate) nanoparticles coated with polysaccharide. J Nanosci Nanotechnol 6(9–10):3102–3109
Bietz JA, Wall JS (1980) Identity of high molecular weight gliadin and ethanol soluble glutenin subunits of wheat: relation to gluten structure. Cereal Chem 57(6):415–421
Davies J, Nunnerley CS, Brisley AC, Edwards JC, Finlayson SD (1996) Use of dynamic contact angle profile analysis in studying the kinetics of protein removal from steel, glass, polytetrafluoroethylene, polypropylene, ethylenepropylene rubber, and silicone surfaces. J Colloid Interface Sci 182:437–443
Fattal E, Peracchia T, Couvreur P (1997) Poly(alkylcyanoacrylates). In: Domb AJ, Kost J, Wiseman DM (eds) Handbook of biodegradable polymers. Harwood, Amsterdam
Gao H, Matyjaszewski K (2009) Synthesis of functional polymers with controlled architecture by CRP of monomers in the presence of cross-linkers: from stars to gels. Progr Polym Sci 34(4):317–350
Hussain A, Lukow OM (1997) Influence of gliadin-rich subfractions of glenlea wheat on the mixing characteristics of wheat flour. Cereal Chem 74(6):791–799
Irache JM, Esparza I, Gamazo C, Agueros M, Espuelas S (2011) Nanomedicine: novel approaches in human and veterinary therapeutics. Vet Parasitol 180:47–71
Jackson EA, Holt LM, Payne PI (1983) Characterisation of high molecular weight gliadin and low-molecular-weight glutenin subunits of wheat endosperm by two-dimensional electrophoresis and the chromosomal localisation of their controlling genes. Theor Appl Genet 66:29–37
Kim S (2008) Processing and properties of gluten/zein composite. Bioresour Technol 99:2032–2036
Kim S (2011) Production of composites by using gliadin as a bonding material. J Cereal Sci 54:168–172
Kim S, Xu J (2008) Aggregate formation of zein and its structural inversion in aqueous ethanol. J Cereal Sci 47:1–5
Kim S, Xu J, Liu S (2010) Production of biopolymer composites by particle bonding. Compos Part A 41:146–153
Kim S, Evans K, Biswas A (2013) Production of BSA-poly(ethyl cyanoacrylate) nanoparticles as a coating material that improves wetting property. Colloid Surface B 107:68–75
Lander LM, Siewierski LM, Brittain WJ, Vogler EA (1993) A systematic comparison of contact angle methods. Langmuir 9:2237–2239
Morihara K, Oda K (1992) Microbial degradation of proteins. In: Winkelmann G (ed) Microbial degradation of natural products. VCH Verlagsgesellschaft, Weinheim/VCH Publishers, New York
Nicolas J, Couvreur P (2009) Synthesis of poly(alkyl cyanoacrylate)-based colloidal nanomedicines. Wiley Interdiscipl Rev Nanomed Nanobiotechnol 1(1):111–127
Robertson GH, Cao TK, Orts WJ (2004) Wheat proteins extracted from flour and batter with aqueous ethanol at subambient temperatures. Cereal Chem 84(5):497–501
Shukla R, Cheryan M (2001) Zein: the industrial protein from corn. Ind Crop Prod 13:171–192
Sommerfeld P, Schroeder U, Sabel BA (1997) Long-term stability of PBCA nanoparticle suspensions suggests clinical usefulness. Int J Pharm 155:201–207
Vauthier C, Dubernet C, Fattal E, Pinto-Alphandary H, Couvreur P (2003) Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv Drug Deliver Rev 55:519–548
Wieser H (2007) Chemistry of gluten proteins. Food Microbiol 24(2):115–119
Acknowledgments
The authors would like to express appreciation to Mr. Jason Adkins for his technical support during this experiment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Names are necessary to report factually on available data, however, the USDA neither guarantees nor warrants the standard of the product, and the use of the name by USDA implies no approval of the product to the exclusion of others that may also be suitable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 19474 kb)
Rights and permissions
About this article
Cite this article
Kim, S., Kim, Y.S. Production of gliadin-poly(ethyl cyanoacrylate) nanoparticles for hydrophilic coating. J Nanopart Res 16, 2277 (2014). https://doi.org/10.1007/s11051-014-2277-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2277-6