Abstract
Candida albicans persisters have so far been observed only in biofilm environment; the biofilm element(s) that trigger(s) persister formation are still unknown. In this study, we tried to further elucidate the possible relationship between C. albicans persisters and the early phases of biofilm formation, especially the surface adhesion phase. Three C. albicans strains were surveyed for the formation of persisters. We tested C. albicans persister formation dynamically at different time points during the process of adhesion and biofilm formation. The number of persister cells was determined based on an assessment of cell viability after amphotericin B treatment and colony-forming unit assay. None of the planktonic cultures contained persisters. Immediately following adhesion of C. albicans cells to the surface, persister cells emerged and the proportion of persisters reached a peak of 0.2–0.69 % in approximately 2-h biofilm. As the biofilm matured, the proportion of persisters decreased and was only 0.01–0.02 % by 24 h, while the number of persisters remained stable with no significant change. Persisters were not detected in the absence of an attachment surface which was pre-coated. Persisters were also absent in biofilms that were scraped to disrupt surface adhesion prior to amphotericin B treatment. These results indicate that C. albicans antifungal-tolerant persisters are produced mainly in surface adhesion phase and surface adhesion is required for the emergence and maintenance of C. albicans persisters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Candida albicans is the most prevalent opportunistic human fungal pathogen and is particularly problematic in immuno-compromised patients such as those with AIDS and cancer, or in organ transplant patients [1]. Candida infections are often untreatable and sometimes can result in life-threatening diseases with a high rate of mortality approaching 40–70 % [2–9]. It is believed that biofilm formation and drug tolerance can lead to the recalcitrant and untreatable nature of some fungal infectious diseases [7, 10]. Biofilm-related drug tolerance may be due to high density and low growth rate within the biofilm, binding of large molecule antibiotics to the exopolymer matrix, and up-regulation of biofilm-associated genes such as MDR1, CDR1 and CDR2 [11–15]. Indeed, increasing evidence indicates that the mechanism of biofilm-related drug tolerance is multifactorial [7, 10].
In 2006, LaFleur and colleagues found that only a small population in the C. albicans biofilm could survive when challenged with lethal antifungal treatment [16]. The subpopulation of survivors is known as persisters [16–21]. Persisters can reconstitute a new biofilm with a similar proportion of persisters and an equal susceptibility to antifungal treatment when they are harvested and re-incubated [10, 16, 21–23]. Just as for their bacterial counterparts, these C. albicans persisters exhibit a non-hereditary, multi-drug tolerance [16, 23–25]. Therefore, persisters were implicated as the main determinants of high biofilm tolerance to antifungal and to the recurring symptoms of fungal infectious diseases [18, 24].
Intriguingly, while bacterial persisters can be detected in both planktonic and biofilm conditions [24, 26–28], C. albicans persisters have so far been observed only in biofilms [16, 17, 20]. Persisters are thought to be dormant phenotypic variants of the regular cell population [10, 16, 17, 21, 23, 24]. Although investigations into the mechanism of fungal persister cell formation are at an early stage, some studies have investigated this clinically important area. Biofilms seem to be essential for the formation of Candida persisters, but some mutants that cannot form mature biofilms, such as Δefg1/Δcph1, can still produce wild-type levels of persister cells [16]. This result indicates that the maturation of biofilms is not the essential condition for Candida persister formation. However, the previous studies have been focused on the persister formation in mature biofilms (48 h) and none of these studies have been conducted to investigate persister formation in the early phases of biofilms [16–20]. Thus, these previous results are unsatisfactory and further studies are still necessary.
To search for the possible determinants, we dynamically tested the formation of C. albicans persisters at different time points (0–24 h) during biofilm development. C. albicans persisters were first reported to occur following treatment with a high concentration of amphotericin B or chlorhexidine [16]. Since their identification, dose-dependent killing has been the only effective and straightforward method of isolating persisters [16–20]. In the present study, we used a similar protocol with amphotericin B to measure persisters and three C. albicans strains were tested. The persister levels were also tested in biofilms grown in the pre-coated wells of microtiter plates, of which the surface had been previously occupied by other strains. Additionally, we tested the persister levels in biofilms that were scraped to disrupt surface adhesion prior to amphotericin B treatment.
Materials and Methods
Strains and Growth Conditions
Candida albicans strains 3153A [16], SC5314 [29, 30] and YEM30 [31] were tested for the presence of persisters after exposure to amphotericin B, and C. albicans strain CAI-4 (ura3Δ::imm434/ura3Δ::imm434) [16] was used to pre-coat the attachment surface in this study. C. albicans strain CAI-4 is URA3 null mutant, and C. albicans strains 3153A, SC5314 and YEM30 are wild types. Stock cultures of C. albicans strains were routinely propagated in yeast extract peptone dextrose [YPD; 1 % (w/v) yeast extract, 2 % (w/v) peptone, 2 % (w/v) glucose] solid medium containing 1.5 % (w/v) agar. The inocula of yeast cells were prepared by transfer of a single colony from YPD solid medium to YPD medium and incubated at 37 °C for 12 h in an incubator shaker at about 100 rpm.
Antifungal Susceptibility Assay
The minimal inhibitory concentration (MIC) of the tested C. albicans strains to amphotericin B (Amresco, USA) was determined by the broth microdilution method based on the CLSI M27-A2 guidelines [32]. Briefly, cells were harvested by centrifugation at 6000×g for 3 min from overnight culture, washed twice in sterile PBS and re-suspended in RPMI 1640 medium. Cells were cultured in 96-well microtiter plates (Corning Costar, USA) at a density of (1–5) × 103 cells ml−1. The cell suspensions were treated with a series of twofold dilutions of amphotericin B in RPMI 1640 medium, while the negative controls were conducted with the same amount of vehicle. The MIC was determined by incubating the inoculated cultures for 24 h at 37 °C and observing inhibition of growth based on turbidity. Experiments were performed with at least four independent cultures.
Preparation and Amphotericin B Challenge of Planktonic Cultures
Planktonic cultures of C. albicans were prepared according to the methods described by Al-Dhaheri and Douglas [17]. Briefly, C. albicans cells were harvested by centrifugation, washed twice in sterile PBS and re-suspended in RPMI 1640 medium plus MOPS at 1.0 × 107 cells ml−1. The planktonic cells were aliquoted into wells of microtiter plates at 100 µl per well and treated for 24 h with amphotericin B (100 µg ml−1).
Candida albicans Biofilm Development on the Surfaces of Wells of Microtiter Plates
Candida albicans biofilm development on the surfaces of wells of flat-bottom 96-well microtiter plates was induced as described by Ramage et al. [33, 34]. Briefly, cells were harvested by centrifugation, washed twice in sterile PBS and re-suspended in RPMI 1640 medium plus 0.165 M MOPS. The suspension was adjusted to the desired density of 1.0 × 106 cells ml−1 after counting with a hemocytometer. The cell suspension was dispensed into the wells of microtiter plates at 100 μl per well, and the plates were incubated at 37 °C in an incubator shaker at approximately 100 rpm. Biofilms were formed over a series of time intervals (0.5, 1, 2, 4, 6, 8, 12 and 24 h).
Preconditioning the Surfaces of Microtiter Plates with Candida albicans Strain CAI-4
Candida albicans strain CAI-4 culture was incubated overnight at 37 °C in YPD medium. Cells were harvested by centrifugation, washed twice in sterile PBS and re-suspended in RPMI 1640 medium plus 0.165 M MOPS and adjusted to the desired density of (3–5) × 107 cells ml−1 after counting with a hematocytometer. The cell density was chosen to ensure that the surface of microtiter plates was entirely coated after preconditioning 24 h with C. albicans CAI-4. The cell suspension was aliquoted into the wells of 96-well microtiter plates at 100 μl per well, and the plates were incubated at 37 °C for 24 h in an incubator shaker at approximately 100 rpm. Then, the medium was aspirated and the wells were washed twice with sterile PBS to remove nonadherent cells. Subsequently, one hundred microliters of above cell suspension at a concentration of 1.0 × 106 cells ml−1 was dispensed into the wells of 96-well microtiter plates and incubated at 37 °C at approximately 100 rpm over a series of time intervals (0.5, 1, 2, 4, 6, 8, 12 and 24 h).
Amphotericin B Challenge of Candida albicans biofilm
Amphotericin B was dissolved in RPMI 1640 medium at 100 μg ml−1, which exceeded 10× MIC to minimize survival of potential spontaneous resistant mutants. After biofilm formation, the RPMI 1640 medium was discarded and nonadherent cells were removed by washing twice in sterile PBS. The biofilms were then randomly categorized into three groups: The first group was directly challenged with 100 μg ml−1 amphotericin B for 24 h at 37 °C; the second group was scraped to disrupt surface adhesion before challenged with 100 μg ml−1 amphotericin B for 24 h at 37 °C; and the third group was used as an untreated control to determined the growth levels of C. albicans in biofilms by colony-forming unit assay.
Candida albicans Persisters Determination
Selective SC-Ura medium (SC minus Uracil; Sigma-Aldrich, USA) was employed in this experiments because C. albicans CAI-4 could not grow on this medium. The quantification of persisters was performed as previously described by LaFleur et al. [16]. Briefly, the biofilms challenged with amphotericin B for 24 h were washed twice with sterile PBS, scraped from the surface of wells of microtiter plates and re-suspended in 100 µl PBS. Then, the suspensions were serially diluted in PBS, and viable cells were counted by plating 5 μl drops on the SC-Ura medium agar plates and incubated at 37 °C for 48 h. The percentage of persisters was measured by comparing the number of viable cells with that in control biofilm at the same time point.
Live/Dead Cell Analysis and Confocal Laser Scanning Microscopy (CLSM)
LIVE/DEAD FungaLight yeast viability kit (Invitrogen, USA) employed in the live/dead cells analysis. One microliter of SYTO®9 dye and 1 μl of propidium iodide solution were added to 1 ml of 1 M Tris–HCl buffer (pH 6.8) and blended; the mixtures were then applied to stain the samples of C. albicans biofilms. After biofilm formation or antifungal treatment, RPMI 1640 medium and nonadherent cells were removed from the microtiter plate by thoroughly washing the biofilms twice with sterile Tris–HCl buffer. Dye mixtures (100 μl) were added to each sample and incubated at room temperature or 37 °C and protected from light for 15–30 min. After incubation with the dyes, stained biofilms were visualized with Zeiss LSM780 confocal laser scanning microscope.
Statistical Analysis
Statistical significances were determined by Student’s t test. All statistical analyses were computed using SPSS 17.0. Data were expressed as the mean ± standard deviation (SD). The level of statistical significance for all tests was set at p values <0.05.
Results
Presence of Persisters in Candida albicans Biofilms and Planktonic Cultures
Biofilms and planktonic cells of three C. albicans strains were surveyed for the presence of persisters after amphotericin B treatment. There were no detectable survivors in any planktonic cultures of the tested C. albicans strains. However, persisters were found in biofilms of all tested C. albicans strains. For all tested C. albicans strains, the persister population was small, representing approximately 0.021, 0.014 and 0.012 % of the total population of mature 24-h biofilms of C. albicans 3153A, SC5314 and YEM30, respectively.
Formation of Candida albicans Persisters During Biofilm Formation
We dynamically tested persister formation during surface adhesion and biofilm development. The persister cells emerged immediately after C. albicans cells adhered to surface and significantly increased at 0–2 h (p < 0.05; Fig. 1). The proportion of persisters reached a peak (0.2–0.69 %) in approximately 2-h biofilm. The number of persisters in 2 h biofilms was 295.0 ± 11.90, 103.75 ± 11.43 and 227.5 ± 18.43 (cell/well), representing approximately 0.69, 0.25 and 0.36 % of the total population of biofilms of C. albicans 3153A, SC5314 and YEM30, respectively. During fungal cell growth, proliferation, aggregation, hyphae formation and biofilm maturation, the number of persister cells in the biofilm remained stable without significant change (p > 0.05; Fig. 1). As a result, the proportion of persisters declined, yielding a fraction of only 0.01–0.02 % in 24-h biofilm. The persister population in 24-h biofilms was 292.5 ± 8.34, 103.75 ± 8.00 and 218.75 ± 19.87 (cell/well), representing approximately 0.021, 0.014 and 0.012 % of the total population of biofilms of C. albicans 3153A, SC5314 and YEM30, respectively. Interestingly, persister cells were absent in biofilms that were scraped to disrupt surface adhesion prior to amphotericin B treatment.
Persister formation during biofilm development. Biofilms are incubated over a series of time intervals as indicated and challenged with amphotericin B. Growth levels and persister levels in biofilms of C. albicans 3153A (a), C. albicans sc5314 (b) and C. albicans YEM30 (c). Error bars indicate standard deviations, and results are from 16 independent experiments
Since killing of C. albicans biofilms by amphotericin B followed a biphasic pattern [16], with persisters surviving extended periods of time, exhibiting slow, steady cell death, we tested the survivors of C. albicans to see whether they showed this characteristic of persisters. Hence, we exposed the biofilms of C. albicans to amphotericin B for a prolonged period, quantifying the survivors at several time points. Our results reflect slow, steady cell death for up to 8 h of amphotericin B treatment (Fig. 2), which is characteristic of persisters. Therefore, the persisters detected in the present study are “true” persisters.
Prolonged amphotericin B treatment of C. albicans biofilms. Persister levels in biofilms of C. albicans 3153A, SC5324 and YEM 30 during prolonged amphotericin B treatment. Persister levels during the prolonged amphotericin B treatment are not changed significantly (p > 0.05). Error bars indicate standard deviations, and results are from four independent experiments
Effect of Pre-coated Surface on Persister Formation in the Biofilm
Given that C. albicans persisters only seemed to arise after adhesion to the attachment surface, we investigated whether pre-coated attachment surface would effect the formation of C. albicans persisters. C. albicans strain CAI-4 was used in this study, which does not grow on SC-Ura medium. Accordingly, we pre-coated the surfaces of wells of microtiter plates with C. albicans CAI-4 before incubating biofilms of the tested C. albicans strains. The growth levels of C. albicans in biofilms were significantly elevated at 0–2 h (p < 0.05; Fig. 3), whereas no significant differences were observed at 4–24 h (p > 0.05; Fig. 3). However, biofilms grown on the pre-coated surface, like the planktonic cultures of the tested C. albicans strains, appeared to lack persisters completely, in which there were no detectable survivors after amphotericin B treatment.
Effect of pre-coated surfaces on C. albicans growth in the biofilm. Biofilms are incubated on the pre-coated surface of microtiter plates. Growth levels in biofilms of C. albicans 3153A (a), C. albicans sc5314 (b) and C. albicans YEM30 (c). Error bars indicate standard deviations, and results are from 16 independent experiments
CLSM Visualization of Candida albicans Biofilms and Persisters
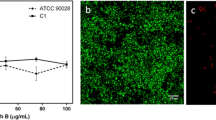
We stained the biofilms of C. albicans 3153A with a LIVE/DEAD FungaLight yeast viability kit, which contains two dyes, SYT0®9 and propidium iodide. Yeasts with intact cell membranes stain fluorescent green, whereas yeasts with damaged membranes stain fluorescent red.
Our data showed that C. albicans biofilm formation on the surface of wells of microtiter plates proceeded in four distinct developmental phases: adhesion of C. albicans cells to the surface; proliferation and co-aggregation; production and release of exopolymer matrix; and biofilm maturation. In the adhesion phase (0–2 h), C. albicans cells, in the form of blastospores, adhered to the surface in a random manner (Fig. 4a). At 2–4 h, co-aggregation among C. albicans cells led to the emergence of microcolonies on the surface of the microtiter plates, following which the yeast cells budded and started to form hyphae (Fig. 4b). At 4–6 h, C. albicans cells presented as yeast cells, pseudohyphae and hyphae and tended to aggregate along the surface irregularities. By 6 h, the emergence of exopolymer matrix was apparent in the form of a “mist-like” covering of the microcolonies. At 8–12 h, cell density increased, and multilayer cells containing all fungal morphologies covered the attachment surface in an intricate network of spatially scattered, intertwined hyphae (Fig. 4c). As the biofilm matured after 24 h of growth, a dense network of yeast cells aggregating along the hyphae covered the entire well surface (Fig. 4d). Fluorescent red was rarely detected in biofilms.
Live/dead cell analysis of C. albicans biofilms C. albicans 3153A. Biofilms are incubated in the wells of microtiter plates over a series of time intervals (2, 4, 8 and 24 h). CLSM micrographs are taken at ×200 magnification and scale bar equals 20 μm. Images of biofilms (a–d) and biofilms after amphotericin B treatment (e–h). Live cells stain fluorescent green, whereas dead cells stain fluorescent red
After exposure to amphotericin B, the majority of biofilm cells were killed and a very small fraction of cells survived, which were persister cells (Fig. 4e–h). These rare surviving persisters were morphologically unremarkable and looked like regular cells in biofilms untreated with amphotericin B. The results showed that C. albicans persisters emerged immediately upon adhesion to the surface (0–2 h; Fig. 4e). As the biofilms matured, fluorescent green was still rarely detected in the treated biofilms (2–24 h; Fig. 4e–h).
Discussion
Persisters with high tolerance to antibiotics are described as dormant variants of regular cells that are produced stochastically in microbial populations [10, 16, 21, 23, 24]. These cells may be an unappreciated source for the recalcitrance of chronic infectious disease [18, 24]. Now there were some landmark studies demonstrated that the level of persister formation was closely associated with the duration of microbes in vivo and that persisters were directly linked to the clinical manifestation of disease [18, 35]. However, the mechanism underlying persister formation remains unclear.
Previous studies have reported that C. albicans persisters, in contrast to their bacterial counterparts, could only be detected in the biofilm state [16, 17, 20]. In the present study, we again confirmed that C. albicans persisters were observed only in biofilms while the planktonic cultures lacked persisters completely. However, the persister population was small and accounted for only 0.01–0.02 % of the total cell population of the mature 24-h biofilms. The proportion of persisters is similar to reports by Al-Dhaheri and Douglas but much less than that detected by LaFleur et al. [16, 17]. The reason for the disparity may be due to the different C. albicans strains employed in the two studies.
However, it is worth noting that in the present study, persister population was discovered in biofilms of C. albicans strain SC5314, representing approximately 0.014 % of the total cell population of mature 24-h biofilms. The result is diametrically opposite to that of Al-Dhaheri and Douglas, who demonstrated that persisters were absent in biofilms formed by C. albicans SC5314 [17, 19]. But the previous studies only tested the mature 48-h biofilms, whereas our kinetic study has more discriminatory power.
Candida species biofilm formation has been reported to proceed through early, intermediate and maturation phases [34, 36]. Similar to previous findings [34], we found that C. albicans biofilm formation on the surface of microtiter plates proceeded through adhesion (0–2 h), proliferation and co-aggregation (~2–6 h), matrix formation (~6 h) and maturation (~24 h) phases. During our dynamic monitoring of persister formation in biofilm, we found that once they adhered to the surface, C. albicans persisters emerged rapidly, and the number of persisters reached a plateau by 2 h (Fig. 1). In our study, CLSM images revealed that C. albicans cells were present as blastospores adhering to the surface at 0–2 h (adhesion phases) and that beyond 2 h, some of the fungal cells began to aggregate and formed microcolonies, which then merged and produced a three-dimensional structure surrounded by exopolymer matrix [4, 7, 36]. With the maturation of biofilm, the number of persisters did not significantly increase. The results indicate that C. albicans persisters are mainly produced during the adhesion phase.
Unexpectedly, we observed that for all tested C. albicans strains, persisters were completely absent in biofilms grown on the pre-coated surface with C. albicans strain CAI-4. We do not understand why persisters were absent from biofilms grown on the “yeast layer,” but it did. The results suggested that the formation of C. albicans persisters was closely related to the adhesion of C. albicans cells to the surface, rather than the complex architecture or other steps of biofilm formation, such as aggregation, morphogenetic transition and secretion of exopolymer matrix. This finding is consistent with a previous study that suggested some mutant strains defective in both hyphal and biofilm formation were able to produce wild-type levels of persisters [16]. Considering these findings, we speculate that surface adhesion, rather than other steps of biofilm formation, is closely related to the formation of C. albicans persisters.
In the present study, we also found persister cells were completely absent in biofilms that were scraped to disrupt surface adhesion prior to amphotericin B treatment. This finding suggests that persisters are revived from a dormant state and lose their antifungal tolerance when surface adhesion is disrupted. The revival of persisters has been mentioned previously, although the underlying mechanisms are unknown [24]. The above results demonstrate that surface adhesion is required not only for the formation of C. albicans persisters but also for their maintenance.
In summary, we speculate that fungal adhesion to surface triggers cell signal transduction systems prior to biofilm formation. As a result, a subpopulation of cells transfers to persister cells, which is controlled by unknown mechanism. This special state protects cells from lethal interactions between antifungal and their targets. Further studies are required to determine differential gene expression profiles from persisters and regular cells in order to elucidate the molecular basis required for persisters.
Conclusion
This study demonstrates that C. albicans antifungal-tolerant persister cells are produced mainly in the surface adhesion phase of biofilm formation. Furthermore, our findings indicate that that surface adhesion, rather than other steps of biofilm formation, is required for the formation and maintenance of C. albicans persisters.
References
Selmecki A, Forche A, Berman J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science. 2006;313(5785):367–70. doi:10.1126/science.1128242.
Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11(1):30–6.
Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med. 1988;148(12):2642–5.
Seneviratne CJ, Jin L, Samaranayake LP. Biofilm lifestyle of Candida: a mini review. Oral Dis. 2008;14(7):582–90. doi:10.1111/j.1601-0825.2007.01424.x.
Colombo AL, Nucci M, Park BJ, Nouer SA, Arthington-Skaggs B, da Matta DA, et al. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J Clin Microbiol. 2006;44(8):2816–23. doi:10.1128/JCM.00773-06.
Wenzel RP, Gennings C. Bloodstream infections due to Candida species in the intensive care unit: identifying especially high-risk patients to determine prevention strategies. Clin Infect Dis. 2005;41(Suppl 6):S389–93. doi:10.1086/430923.
Mathe L, Van Dijck P. Recent insights into Candida albicans biofilm resistance mechanisms. Curr Genet. 2013;59(4):251–64. doi:10.1007/s00294-013-0400-3.
Bouza E, Burillo A, Munoz P, Guinea J, Marin M, Rodriguez-Creixems M. Mixed bloodstream infections involving bacteria and Candida spp. J Antimicrob Chemother. 2013;68(8):1881–8. doi:10.1093/jac/dkt099.
Peng S, Lu Y. Clinical epidemiology of central venous catheter-related bloodstream infections in an intensive care unit in China. J Crit Care. 2013;28(3):277–83. doi:10.1016/j.jcrc.2012.09.007.
Taff HT, Mitchell KF, Edward JA, Andes DR. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013;8(10):1325–37. doi:10.2217/fmb.13.101.
Qu Y, Daley AJ, Istivan TS, Rouch DA, Deighton MA. Densely adherent growth mode, rather than extracellular polymer substance matrix build-up ability, contributes to high resistance of Staphylococcus epidermidis biofilms to antibiotics. J Antimicrob Chemother. 2010;65(7):1405–11. doi:10.1093/jac/dkq119.
Baillie GS, Douglas LJ. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob Agents Chemother. 1998;42(8):1900–5.
Baillie GS, Douglas LJ. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J Antimicrob Chemother. 2000;46(3):397–403.
de Micheli M, Bille J, Schueller C, Sanglard D. A common drug-responsive element mediates the upregulation of the Candida albicans ABC transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol Microbiol. 2002;43(5):1197–214.
Ramage G, Bachmann S, Patterson TF, Wickes BL, Lopez-Ribot JL. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother. 2002;49(6):973–80.
LaFleur MD, Kumamoto CA, Lewis K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother. 2006;50(11):3839–46. doi:10.1128/aac.00684-06.
Al-Dhaheri RS, Douglas LJ. Absence of amphotericin B-tolerant persister cells in biofilms of some Candida species. Antimicrob Agents Chemother. 2008;52(5):1884–7. doi:10.1128/aac.01473-07.
LaFleur MD, Qi Q, Lewis K. Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob Agents Chemother. 2009;54(1):39–44. doi:10.1128/aac.00860-09.
Al-Dhaheri RS, Douglas LJ. Apoptosis in Candida biofilms exposed to amphotericin B. J Med Microbiol. 2010;59(Pt 2):149–57. doi:10.1099/jmm.0.015784-0.
Bink A, Vandenbosch D, Coenye T, Nelis H, Cammue BP, Thevissen K. Superoxide dismutases are involved in Candida albicans biofilm persistence against miconazole. Antimicrob Agents Chemother. 2011;55(9):4033–7. doi:10.1128/AAC.00280-11.
Dawson CC, Intapa C, Jabra-Rizk MA. “Persisters”: survival at the cellular level. PLoS Pathog. 2011;7(7):e1002121. doi:10.1371/journal.ppat.1002121.
Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–31.
Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5(1):48–56. doi:10.1038/nrmicro1557.
Lewis K. Persister cells. Annu Rev Microbiol. 2010;64(1):357–72. doi:10.1146/annurev.micro.112408.134306.
Lewis K. Persister cells: molecular mechanisms related to antibiotic tolerance. Handb Exp Pharmacol. 2012;211:121–33. doi:10.1007/978-3-642-28951-4_8.
Spoering AL, Lewis K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol. 2001;183(23):6746–51. doi:10.1128/JB.183.23.6746-6751.2001.
Keren I, Minami S, Rubin E, Lewis K. Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. MBio. 2011;2(3):e00100–11. doi:10.1128/mBio.00100-11.
Kwan BW, Valenta JA, Benedik MJ, Wood TK. Arrested protein synthesis increases persister-like cell formation. Antimicrob Agents Chemother. 2013;57(3):1468–73. doi:10.1128/AAC.02135-12.
Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198(1):179–82.
Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90(5):939–49.
Yang C, Gong W, Lu J, Zhu X, Qi Q. Antifungal drug susceptibility of oral Candida albicans isolates may be associated with apoptotic responses to Amphotericin B. J Oral Pathol Med. 2010;39(2):182–7. doi:10.1111/j.1600-0714.2009.00811.x.
Silici S, Koc AN. Comparative study of in vitro methods to analyse the antifungal activity of propolis against yeasts isolated from patients with superficial mycoses. Lett Appl Microbiol. 2006;43(3):318–24. doi:10.1111/j.1472-765X.2006.01949.x.
Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45(9):2475–9.
Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. Biofilm formation by Candida dubliniensis. J Clin Microbiol. 2001;39(9):3234–40.
Mulcahy LR, Burns JL, Lory S, Lewis K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J Bacteriol. 2010;192(23):6191–9. doi:10.1128/JB.01651-09.
Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183(18):5385–94. doi:10.1128/jb.183.18.5385-5394.2001.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant Nos 30973310, 81371158). Our laser confocal scanning work was performed at The Microscopy Characterization Facility, Shandong University. We are also indebted to Professor K. Lewis for supplying the C. albicans strains.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, J., Li, Z., Chu, H. et al. Candida albicans Amphotericin B-Tolerant Persister Formation is Closely Related to Surface Adhesion. Mycopathologia 181, 41–49 (2016). https://doi.org/10.1007/s11046-015-9894-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-015-9894-1