Abstract
Chromoblastomycosis (CBM) is an implantation mycosis mainly occurring in tropical and subtropical zones worldwide. If not diagnosed at early stages, patients with CBM require long-term therapy with systemic antifungals flanked by various physical treatment regimens. As in other neglected endemic mycoses, comparative clinical trials have not been performed for this disease; nowadays, therapy is mainly based on a few open trials and on expert opinions. Itraconazole, either as monotherapy or associated with other drugs, or with physical methods, is widely used. Recently, photodynamic therapy has been employed successfully in combination with antifungals in patients presenting with CBM. In the present paper, the most used therapeutic options against CBM are reviewed as well as the several factors that may have impact on the patient’s outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromoblastomycosis is one of the most prevalent implantation mycosis in tropical and subtropical zones. Lesions related to this disease may be recalcitrant and extremely difficult to eradicate. Except for the initial lesions, which should be surgically removed, CBM lesions constitute a true therapeutic challenge. Almost a century has passed since Max Rudolph first reported the disease in 1914. Since then, several therapeutic regimens have been proposed, including physical therapeutic methods and chemotherapy with antifungals [1, 2]. As comparative trials on this disease are lacking, evidence that helps to select optimal therapy is based on a few open clinical studies and many expert opinions [3–7]. No “gold standard” therapy for CBM is available, but several treatment options [7, 8].
Microbiological and histological diagnoses are advised in order to achieve optimal therapeutic outcome. Chromoblastomycosis is caused by several species of melanized (‘dematiaceous’) fungi, almost all being members of the black yeast relationship included in the ascomycete family Herpotrichiellaceae. This group of fungi is known from unusual, hostile habitats such as those extremely poor in nutrients, or toxic due to the presence of monoaromatic hydrocarbons [9–11]. Agents of CBM are found on plants thorns or debris. They mainly belong to Fonsecaea and Cladophialophora and to a less extent to Rhinocladiella, while scattered cases have been reported in Phialophora and Exophiala [9–15]. Most agents are filamentous in culture and present with muriform cells in human tissue. Muriform cells are the hallmark for the diagnosis of CBM and are considered a biologic and physiologic adaptation to stress elicited by the host’s response to transcutaneous implantation of foreign bodies [12, 16, 17]. This protective structure also enhances resistance to antifungal drugs [18, 19]. Muriform cells are characterized by thick melanized cell walls, isodiametric expansion and unordered septum formation. In main traits, Cladophialophora carrionii and Phialophora verrucosa are more sensitive to antifungals than Fonsecaea pedrosoi [20], while also differences in susceptibility have been reported between the species of Fonsecaea, viz. F. pedrosoi, F. monophora and F. nubica [21, 22]. Prior to exact identification of the etiologic agent, therapy can be started at observation of muriform cells in wet mounts and/or histopathology. Clinical manifestations are highly variable but no data are available on correlation of lesion type and etiologic agent [8, 16]. The present article reviews the main therapeutic modalities for patients with CBM ad discuss the main therapeutic challenges to clinicians in the control of the disease.
Clinical Classification, Severity Grade and Length of Therapy
Except for its initial lesions, developed lesions of CBM require long-term continuous systemic antifungal therapy. The duration of therapy may range from months to years and even more than one decade [3–5, 20, 23]. In order to achieve optimal results in terms of clinical and microbiological efficacy as well as patient’s compliance, it is recommended that lesions are classified according to clinical type and graded according to degree of severity (Table 1). During the last century, several clinical classifications were proposed by many authors to better describe the clinical polymorphism of CBM lesions. The one modified after Carrión is well accepted in the clinical practice. It uses basic dermatologic lesions types: nodular, tumorous, verrucous, plaque and scarring (Table 1, Fig. 1) [9, 16, 24, 25]. More recently, other clinical types of lesions have been described: pseudo-vacuole and eczematous types (Figs. 1F, I). These lesions were described by Lu et al. [26] and refer to mild to moderate cases depicting short duration in years and good response to therapy. In advanced cases, more than one type of lesion can be observed in the same patient and one type may predominate to the others (Fig. 1E) [9]. Although CBM usually spreads by contiguity and auto-implantation, lymphatic dissemination has been described in a few cases [27, 28]. Success rates are related to factors including etiologic agent, duration and severity of the disease and host individual response. Severe lesions tend to respond slowly or even become non-responding to antifungal drugs (Table 1) [3, 5]. Mean duration of therapy with antifungal drugs, physical methods or combination of both, should be guided by clinical, mycological and histological criteria (Table 1) [2, 9, 28].
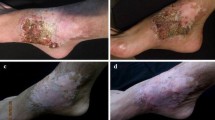
Initial lesion of chromoblastomycosis: A scaly papulous ulcerative lesion on the knee, after six months evolution (1A). Nodular lesions on the lower leg (1B). Verrucous lesion of the foot (1C). Plaque lesion on the sholder (1D). Mixed lesion composed by plaque, nodular and verrucous lesions involving the lower limb (1E).Pseudo vacuole lesions involving the hand and forearm (1F)*. Cicatritial lesions of the knee and upper leg (1G). Tumoral lesions on the thigh and knee (1H). Eczymatous lesions of the hand and wrist (1I) * Courtesy of Prof Liyan Xi, Sun Yat Sen University, Guangzhou, China
Antifungal Susceptibility
Based on non-comparative trials, patients are mostly treated with itraconazole (ITZ), terbinafin (TBF) or with a combination of both [3–7]. To date, no standardization of in vitro susceptibility is available, and break points values have not been determined for melanized fungi [11, 29, 30]. Andrade et al. [31] suggested some correlation between in vitro and in vivo data. Susceptibility profiles are particularly significant in patients not or insufficiently responding to antimycotic therapy. Earlier studies have documented that F. pedrosoi isolates is susceptible to ITZ, but has high minimal inhibitory concentration (MICs) to amphotericin B (AMB), fluconazole (FCZ) and 5-flucytosine (5-FC) [29, 30]. It was suggested that ITZ resistance might be acquired in patients under chronic use of this drug [29, 31]. Najafzadeh et al. [32] described the sensitivity test in three Fonsecaea species in accordance with the CLSI document M38-A2. All isolates had low MICs for posaconazole (POS), ITZ, isavuconazole (ISA) and voriconazole (VCZ). High MICs were documented against AMB, caspofungin (CAS), anidulafungin (ANI) and FCZ. MICs to VCZ, POS and ISA were 1 or 2 dilutions higher in F. pedrosoi than F. monophora and F. nubica [32]. Similar to Fonsecaea, C. carrionii showed sensitivity to ITZ, VCZ and TBF. Testing environmental isolates of C. carrionii complex demonstrated that C. carrionii strains from cactus debris and C. yegresii strains from living cactus were indistinguishable in their response to antifungals [33, 34].
The second most commonly used drug for CBM is TBF, an allylamine derivative causing fungicidal effects through blockade of lanosterol synthesis, an ergosterol precursor. Terbinafin does not interfere with P450 cytochrome, leading to less drug-to-drug interaction than ITZ [23, 75–79]. One report suggested that TBF has significant antifibrotic effect in vitro, but unfortunately this was not substantiated in vivo [4]. According to published data, cure rates with ITZ or TBF may range from 15 to 80 %, depending on the etiologic agent, severity of the disease and criteria of cure used for therapy evaluation [3, 5, 9].
Combination Therapy
Combination therapy with antifungal drugs is common in the setting of severe and invasive infections. Few studies have been conducted with melanized fungi. Yu et al. [35] studied 53 isolates, which included 22 C.carrionii, 20 P. verrucosa and 11 F. pedrosoi isolates. The in vitro evaluation of the combination of antifungal drugs against CBM agents was made with D-AMB, ITZ and TBF. No synergic or antagonistic results were observed with all isolates when D-AMB was combined with TBF or ITZ. A synergic interaction was noted only to one isolate of C. carrionii when TBF and ITZ were combined. Neither synergy nor antagonism was found with the remaining isolates of C. carrionii and all isolates of P. verrucosa and F. pedrosoi [35]. According to the clinical response achieved in patients from Guyana and Brazil, the combination of ITZ and 5-FC is effective and should be evaluated in in vitro tests [36–38].
In summary, D-AMB, FCZ and the echinocandins should not be used for the primary therapy of patients with CBM. Primary resistance is rare, and it is not an important problem to start treatment of patients with CBM. It is important to note that most of in vitro studies involving CBM agents were conducted with the mycelial phase propagules (hyphae and conidia) but not with the pathogenic (muriform cells) phase. So the ‘in vitro’ data may not represent the ‘in vivo’ reality. Although methods to obtain in vitro muriform cells have been reported as well as an experimental model in Wistar rats, to date, there are no data on experimental therapy for CBM lesions, neither in in vitro nor in in vivo models [39, 40]. As muriform cells have not been tested for antifungal drugs in vitro yet, further studies with a better methodology simulating host conditions are necessary.
Adjuvant Therapy
Innate immunity cells with subsequent activation of humoral pathways conserved molecular structures, including Toll-like receptors (TLRs) and lectins, are accepted as the initial response mediators against several fungal agents [41]. Despite the evolution in the immunopathology of fungal diseases, classical aspects of fungal infections and associated diseases are explained in terms of Th1/Th2 activation patterns. CD4 + T cells can be subdivided into two distinct effector populations, Th1 and Th2, and this differentiation is important for defining the host susceptibility or resistance to fungal agents [42, 43]. The Th1 response is characterized by the production and action of cytokines such as interferon-γ (INF-γ), interleukin-6 (IL-6) and IL-12. The Th2 response is characterized by overproduction of IL-4 and IL-10 [44].
Although studies of the immunopathogenesis of infections caused by Aspergillus, Candida and Cryptococcus are well characterized in the literature, few studies about immunopathogenesis of melanized and dimorphic fungal infections are available [42, 43]. Mazo et al. [45] described the relationship between severity of clinical forms of CBM and Th1/Th2 activation pattern. They characterized two distinct clinical populations of patients based on the severity of the disease: a group with mild forms that developed Th1 response with high production of INF-γ and other group that developed severe forms of CBM with Th2 response and high production of IL-10 and low production of INF-γ. So, this study suggests that high levels of INF-γ and low levels of IL-10 are important in the control of CBM infection. The chronicity of CBM has a large impact on its treatment. Currently, immunological aspects are directly related to the pattern of chronic disease. Sousa et al demonstrated that in murine models Fonsecaea pedrosoi not primarily recognized by Toll-like Receptors (TLR), resulting in defective induction of pro-inflammatory cytokines and promoting a chronic disease. Perhaps the exogenous administration of TLR agonists may be used in the treatment of CBM. In addition, the enhancement of inflammatory responses may be responsible for the anti-infective activities and the immunostimulants agents (glucan and Imiquimod) can be used as potent arsenal in therapy of CBM [46].
Over the time, CBM lesions may became fibrotic and reduce the availability of antifungal drugs to the site of infection [9]. At this scenario, immunomodulatory therapies may constitute an interesting strategy in the management of severe forms of the disease. Immunostimulant drugs represent the main form to modify the management of fungal and parasitic infections. Glucan (beta-1,3-polyglucose from Saccharomyces cerevisiae) had been used as an adjuvant in the experimental immunization against Leishmania donovani [47]. Meira et al. [48] reported a group of 10 patients with paracoccidioidomycosis that received intravenous glucan as an immunostimulant and they observed that the patients who received glucan had a more favorable response to antifungal therapy. There is only one report of adjuvant treatment with glucan for CBM. Azevedo et al. [49] described a severe and disseminated form of CBM with facial, cervical and trunk involvement. This patient had Th2 activation pattern with high production of IL-10 and low levels of INF-γ. The association of glucan with the ITZ regimen allowed after 6 months a decrease in the production of IL-10 and an increase in the production of INF-ϒ. The use of immunostimulant drugs (glucan) improved the cellular immune response of the patient and allowed the regression of the disease.
The Role of Physical Methods
During the last decades, a series of non-chemotherapeutic methods were described for the therapy of CBM (Table 2). The best of these is surgical excision, but can be used for initial lesions only. Chromoblastomycosis initial lesions are scarcely observed. They start as solitary papular lesions that may evolve with a scaly nodular appearance with well-delimited edges and clear-cut planes of cleavage. Usually, these characteristics are present during the first one to 3 months of evolution (Fig. 1). If not removed at this phase, the initial lesion will evolve and assume one or more of the basic clinical types with different severity grades, mild, moderate or severe, and should not be treated with surgery anymore. Differently from phaeohyphomycosis (PHM) cystic lesions, CBM lesions are not encapsulated and thus they do not depict a clear cleavage limit [50–52]. In addition, relapses are frequent when non-initial lesions are submitted to resection methods, including surgery, curettage and electro-cautery [17, 51, 53, 54]. The first reported physical method used for CBM lesions was iontophoresis with copper sulfate [51]. This was a long, ineffective and painful therapeutic mode, which has been abandoned as many others therapeutic methods in the past [52]. There are several other physical methods previously used by different authors in the past (Table 2). The recommendations for using physical methods in CBM are based on case reports or case series only. In contrast to antifungal chemotherapy that has been evaluated in non-comparative clinical trials, patients submitted to therapy with physical methods were not followed properly according to clinical, mycological and histological defined criteria of cure (Table 1) [3, 5]. For this reason, physical methods must be always associated with antifungals when treating mild, moderate or severe forms of the CBM lesions.
The most used physical methods for non-initial lesions are thermotherapy and photodynamic therapy (PDT). It is important to note that these methods may short the duration of therapy when combined to systemic antifungals. Thermotherapy encompasses heat and cold therapy. In the former, thermal devices are used to increase tissue local temperature local applications in a continuous way [55, 56]. Some studies showed no growth of F. pedrosoi on Sabouraud dextrose agar when they were incubated at 42.5 °C [57]. Local heat therapy can promote vasodilation and diffusion of antifungal drugs in tissues, and it can have a great value as adjuvant therapy of CBM lesions. However, it can cause skin burns if it is not well monitored [58]. Cold therapy or cryosurgery method is based on the local application of liquid nitrogen allowing localized tissue freezing, necrosis and inflammation. Tissue reactions are the main healing factor for the improvement of the CBM lesions. There is evidence showing that extremely cold environments do not kill the muriform cells in vivo, nor inhibit the filamentous phase in vitro [59]. On the other hand, tissue damage induced by cold such as necrosis and inflammation is responsible for the destruction of fungi after some days or weeks [59, 60]. Tissue necrosis can be severe, causing pain and secondary bacterial infections. Furthermore, it cannot be performed in some areas like face, fingers and areas around the joints. Cryotherapy should be used in combination with antifungal drugs such as ITZ or TBF, and more studies are needed to assess this association.
Photodynamic Therapy
Photodynamic therapy is a therapeutic modality with non-toxic, light-sensitive compounds that are exposed to specific light, with a conversion into toxic compounds that are toxic to malignant cells or infectious agents. It is a minimally invasive modality of treatment with few side effects, and classically, it is used in the treatment of neoplastic skin tumors (cutaneous T cell lymphoma), tumors localized in the oral cavity and non-melanoma skin cancer, actinic keratosis, acne vulgaris, photo-rejuvenation and suppurative hydradenitis [61–64]. Toxic substances are produced by the interaction between light and tissue leading to oxidative stress. Reactive oxygen species produce such peroxide or superoxide anions, which damage cellular targets of host and possible infectious agents [61–63]. Although PDT has been created for the adjuvant treatment of malignant processes, its usefulness in the management of infectious diseases has been increasing in recent years. Concern about antimicrobial resistance has led the creation of alternative mechanisms of treatments with good efficacy and low toxicity. In the setting of fungal diseases, PDT has been useful in the management of mucocutaneous candidiasis, pityriasis versicolor, dermatophytosis (T. rubrum) and onychomycosis [65].
Lyon et al. [66] selected ten patients with CBM, diagnosed by identification of muriform cells in tissue and no description of the species involved, without any antifungal treatment in the last 6 months and submitted them to PDT with a 20 % methylene blue preparation in cream. All of ten patients presented reduction in the CBM lesions after six applications, with at least 80 % of improvement in clinical and microscopic analysis. The authors concluded that PDT is a promising modality and a well-tolerated therapy. The same authors also evaluated the in vitro PDT method, using methylene blue as photosensitizer against two isolates of F. pedrosoi and two isolates of C. carrionii. In this therapy, light-emitting diodes were efficient in reducing the growth of all samples tested [67]. These findings were shared by Yang et al. [68] described a refractory case of CBM caused by a multidrug sensitive strain of F. monophora treated with ITZ and TBF alone or in combination. After the start of PDT with 5-aminolevulinic acid (ALA) and combined therapy with TBF and VCZ, they observed a greatly reduction the size of CBM lesion. In recent years, the employment of PDT as a co-adjuvant therapy for CBM has been increasing [66–68].
Despite the remarkable advances in molecular characterization of CBM agents and in understanding their worldwide epidemiology in recent years, studies are needed in order to develop drugs with good pharmacokinetic profiles and substances that may exert immunomodulatory roles, such as INF-γ and glucan. Furthermore, thermotherapy and PDT, especially the latter, can represent potential modalities of adjuvant therapy in the management of this disease, where other clinical variables cannot be modified.
The role of Antifungal Chemotherapy
Topic Therapy
All CBM patients not depicting initial lesions should undergo systemic antifungal chemotherapy independently of lesion type or severity. Only one comparative trial of two topic antifungal therapies has been reported in patients with mild CBM lesions [69]. In this study, ajoene (4,5,9-trithiadodeca-1,6,11-triene-9-oxide), a garlic-derived compound, was compared to 5-fluoracil (5-FU) [70]. In this Venezuelan study, patients in whom C. carrionii caused the disease were randomized and allocated to receive either ajoene (0.5 % gel; n = 19) or 5-FU (1 % cream; n = 18) topically with occlusion, for 12–16 weeks. Efficacy was evaluated by clinical and mycological criteria, but not histologically. Complete clinical and mycological remission was achieved in 74 % of patients treated with ajoene and in 78 % of patients submitted to 5-FU [69]. There was no statistical difference in terms of responses or relapses. Both regimens were safe and effective. This original trial was not reproduced in patients presenting CBM mild lesions due do Fonsecaea spp. In addiction, this substance is a biologic compound and is not available in a chemically defined formulation.
Systemic Antifungals
For all patients where the initial lesions were not diagnosed or surgically removed, systemic antifungal therapy is validated. Among several compounds that were used and abandoned, ITZ or TBF are indicated as first-line therapies and PCZ for refractory cases (Table 1). Itraconazole is a fungistatic first generation triazole, which inhibits the cell membrane ergosterol via 14-alpha-demethylase blockage [71]. Itraconazole is the most experienced drug in CBM to date [3, 5, 20, 23, 72, 73]. Although this drug has a safe profile, even when used in long-term course, ITZ is available only in capsule formulation in most of the endemic regions for CBM [74]. This may be an issue and the explanation for unsuccessful responses in that some patients may present poor gut absorption and consequently low plasma and tissue levels [72]. Another important issue is the several ITZ drug-to-drug interactions that may contribute to treatment failures (Table 3) [75].
The second most used drug for CBM is TBF, an allylamine derivative causing a fungicidal effect through lanosterol synthesis, an ergosterol precursor, though blockade of squalene epoxidades. Terbinafine does not interfere with P450 cytochrome, leading to less drug-to-drug interaction than ITZ [23, 76–80]. Finally, there is one report suggesting that TBF shows a significant antifibrotic effect in vitro. Unfortunately, this finding has not been studied in vivo, at histopathologic level [4]. According to the published data, cure rates with ITZ or TBF may range from 15 to 80 %, depending on the etiologic agent, severity of the disease and criteria of cure used for therapy evaluation. As expected, as severity increases, cure rate decreases and relapses are frequent [3, 5, 9].
Combination Therapy
Combination therapy is also an option for refractory or severe clinical forms of the disease. In the past, the association of 5-FC plus D-AMB and 5-FC was used, but not nowadays [80, 81]. Although the in vitro combination of ITZ and TBF did not show synergism, the clinical effect in vivo was clearly demonstrated in patients presenting refractory CBM [6]. The combination of ITZ and 5-FC was used in a few moderate to severe cases with excellent results [36–38].
The Future
The emergence of new antifungal drugs in the setting of invasive mycosis has allowed their use in the treatment of several endemic mycoses, including CBM. Among the recently licensed antifungals, PCZ is an attractive option for severe or refractory forms of CBM [82, 83]. Posaconazole presents the broadest in vitro antifungal spectrum, also encompassing the melanized fungi responsible for CBM and PHM. In addition, PCZ oral solution has a better pharmacokinetic and pharmacodynamic profile than ITZ in capsules. It is expected that in the future, new PCZ formulations, that is, oral tablets and intravenous solutions, may also play a role in the therapy of patients with CBM. The combination of PCZ with TBF and 5-FC may be a potential option for refractory cases. Although the in vitro data suggested that the echinocandins are not effective against the melanized fungi, their association with amphotericin B or with triazoles was tried in patients with PHM and cancer [84]. A less used option for refractory disease is oral VCZ. It was used to treat a few refractory patients with good results, but VCZ may be associated with adverse events such photosensitive dermatitis and visual abnormalities [85]. Isavuconazole, a new broad-spectrum triazole, was also evaluated for safety and efficacy in a few patients with CBM and PHM who were enrolled in a global antifungal clinical trial. It may be another therapeutic option in the future [86].
Major recent advances in knowledge on CBM are the employment of molecular methods in the taxonomy of the etiologic agents and in the epidemiology of this disease. However, this scientific progress does not seem to impact the management of patients around the world [18]. Patients with CBM still represent a true therapeutic challenge for clinicians. Early clinical suspicion and adequate therapy are still fundamental to improve patient’s quality of life.
References
Rudolph M. Über die brasilianische ‘Figueira’ (Vorläufige Mitteilung). Archiv Schiffs- und Tropen-Hyg. 1914;18:498–9.
Castro RM, Castro LGM. On the priority of description chromomycosis. Mykosen. 1987;30(9):397–403.
Restrepo A, Gonzalez A, Gomez I, Arango M. de BC. Treatment of chromoblastomycosis with itraconazole. Ann N Y Acad Sci. 1988;544:504–16.
Esterre P, Inzan CK, Rtasioharana M, et al. A multicenter trial of terbinafine in patients with chromoblastomycosis: effects on clinical and biological criteria. J Dermatol Treat. 1998;9:529–34.
Queiroz-Telles F, Purim KS, Fillus JN, Bordignon GF, Lameira RP, Van Cutsem J, Cauwenbergh G. Itraconazole in the treatment of chromoblastomycosis due to Fonsecaea pedrosoi. Int J Dermatol. 1992;31(11):805–12.
Gupta AK, Taborda PR, Sanzovo AD. Alternate week and combination itraconazole and terbinafine therapy for chromoblastomycosis caused by Fonsecaea pedrosoi in Brazil. Med Mycol. 2002;40(5):529–34.
Bonifaz A, Carrasco-Gerard E, Saul A. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses. 2001;44(1–2):1–7.
Queiroz-Telles F, Esterre P, Perez-Blanco M, Vitale RG, Salgado CG, Bonifaz A. Chromoblastomycosis: an overview of clinical manifestations, diagnosis and treatment. Med Mycol. 2009;47(1):3–15.
Rippon JW. Chromoblastomycosis and related dermal infections caused by dematiaceous fungi. In: Mycology Medical, editor. The Pathogenic Fungi and the Pathogenic Actinomycetes. 2nd ed. JW Rippon, Philadelphia: WB Saunders; 1982. p. 249–76.
Queiroz-Telles F, Nucci M, Colombo AL, Tobón A, Restrepo A. Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol. 2011;49(3):225–36.
Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23(4):884–928.
Queiroz-Telles F, Nucci M, Colombo AL, Tobón A, Restrepo A. Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol. 2011;49(3):225–36.
Borelli D. Acrotheca aquaspersa nova, new species agent of chromomycosis. Acta Cient Venez. 1972;23(6):193–6.
Barba-Gómez JF, Mayorga J, McGinnis MR, González-Mendoza A. Chromoblastomycosis caused by Exophiala spinifera. J Am Acad Dermatol. 1992;26(2 Pt 2):367–70.
Naka W, Harada T, Nishikawa T, Fukushiro R. A case of chromoblastomycosis: with special reference to the mycology of the isolated Exophiala jeanselmei. Mykosen. 1986;29(10):445–52.
Matsumoto T, Matsuda T, McGinnis MR, Ajello L. Clinical and mycological spectra of Wangiella dermatitidis infections. Mycoses. 1993;36(5–6):145–55.
McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8(1):1–16.
Esterre P, Queiroz-Telles F. Management of chromoblastomycosis: novel perspectives. Curr Opin Infect Dis. 2006;19(2):148–52.
Rosen T, Overholt M. Persistent viability of the Medlar body. Int J Dermatol. 1996;35(2):96–8.
Borelli D. A clinical trial of itraconazole in the treatment of deep mycoses and leishmaniasis. Rev Infect Dis. 1987;9(Suppl 1):S57–63.
Najafzadeh MJ, Gueidan C, Badali H, Van Den Ende AH, Xi L, De Hoog GS. Genetic diversity and species delimitation in the opportunistic genus Fonsecaea. Med Mycol. 2009;47(1):17–25.
Najafzadeh MJ, Sun J, Vicente V, Xi L, van den Ende AH, De Hoog GS. Fonsecaea nubica sp. nov, a new agent of human chromoblastomycosis revealed using molecular data. Med Mycol. 2010;48(6):800–6.
Bonifaz A, Paredes-Solis V, Saul A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5(2):247–54.
Carrion AL. Chromoblastomycosis. Ann N Y Acad Sci. 1950;50(10):1255–82.
Xi L, Lu C, Sun J, Li X, Liu H, Zhang J, Xie Z, De Hoog GS. Chromoblastomycosis caused by a meristematic mutant of Fonsecaea monophora. Med Mycol. 2009;47(1):77–80.
Lu S, Lu C, Zhang J, Hu Y, Li X, Xi L. Chromoblastomycosis in Mainland China: A Systematic Review on Clinical Characteristics. Mycopathologia. 2012 Oct 20. [Epub ahead of print].
Badali H, Bonifaz A. Barrón-Tapia et al. Rhinocladiella aquaspersa, proven agent of verrucous skin infection and a novel type of chromoblastomycosis. Med Mycol. 2010;48(5):696–703.
Bayles MA. Chromomycosis. In: Hay RJ, (ed.), Baillière’s clinical tropical medicine and communicable diseases. tropical fungal infections. London: WB Saunders; 1986;4(1):45–70.
McGinnis MR, Pasarell L. In vitro evaluation of terbinafine and itraconazole against dematiaceous fungi. Med Mycol. 1998;36(4):243–6.
Caligiorne RB, Resende MA, Melillo PH, Peluso CP, Carmo FH, Azevedo V. In vitro susceptibility of chromoblastomycosis and phaeohyphomycosis agents to antifungal drugs. Med Mycol. 1999;37(6):405–9.
Andrade TS, Castro LG, Nunes RS, Gimenes VM, Cury AE. Susceptibility of sequential Fonsecaea pedrosoi isolates from chromoblastomycosis patients to antifungal agents. Mycoses. 2004;47(5–6):216–21.
Najafzadeh MJ, Badali H, Illnait-Zaragozi MT, De Hoog GS, Meis JF. In vitro activities of eight antifungal drugs against 55 clinical isolates of Fonsecaea spp. Antimicrob Agents Chemother. 2010;54(4):1636–8.
de Hoog GS, Nishikaku AS, Fernandez-Zeppenfeldt G, Padín-González C, Burger E, Badali H, Richard-Yegres N. van den Ende AH. Molecular analysis and pathogenicity of the Cladophialophora carrionii complex, with the description of a novel species. Stud Mycol. 2007;58:219–34.
Vitale RG, Perez-Blanco M, De Hoog GS. In vitro activity of antifungal drugs against Cladophialophora species associated with human chromoblastomycosis. Med Mycol. 2009;47(1):35–40.
Yu J, Li R, Zhang M, Liu L, Wan Z. In vitro interaction of terbinafine with itraconazole and amphotericin B against fungi causing chromoblastomycosis in China. Med Mycol. 2008;46(7):745–7.
Pradinaud R, Bolzinger T. Treatment of chromoblastomycosis. J Am Acad Dermatol. 1991;25(5 Pt 1):869–70.
Bolzinger T, Pradinaud R, Sainte-Marie D, Dupont B, Chwetzoff E. Traitement de quatre cas de chromomycose à Fonsecaea pedrosoi par l’association 5-fluorocytosine-itraconazole. Nouv Dermatol. 1991;10:462–6.
Antonello VS, Appel da Silva MC, Cambruzzi E, Kliemann DA, Santos BR, Queiroz-Telles F. Treatment of severe chromoblastomycosis with itraconazole and 5-flucytosine association. Rev Inst Med Trop Sao Paulo. 2010;52(6):329–31.
da Silva MB, da Silva JP. Sirleide Pereira Yamano S, Salgado UI, Diniz JA, Salgado CG. Development of natural culture media for rapid induction of Fonsecaea pedrosoi sclerotic cells in vitro. J Clin Microbiol. 2008;46(11):3839–41.
Xie Z, Zhang J, Xi L, Li X, Wang L, Lu C. Sum J A chronic chromoblastomycosis model by Fonsecaea monophora in Wistar rat. Med Mycol. 2010;48:201–6.
Romani L. Cell mediated immunity to fungi: a reassessment. Med Mycol. 2008;46(6):515–29.
Shoham S, Huang C, Chen JM. Golenbock D T, Levitz SM. Toll-like receptor 4 mediates intracellular signaling without TNF-α release in response to Cryptococcus neoformans polysaccharide capsule. J Immunol. 2001;166(7):4620–6.
Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11(4):275–88.
Shoham S, Levitz SM. The immune response to fungal infections. Br J Haematol. 2005;129(5):569–82.
Mazo Fávero Gimenes V, de Da Glória Souza M, Ferreira KS, Marques SG, Gonçalves AG, de Vagner Castro Lima Santos D, de Pedrosoe Silva CM, Almeida SR. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7(4):708–13.
Sousa MG, Reid DM, Schweighoffer E, Tybulewicz V, Ruland J, Langhorne J, Yamasaki S, Taylor PR, Almeida SR, Brown GD. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe. 2011;9(5):436–43.
Di Luzio NR, Williams DL. The role of glucan in the prevention and modification of microparasitic diseases. Prog Clin Biol Res. 1984;161:443–56.
Meira DA, Pereira PC, Marcondes-Machado J, Mendes RP, Barraviera B, Pellegrino Júnior J, Rezkallah-Iwasso MT, Peracoli MT, Castilho LM, Thomazini I, Da Silva CL, Foss NT, Curi PR. The use of glucan as immunostimulant in the treatment of paracoccidioidomycosis. Am J Trop Med Hyg. 1996;55(5):496–503.
de Azevedo CM, Marques SG, Resende MA, Gonçalves AG, Santos DV, da Silva RR, de Sousa Mda G, de Almeida SR. The use of glucan as immunostimulant in the treatment of a severe case of chromoblastomycosis. Mycoses. 2008;51(4):341–4.
Cachão P, Rocha MM, Cabrita J, Rodrigo JG. Chromomycosis. Med Cutan Ibero Lat Am. 1987;15(5):403–6.
Milan CP, Fenske NA. Chromoblastomycosis. Dermatol Clin. 1989;7(2):219–25.
Yeguez-Rodriguez JF, Richard-Yegres N, Yegres F. Estado actual de las alternativas terapeuticas para combater la cromomicosis en Venezuela y en el mundo (parte II). Med Priv. 1992;8(3):54–5.
Fader RC, McGinnis MR. Infections caused by dematiaceous fungi: chromoblastomycosis and phaeohyphomycosis. Infect Dis Clin North Am. 1988;2(4):925–38.
Martin DS, Baker RD, Conant NF. A case of verrucous dermatitis caused by Hormodendrum pedrosoi (Chromoblastomycosis) in North Carolina. Am J Trop Med Hyg. 1936;s1-16(5):593–619.
Hiruma M, Kawada A, Yoshida M, Kouya M. Hyperthermic treatment of chromomycosis with disposable chemical pocket warmers. Report of a successfully treated case, with a review of the literature. Mycopathologia. 1993;122(2):107–14.
Tagami H, Ohi M, Aoshima T, Moriguchi M, Suzuki N, Yamada M. Topical heat therapy for cutaneous chromomycosis. Arch Dermatol. 1979;115(6):740–1.
Tagami H, Ginoza M, Imaizumi S, Urano-Suehisa S. Successful treatment of chromoblastomycosis with topical heat therapy. J Am Acad Dermatol. 1984;10(4):615–9.
Kinbara T, Fukushiro R, Eryu Y. Chromomycosis. Report of two cases successfully treated with local heat therapy. Mykosen. 1982;25(12):689–94.
Castro LGM, Salebian A, Lacaz CS. Células fúngicas permanecem viáveis por até doze dias em lesões de cromomicose tratadas pela criocirurgia com nitrogênio líquido. An Bras Dermatol. 2003;78(3):279–82.
Castro LGM. Mecanismo de cura da cromomicose pela criocirurgia com nitrogênio líquido. An Bras Dermatol. 1989;64(6):297–300.
Moreira LM, Santos FV, Lyon JP, Maftoum-Costa M, Pacheco-Soares C, Silva NS. Photodynamic therapy: porphyrins and phthalocyanines as photosensitizers. Aus J Chem. 2008;61(10):741–54.
Dai T, Fuchs BB, Coleman JJ, et al. Concepts and Principles of Photodynamic Therapy as an Alternative Antifungal Discovery Platform. Front Microbiol. 2012;3:120.
Wainwright M. Photodynamic antimicrobial chemotherapy. J Antimicrob Chemother. 1998;42(1):13–28.
Zeina B, Greenman J, Purcell WM, Das B. Killing of cutaneous microbial species by photodynamic therapy. Br J Dermatol. 2001;144(2):274–8.
Calzavara-Pinton PG, Venturini M, Sala R. A comprehensive overview of photodynamic therapy in the treatment of superficial fungal infections of the skin. J Photochem Photobiol B. 2005;78(1):1–6.
Lyon CM, Pedroso e Silva Azevedo JP, Moreira LM, et al. Photodynamic antifungal therapy against chromoblastomycosis. Mycopathologia. 2011;172(4):293–7.
Lyon JP, Moreira LM, de Moraes PC, et al. Photodynamic therapy for pathogenic fungi. Mycoses. 2011;54(5):265–71.
Yang Y, Hu Y, Zhang J, et al. A refractory case of chromoblastomycosis due to Fonsecaea monophora with improvement by photodynamic therapy. Med Mycol. 2012;50(6):649–53.
Pérez-Blanco M, Valles RH, Zeppenfeldt GF, Apitz-Castro R. Ajoene and 5-fluorouracil in the topical treatment of Cladophialophora carrionii chromoblastomycosis in humans: a comparative open study. Med Mycol. 2003;41(6):517–20.
Hassan HT. Ajoene (natural garlic compound): a new anti-leukaemia agent for AML therapy. Leuk Res. 2004;28(7):667–71.
Grant SM, Clissold SP. Itraconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in superficial and systemic mycoses. Drugs. 1989;37(3):310–44.
Heyl T. Treatment of chromomycosis with itraconazole. Br J Dermatol. 1985;112(6):728–9.
Lavalle P, Suchil P, De Ovando F, Reynoso S. Itraconazole for deep mycoses: preliminary experience in Mexico. Rev Infect Dis. 1987;9(suppl 1):S64–70.
Queiroz-Telles F, Purim KS, Boguszewski CL, Afonso FC, Graf H. Adrenal response to corticotrophin and testosterone during long-term therapy with itraconazole in patients with chromoblastomycosis. J Antimicrob Chemother. 1997;40(6):899–902.
Shear N, Drake L, Gupta AK, Lambert J, Yaniv R. The implications and management of drug interactions with itraconazole, fluconazole and terbinafine. Dermatology. 2000;201(3):196–203.
Esterre P, Inzan CK, Ramarcel ER, Andriantsimahavandy A, Ratsioharana M, Pecarrere JL, Roig P. Treatment of chromomycosis with terbinafine: preliminary results of an open pilot study. Br J Dermatol. 1996;134(Suppl 46):S33–6.
Esterre P, Inzan CK, Ratsioharana M. Andriantsimahavandy A, Raharisolo C, Randrianiaina E, Roig P. A multicenter trial of terbinafine in patients with chromoblastomycosis: effects on clinical and biological criteria. J Dermatol Treat. 1998;9(Suppl1):S29–34.
Bonifaz A, Saul A, Paredes-Solis V, Araiza J, Fierro-Arias L. Treatment of chromoblastomycosis with terbinafine: experience with four cases. J Dermatol Treat. 2005;16(1):47–51.
Xibao Z, Changxing L, Quan L, Yuqing H. Treatment of chromoblastomycosis with terbinafine: a report of four cases. J Dermatol Treat. 2005;16(2):121–4.
Astorga B, Bonilla E, Martínez C, Mora W. Tratamiento de la cromomicosis con anfotericina B y 5-fluorcitosina. Med Cut ILA. 1981;9(2):125–8.
Silber JG, Gombert ME, Green KM, Shalita AR. Treatment of chromomycosis with ketoconazole and 5-fluorocytosine. J Am Acad Dermatol. 1983;8(2):236–8.
Negroni R, Tobon A, Bustamante B, et al. Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Rev Inst Med Trop Sao Paulo. 2005;47(6):339–46.
Queiroz-Telles F, Almeida B, Breda G, et al. Therapeutic issues in patients with refractory chromoblastomycosis. Poster presented at the 18th Congress of the International Society for Human and Animal Mycology (ISHAM). Mycoses. 2012;55(suppl4):101.
Ben-Ami R, Lewis RE, Raad II, Kontoyiannis DP. Phaeohyphomycosis in a tertiary care cancer center. Clin Infect Dis. 2009;48(8):1033–41.
Criado PR, Careta MF, Valente NY, Martins JE, Rivitti EA, Spina R, Belda W Jr. Extensive long-standing chromomycosis due to Fonsecaea pedrosoi: three cases with relevant improvement under voriconazole therapy. J Dermatolog Treat. 2011;22(3):167–74.
www.clinicaltrials.gov. Isavuconazole in the Treatment of Renally Impaired Aspergillosis and Rare Fungi—A phase III study.
Acknowledgments
We thank Professor Sybren de Hoog for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Queiroz-Telles, F., de C L Santos, D.W. Challenges in the Therapy of Chromoblastomycosis. Mycopathologia 175, 477–488 (2013). https://doi.org/10.1007/s11046-013-9648-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-013-9648-x