Abstract
Chaetomium globosum Kunze:Fr is a dermatophytic, dematiaceous fungus that is ubiquitous in soils, grows readily on cellulolytic materials, and is commonly found on water-damaged building materials. Chlorate affects nitrogen metabolism in fungi and is used to study compatibility among anamorphic fungi by inducing nit mutants. The effect of chlorate toxicity on C. globosum was investigated by amending a modified malt extract agar (MEA), oat agar, and carboxymethyl cellulose agar (CMC) with various levels of potassium chlorate (KClO3). C. globosum perithecia production was almost completely inhibited (90–100 %) at low levels of KClO3 (0.1 mM) in amended MEA. Inhibition of perithecia production was also observed on oat agar and CMC at 1 and 10 mM, respectively. However, hyphal growth in MEA was only inhibited 20 % by 0.1–100 mM KClO3 concentrations. Hyphal growth was never completely inhibited at the highest levels tested (200 mM). Higher levels of KClO3 were needed on gypsum board to inhibit perithecia synthesis. In additional experiments, KClO3 did not inhibit C. globosum, Fusarium oxysporum, Aspergillus niger, Penicillum expansum, and airborne fungal spore germination. The various fungal spores were not inhibited by KClO3 at 1–100 mM levels. These results suggest that C. globosum perithecia synthesis is more sensitive to chlorate toxicity than are hyphal growth and spore germination. This research provides basic information that furthers our understanding about perithecia formation and may help in developing control methods for fungal growth on building materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Indoor air quality is a major health concern in public buildings and homes. Molds (fungi) have been implicated as primary allergens in homes that can cause a variety of human respiratory problems [5, 13, 17, 23, 27]. Indoor mold of greatest concern is directly associated with water-damaged building materials [4, 17]. Approximately 30 % of the homes in America and Europe have excess moisture problems that allow considerable mold growth [24]. The most common symptom of a mold disorder is the allergic response due to IgE antibody production because of exposure to fungal hyphae, spores, and proteins [7, 17].
Most members of the Chaetomiaceae are cellulolytic and grow on a variety of carbon sources including building materials, paper, and cotton fabrics [1]. C. globosum Kunze:Fr is a fungus placed in the Phylum Ascomycota [1] and is in the Family Chaetomium. C. globosum is identified by the dark ostiolate perithecium with numerous hairs extending from the perithecium [20] that can be visualized on wall board (Fig. 1). The ascospores within the perithecia are the primary agents of dissemination (Fig. 1). Both hyphae and fungal spores can be allergenic. In rare cases, C. globosum has been associated with phaeohyphomycosis and opportunistic infections [2, 19], dermatomycoses [38] contamination of dialysis fluid [14], onychomycosis [21], and infection of dystrophic nails [36]. Mycotoxins of C. globosum have been characterized [15, 16] and found toxic to laboratory animals [29]. In addition, Wijeratne [40] has shown that C. globosum synthesizes anti-cancer compounds.
Chaetomum globosum is commonly found as a saprophyte on straw and dung [1]. Because of the ability to grow on cellulose- and lignin-rich substrates, C. globosum has been investigated as a potential biocontrol against several other plant pathogenic fungi [10, 26]. Strains of C. globosum have shown competition for the cellulolytic substrates in which the plant pathogens often reside and have exhibited antibiosis [11] and mycoparasitism. As a cellulolytic fungus, C. globosum is known for discoloring paper in books and other artifacts [32, 37]. It is commonly found on building materials that have been exposed to water [3, 22, 33].
Sensitivity of fungi to chemicals in the form of fungicides and disinfectants is well known. Chlorate is a nitrate analogue and interferes with nitrate reduction to ammonium and has been used to study nitrate assimilation in fungi, bacteria, and algae [12, 39]. Others postulate that chlorate is converted to chlorite by nitrate reductases and the toxic compound is chlorite [39]. Cove [9] found that chlorate toxicity affecting Aspergillus nidulans was caused by the interference of chlorate with the breakdown of organic sources that would provide nitrogen to the organism. Preliminary studies in our laboratory indicated that chlorate was toxic to C. globosum perithecia production at very low concentrations on both media and gypsum board [42]. By stimulating nitrate nonutilizing (nit) mutants with KClO3, vegetative compatibility and relatedness may be determined. It has been used to test for vegetative compatibility among Fusarium oxysporum isolates [8, 31]. According to van Wijk [39], chlorate is nontoxic to most aquatic organisms, except for a small group of marine brown algae.
Our laboratory is interested in the basic biology of fungi and factors that affect fungal growth and reproduction. In the process of screening several chemicals to determine their effect on C. globosum, we discovered that KClO3, utilized in stimulating nit mutants, inhibited perithecia production. The purpose of this study was to determine the amount of spore germination, hyphal growth, and perithecia synthesis by C. globosum when exposed to various concentrations of potassium chlorate (KClO3). This information will increase our basic knowledge of perithecial growth of C. globosum and may provide useful information to help control the growth of this fungus in indoor environments. Hyphal growth and perithecia synthesis were observed on several media including MEA, CMC, and oat agar using various isolates of C. globosum. Spore germination was observed on MEA amended with KClO3, and C. globosum spore germination was compared with other fungi commonly found in water-damaged buildings. Perithecia synthesis of C. globosum on MEA was compared with perithecia synthesis on gypsum board, where C. globosum often grows in water-damaged buildings.
Materials and Methods
Obtaining and Maintaining the Fungus
Chaetomium globosum isolates were obtained from various sources. C. globosum ECU 1490 and ECU SC-1 were isolated from healthy Cat Brier (Smilax sp.) leaves in 2008. The NM isolate was obtained from a contaminated piece of gypsum board from a water-damaged house in New Mexico compliments of Dr. Geoffrey Smith, New Mexico State University, Department of Biology, Las Cruces, New Mexico, USA, in 2005. The NMmt isolate was obtained from an inoculated piece of gypsum board at ECU that appeared to have different growth patterns than the NM isolate. PI-932 was obtained from Presque Isle Cultures, Inc., and the CB isolate was obtained from Carolina Biological Company. The ECU-lib C. globosum isolate was obtained from water-damaged carpet at the East Central University Library in Ada, Oklahoma. Aspergillus niger, Penicillium expansum, and F. oxysporum isolates used in the germination studies were obtained from Presque Isle Cultures, Inc. All isolates were grown in pure culture and maintained on malt extract agar or potato dextrose agar (PDA).
The fungal isolates were grown on media plates by taking a plug of the fungus from the malt extract agar (MEA). The 5-mm-diameter plug was placed in the center of the plate with the hyphae side of the plug against the media surface.
Comparison of Growth on Various Media
The fungus, C. globosum, was grown on several types of media to determine which was best for visualization and quantification of perithecia. Potato dextrose agar (PDA) and cornmeal agar (CMA) were from Fisher Scientific (Pittsburg, PA) and BBL™ (Becton, Dickinson and Co., Sparks, MD), respectively. These media were prepared according to the label directions. Materials for the other media are as follows: carboxymethylcellulose (CMC): 10 g carboxymethylcellulose sodium salt (Sigma cat no. C5678), 1 g NH4NO3, 1 g KH2PO4, 0.5 g MgSO4 · 7 H2O, 1 g yeast extract, and 12 g agar/L DI water; Czapek-Dox agar (CZ): 3 g NaNO3, 1 g K2HPO4, 0.5 g MgSO4 · 7H2O, 0.5 g KCl, FeSO4 · 7H2O, 30 g sucrose, 15 g agar/L (Difco™, Becton, Dickinson and Co., Sparks, MD); oat agar (oat) was prepared from whole toasted oats (60 g) from a local grocery store (Walmart, Bentonville, AR), and 600 mL DI water was placed in a Waring blender and homogenized for 5 min. After homogenization, 400 mL DI water and 15 g Difco agar/L were added prior to autoclaving; rice agar (RM): 20 g of rice was homogenized in 600 mL of DI water, 400 mL of DI water; 15 g of Difco agar was added to the slurry before autoclaving; malt extract agar (MEA): 8 g Bacto ™ malt extract (Bacto ™, Becton, Dickinson and Co. Sparks, MD) and 15 g agar/L DI water; SNA: 1 g K2HPO4, 1 g KNO3, 0.5 g MgSO4 · 7H2O, 0.5 KCl, 0.2 g sucrose, 0.2 g glucose, and 20 g Difco agar/L of DI water; quarter strength PDA (QPDA): 6 g PD broth media (Difco ™, Becton, Dickinson and Co., Sparks, MD) and 15 g Difco agar/L of DI water. All media were autoclaved for 15 min at 121 psi and cooled, before pouring into 90-mm Petri dishes. All inoculated media were incubated in the dark at room temperature (25 °C). The optimum temperature for C. globosum growth was 25–30 °C. Growth was completely inhibited at 5 and was reduced in most isolates at 33 and 37 °C (Tables 2, 3). Preliminary experiments indicated that light may inhibit sporulation [43]. Therefore, all treatments were placed in a 24-h dark growth chamber or covered in aluminum foil at room temperature.
Comparison of Chaetomium globosum Growth Incubated at Different Temperatures
Initial experiments were conducted to determine the optimum temperature for growth and perithecia synthesis. Six C. globosum isolates were inoculated onto PDA plates as described above. The plates were then placed in 5, 25, 30, 33, or 37 °C incubators. Diameter was measured every 7 days, and perithecia incidence recorded after 14 days. The experiment was repeated with MEA and five isolates at 25 and 30 °C. Growth was measured every 7 days, and incidence of perithecia recorded after 14 days.
Growth in Malt Extract Broth (MEB) with Varying Amounts of Potassium Chlorate
Studies were conducted to determine whether potassium chlorate inhibited C. globosum (NM isolate) growth in malt extract broth (MEB: 8 g of malt extract per 1,000 mL DI water), when the MEB was amended with differing amounts (0–122 mM) of potassium chlorate. Upon removal from the autoclave, the pH of each broth was standardized at 5.5 using either 1 M NaOH or 1 M HCl. Three flasks of each potassium chlorate level containing 50 mL of broth were inoculated with 2 plugs cut from a plate of C. globosum grown on MEA.
The plugs were grown in the broth for 2 weeks on a platform shaker rotating at 50 rpm at 25 °C (RT). The hyphal strands were separated from the broth using a Büchner funnel with Whatman #1 filter paper that was placed in a side arm flask and a vacuum attached to the side arm. The filter paper was moistened and weighed before filtration, and then weighed immediately after filtration.
Growth on Solid Media Amended with Varying Levels of Potassium Chlorate
Chaetomium globosum (NM isolate) was grown on MEA and oat agar containing various amounts (0–15 g/L; 0–122 mM) of KClO3-amended MEA. A fungal disc (5 mm) of C. globosum was removed from an actively growing edge of a 7- to 14-day MEA culture plate and placed in the middle of a KClO3 amended or control plate (0 mM KClO3). Four to five replications for each treatment were grown for 21 days at 25 °C. The plates were measured every 7 days for hyphal growth, and the number of perithecia was counted after 21 days of growth. Perithecia were counted using a bacterial colony counter magnified approximately 10×. When the density of the perithecia was too high on a single plate (>300), the perithecia on a quadrant of the plate was counted and this figure was used to estimate the total number of perithecia per plate. Due to the high number of perithecia formed on oat (Fig. 2), 10 mL of sterile DI water was placed on each oat Petri dish after 14–21 days of growth. A clean microscope slide was used to gently remove the perithecia with the ascospores from the surface of the agar. The perithecia/ascospore solution was placed in a sterile 50-mL tube and shaken vigorously 25 times. A hemocytometer was utilized to quantify the ascospore production of C. globosum isolates on the various KClO3 treatments.
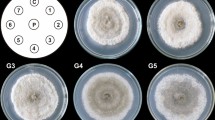
Comparison of C. globosum perithecial growth on MEA, oat agar, and PDA amended with KClO3. a MEA control with 0 mM KClO3 and the bottom plate with 0.1 mM KClO3 after 21 days of growth. b Oat agar control with 0 mM KClO3 and the bottom plate with 10 mM KClO3 after 10 days of growth. c Potato dextrose agar control with 0 mM KClO3 and the bottom plate with 10 mM KClO3 after 21 days of growth
To further confirm the KClO3 inhibitory activity on C. globosum growth and perithecia production, four other C. globosum isolates were grown on 0, 10, and 100 mM KClO3-amended MEA. These experiments were repeated using CMC. Hyphal growth and perithecia were measured as described above for MEA treatments.
Experiments were conducted to determine the KClO3 concentration that would significantly inhibit C. globosum isolates on MEA. Initial experiments using the C. globosum NM isolate utilized MEA plates amended with 0, 0.01, 0.1, 1, 10 mM KClO3. This test was compared to similar concentrations applied to gypsum board. Similar experiments were repeated using MEA plates amended with 0, 0.1, 1. 10, 50, 100, and 200 mM of KClO3 inoculated with three C. globosum isolates. Growth and perithecia were quantified as described previously.
Growth on Gypsum Board
Gypsum board was obtained from a local lumber yard. The gypsum board (wallboard/sheetrock) was cut into 5 cm × 5 cm pieces, placed in glass Petri dishes (90 mm diameter × 20 mm height), and autoclaved for 15 min at 121 psi. Once cooled, either sterile water or 1–10 mM KClO3 was spread over the surface of the gypsum board. The solution was allowed to dry, and then 5 × 105 ascospores were distributed over the sterile sheetrock with a sterile glass rod. Sterile water (1 mL each week) was applied to the bottom of the Petri dish to maintain humidity. All inoculated media and glass Petri dishes were incubated in the dark at room temperature (25 °C). Perithecia were counted after 21 days of growth. Experiments were conducted 3 times with 3–9 replications per treatment.
Chaetomium globosum and Airborne Fungi Growth on Potassium Chlorate-amended Media
Chaetomium globosum (NM isolate) was grown on PDA for 3 weeks allowing abundant perithecia production. Sterile water (10 mL) was gently applied to the surface of the media. A sterile glass slide was used to gently dislodge perithecia/ascospores. The perithecia/ascospore suspension was filtered through a layer of Miracloth™ (Calbiochem-Novabiochem Corp. La Jolla, CA) to separate the ascospores from the hypha and disrupted perithecia. Ascospores were quantified with a hemocytometer and then calibrated to 1,000 spores/mL. One hundred μL of the ascospores suspension was applied to MEA plates with various (1–100 mM) concentrations of KClO3. The same technique was followed using the fungi F. oxysporum, A. niger, and P. expansum. These fungi were chosen because they are often found in water-damaged buildings. The spores were distributed over the media surface with a sterile glass rod. The plates were incubated in the dark at 25 °C, and colonies were counted after 72 h. The experiment was conducted two times with 4–5 replications per treatment.
In another experiment, MEA plates amended with KClO3 (0–100 mM) were exposed to outside air utilizing an Aero-6™ sieve plate instrument (Aerotech laboratories, Inc. Phoenix, AZ). The instrument is very similar to an Andersen sampler and commonly used to quantify viable airborne fungal populations. The plates were placed in the sieve plate instrument, and 75 L of air was pumped through the device onto the KClO3-amended plates for 3 min as recommended by the manufacturer. Plates were incubated in the dark at 25 °C for 72 h, and the fungal colonies counted. Each treatment was replicated 4–5 times, and the experiment was conducted 2 times.
Statistical Analysis
The data were analyzed using Graphpad Instat. Analysis of variance (ANOVA) and Tukey’s comparison of means were conducted on the appropriate data sets emphasized in the results. Ascospore counts on the oat agar were also analyzed using Mann–Whitney paired test to compare the control with the KClO3 treatments. Representative experiments were presented in the tables and figures. Each experiment described above was repeated at least 3 times unless otherwise stated, with 3–5 replicates per treatment.
Results
Growth and Perithecia Production on Various Media
After 6 days of growth on various media, the radial growth of C. globosum was greatest on oat and RM media when compared to the other media. C. globosum hyphae diameter after 6 days was 73 mm on oat and RM (Table 1). The rate of growth for C. globosum on CMC, CMA, MEA, QPDA, V8, PDA, and SNA was less (58–44 mm) than growth on oat and RM. CZ media showed the least growth of all the media tested. Development of perithecia was greatest on oat, then RM followed by V8, CMC, PDA, and MEA. Oat, RM, and PDA stimulated the most luxuriant hyphal growth (Fig. 2, oat and PDA). After 21 days, perithecia were observed in all media plates except CZ (Table 1).
Perithecia grown on MEA appeared on the surface of the media and at a density that could be accurately counted (Fig. 2a). Therefore, quantification of growth and perithecia synthesis was conducted using MEA as the standard media on most of the experiments conducted in this study.
Optimum Temperature Growth of Chaetomium globosum
Optimum growth and perithecial production for isolates appeared to be 25 and 30 °C (Table 2). The two C. globosum isolates found in leaf tissue (ECU 1490 and ECU SC-1) appeared to have a broader temperature range and grew well at 33 °C. The NMmt isolate was the slowest growing isolate at 25 and 30 °C. A significant (P < 0.05) reduction in growth was observed in most isolates at 33 °C. No growth occurred at 5 °C in any of the isolates. At 37 °C, minor hyphal growth was observed in 4 isolates, whereas 2 isolates (CB and ECU SC-1) indicated no growth. Perithecia were observed at 25 °C in all isolates except CB after 19 days of incubation, and all isolates produced perithecia at 30 °C after 19 days. Whereas hyphal growth was significantly reduced in most isolates incubated at 33 °C, 5 of the 6 isolates (NMmt did not) produced perithecia after 19-day incubation.
When comparing growth on PDA and MEA at 25 and 30 °C, there was no significant difference in growth after 14 days among C. globosum isolates (Table 3). Perithecia production was observed in all isolates grown on MEA after 14 days incubated at 25 and 30 °C. Only one isolate (ECU SC-1) produced observable perithecia at 30 °C on PDA after 14-day inoculation. Based upon the information collected in Tables 2 and 3, the KClO3-amended media experiments were conducted at 25 °C and MEA was the standard media for most experiments.
Growth and Perithecia Synthesis of Chaetomium globosum on Solid Media Amended with KClO3
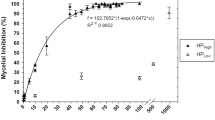
Chaetomium globosum hyphal growth after 7 days in 2 g/L (16 mM) amended MEA was significantly different from the control (Table 4). All KClO3 concentrations (2–15 g/L; 41–122 mM) reduced hyphae growth approximately 6 % after 7 days of growth. Growth of C. globosum in broth culture was reduced approximately 30 % within a concentration range of 16–122 mM (Fig. 3). Perithecia were counted after 21 days of growth. The lowest concentration of KClO3 reduced perithecia formation, and no perithecia were observed on the media in the higher KClO3 concentrations (Table 4, Fig. 2). In further experiments to determine whether lower levels of KClO3 could inhibit perithecia synthesis, perithecia synthesis was inhibited at 0.01 mM and no perithecia grew on the media with 1 mM KClO3 (Fig. 4). A similar experiment was conducted with 3 C. globosum isolates in which the KClO3 range was 0.1–200 mM. All three isolates showed a 10–30 % reduction in hyphal diameter among the 0.1–100 mM KClO3 treatments (Table 5). Perithecial production was reduced almost completely at 0.1 mM KClO3, and no perithecia were observed on plates amended with 10–200 mM KClO3. Hyphal growth was inhibited at 200 mM when compared to the 0.1–100 mM KClO3 treatments in the NM and ECU-lib isolates. When comparing perithecia on MEA and gypsum board, a 10× higher concentration of KClO3 was needed to inhibit perithecia on gypsum board (Fig. 4).
When comparing the KClO3 inhibition of C. globosum (NM isolate) grown on MEA and oat agar, a similar trend was observed (Table 4). However, oat agar produced a profusion of perithecia compared to MEA and PDA (Fig. 2). Therefore, ascospores produced per plate were determined using a hemocytometer. Assuming that the same number of ascospores is produced in each perithecium, the number of ascospores should reflect the relative number of perithecia. Ascospores harvested from the control 0 mM oat agar plates were significantly higher (38–146 times) than the KClO3 treatments. There was no significant difference between the KClO3 treatments.
Experiments were conducted to determine whether the KClO3 inhibition was unique to the NM isolate. Six C. globosum isolates were grown on KClO3 MEA-amended media (Table 6). All isolates showed a similar trend in hyphal growth and perithecia production as the NM isolate. The NMmt isolate produced the highest number of perithecia and the CB isolate produced the least number of perithecia when comparing controls for each isolate (0 mM KClO3 treatment).
An additional media experiment was conducted to determine whether CMC, a highly cellulytic media, would interact with KClO3 in the same manner as MEA and oat agar (Table 7). Four isolates were compared on 0, 10, and 100 mM KClO3. CMC agar stimulated a higher number of perithecia when compared to MEA (Tables 4,5,6). The same trend in the significant perithecia reduction on KClO3-amended CMC was observed on KClO3-amended MEA and oat agar.
Growth of Chaetomium globosum in Broth Culture
Growth of C. globosum in MEA broth was significantly greater in the control (MEA broth only) and 2 g/L (16 mM) KClO3-amended MEA broth, when compared to growth in MEA amended with 5–10 g/L (41–82 mM) KClO3 (Fig. 3). There was no significant difference in the hyphal weight of C. globosum grown in the higher concentrations of KClO3. These results indicate that KClO3 does not completely inhibit hyphal growth of C. globosum in MEA broth at relatively high concentrations (122 mM). This same trend was observed when C. globosum was grown on solid media (Tables 4, 5, 6 and 7 and Figs. 2, 4).
In summary, the experiments represented in Tables 4, 5, 6, and 7 and Figs. 2, 3, and 4 indicate that low levels of KClO3 inhibit C. globosum perithecia production on MEA, oat agar, CMC, and gypsum board. The reduction in hyphal growth and weight was not completely inhibited at the highest levels of KClO3 tested.
Germination of Chaetomium globosum Ascospores, Other Indoor Molds, and Airborne Fungal Spores on Media Amended with Various Concentrations of Potassium Chlorate
Germination of C. globosum ascospores, F. oxysporum conidiospores (primarily macroconidia), A. niger conidia, and P. expansum conidia was not inhibited when exposed to 0–100 mM KClO3 (Table 8). The number of fungal colonies expressed as colony-forming units was not significantly different when comparing the treatment means within the fungal species tested.
MEA plates amended with 0–100 mM were exposed to outdoor air utilizing an Aero-6 sieve plate instrument. The predominant genera appeared to be dematiaceous species. The number of colony-forming units was not significantly different when comparing the means within the different levels of KClO3 tested. These results suggest that KClO3 does not inhibit fungal spore germination on a number of fungal species. However, this aspect needs to be further investigated with more detailed identification.
Discussion
Growth of C. globosum (NM isolate) was significantly (P < 0.05) greater on RM and oat when compared to the other eight media tested. Andersen and Nissen [3] showed that media containing cereals and vegetables stimulated greater growth and increased sporulation of Stachybotrys and Chaetomium isolates. They found consistent growth but variable sporulation on CZ, MEA, and V8 and several other media not tested in this study. The slowest rate of growth in the present study was observed on CZ, which is consistent with the results of Anderson and Nissen [3]. However, we observed consistent perithecia production on MEA (lower concentration of malt extract than normal formula) and V8 agar. MEA was chosen as the media in our experiments because of the quantifiable perithecia production compared to the luxuriant perithecia growing on oat and PDA (Table 1, Fig. 2). MEA enabled the observer to more accurately quantify perithecia production on the media plates. Optimum temperature for C. globosum growth and perithecia production was found to be approximately 25–30 °C for the six isolates tested and corresponds with the fungal growth temperatures used by others [3, 15, 16, 39].
Mycelial growth in broth cultures was not significantly inhibited until KClO3 concentrations were greater than 41 mM (Fig. 3). Increasing KClO3 concentrations tenfold did not significantly reduce hyphal weight, suggesting that C. globosum has a high tolerance for KClO3. Mycelial growth of three C. globosum isolates on solid media (MEA) was inhibited at much lower KClO3 concentrations (0.1 mM). However, there was no significant difference in growth (P < 0.05) among the 0.1 and 100 mM KClO3 concentrations. C. globosum growth rates in broth culture and solid media amended with various KClO3 concentrations align closely to a logarithmic growth curve (R 2 = 0.93).
In contrast to hyphal growth, perithecia production on solid media was significantly reduced when C. globosum was grown on MEA amended with 0.01 mM KClO3, and no perithecia production was observed at 1 mM KClO3. In order to test these effects on building materials, KClO3 liquid suspensions were applied to gypsum board. Applications of 1 mM KClO3 on gypsum board reduced perithecia production and 10 mM completely inhibited perithecia growth (Fig. 4). When oat agar was amended with KClO3, a higher number of perithecia were observed compared to MEA and CMC. In order to quantify the difference between oat agar treatments, ascospores were harvested from the plates and counted. A significant reduction in ascospore synthesis was observed between the 0 mM control and the KClO3 treatments (Table 4). Qualitative differences in C. globosum perithecia were also observed on PDA amended with KClO3 (Fig. 2). However, perithecia were too numerous to count on PDA, and harvesting of ascospores was not successful.
The possibility that KClO3 inhibited ascospore germination rather than perithecia production was tested. C. globosum ascospores were spread over MEA plates and observed for germination and quantified by counting C. globosum colonies. There was no significant reduction in ascospore germination when compared to controls. When further experiments were conducted by exposing media plates amended with 0–100 mM KClO3 to other fungal species often found growing on indoor building materials (Table 8) or to fungal spores collected from outdoor air, the KClO3-amended media (1–100 mM) did not inhibit germination of the other four species tested or the airborne fungal spores. According to these results, it appears that spores of C. globosum and several other fungi are not inhibited by KClO3.
Chaetomium globosum is highly resistant to many environmental stresses. C. globosum exhibits tolerance to salts [6] and has the ability to recolonize and grow on building materials exposed to high levels of radiation [44]. Wilson et al. [41] showed that chlorine dioxide completely inhibited Stachybotrys chartatum, Penicillium chrysogenum, and Cladosporium cladosporium, whereas, 11 % of C. globosum ascospores were not inactivated by the gas. The C. globosum perithecia were implicated as a protective factor against chlorine dioxide infiltration. C. globosum was highly tolerant of LiCl when compared to six other ascomycete fungi [34] and several other imperfect fungi. As the results of this paper indicate, C. globosum appears to be tolerant to relatively high levels of KClO3 in regard to hyphal growth and spore germination.
KClO3 toxicity has been observed in several species including algae, bacteria, and fungi. Toxicity has been associated with the metabolism of chlorate. Nitrate reductase normally reduces nitrate, but can also reduce chlorate to the more toxic chlorite [18, 39]. Strains of Ustilago maydis and Escherichia coli that lack nitrate reductase were found to be resistant to chlorate [28, 30], whereas wild types were chlorate-sensitive. Another toxicity pathway may involve chlorite dismutase that reduces perchlorate to chloride, resulting in a disproportionate amount of chlorite [35]. Cove [9], working with A. nidulans, proposed that chlorate toxicity was associated with the activity of nitrate reductase and the product of the nirA gene. NirA is a up-regulator for nitrate assimilation. Cove [9] also noted that various nitrogen sources affect chlorate sensitivity and that A. nidulans grown in the presence of ammonium reduces nitrogen catabolic activity. Low pH induces toxicity in A. nidulans in a pattern similar to chlorate. However, chlorate up to 500 mM did not affect the pH of the basal media. Fogel [16] observed C. globosum perithecia and ascospores when grown on media with pH of 6–8.6. Sporulation was best in an acidic environment. Our study showed no major change in pH due to addition of KClO3 to the broth or agar media and abundant sporulation at pH 5.5–6. Our study did not indicate that pH played a role in the inhibition of C. globosum perithecia synthesis.
KClO3 has been used to stimulate heterokaryons in several fungi, but most notably in F. oxysporum [8, 31]. When F. oxysporum was grown in 15 g/L of KClO3, 3 distinct phenotypes were classified that presumably were the result of mutations in nitrate reductase (Nit1), nitrate assimilation pathway-specific regulatory locus (Nit3), and molybdenum-cofactor of nitrate reductase (NitM). The presence of nitrate reductase in C. globosum is not known. The present study did indicate that C. globosum hyphal growth was highly resistant to KClO3. Trichoderma hamatum was considered highly resistant to chlorate at ≥7.48 mM [39], whereas C. globosum hyphal growth on 10 mM KClO3 was not significantly less than the control after 4 days of growth, and was never reduced more than 30 % up to 200 mM KClO3. Our results show that many fungi appear to be resistant to chlorate toxicity. Hyphal growth in broth culture showed a similar trend to that observed on agar plates. In contrast, perithecia were inhibited by very low KClO3 levels (0.01 mM KClO3). Johnson [25] predicted that 200–300 genes are involved in perithecia formation. At this time, the mechanism of perithecia inhibition and the perithecia-forming genes that may be mutated by KClO3 are not known.
As indicated above, C. globosum is highly resistant to many environmental stresses. The differential response of hyphal growth and perithecia formation to KClO3 needs further investigation to elucidate the genes involved in reproduction of C. globosum. Further research in the formulation and application of KClO3 to building materials may prove to be an efficient method of inhibiting growth of fungi like C. globosum. Our laboratory is currently investigating the C. globosum proteins expressed when grown in low KClO3 concentrations (0.1–1 mM). The identification of these proteins will lead to genes that may be involved in perithecia inhibition. In additional, further experiments are being conducted on the effect of the type and amount of nitrogen on C. globosum growth and reproduction. This information will help to improve our basic understanding of perithecia formation and, perhaps, provide information that will contribute to the control of fungal growth in water-damaged buildings.
References
Alexopoulos CJ, Mims CW, Blackwell M. Introductory mycology. 4th ed. London: Wiley; 1996.
Anandi V, John TJ, Walter A, Shastry JCM, Lalitha MK, Padhye AA, Ajello L, Chandler FW. Cerebral phaeohyphomycosis caused by Chaetomium globosum in a renal transplant recipient. J Clin Micro. 1989;27:2226–9.
Andersen B, Nissen AT. Evaluation of media for detection of Stachybotrys and Chaetomium species associated with water-damaged buildings. Int Biodeterior Biodegradation. 2000;46:111–6.
Andersson MA, Nikulin M, Köljalg U, Andersson MC, Rainey F, Reijula K, Hintikka E-L, Salkinoja-Salonen M. Bacteria, molds, and toxins in water-damaged building materials. Appl Environ Microbiol. 1997;63:387–93.
Bush RK, Portnoy JM, Saxon A, Terr AI, Wood RA. The medical effects of mold exposure. J Allergy Clin Immunol. 2006;117:326–33.
Cantrell SA, Casillas-Martίnez L, Molina M. Characterization of fungi from hypersaline environments of solar slatterns using morphological and molecular techniques. Mycol Res. 2006;110:962–70.
Chiu A, Fink JN. Fungal allergy and pathogenicity. Introduction. Chem Immunol. 2002;81:1–4.
Correll JC, Klittich CJR, Leslie JF. Nitrate nonutilizing mutants of Fusarium oxysporum and their use in vegetative compatibility tests. Phytopathology. 1987;77:1640–6.
Cove DJ. Chlorate toxicity in Aspergillus nidulans. Mol Gen Genet. 1976;146:147–59.
Dhingra OD, Mizubuti ESG, Santana FM. Chaetomium globosum for reducing primary inoculum of Diaporthe phaseolorum f. sp. meridionalis in soil-surface soybean stubble in field conditions. Biol Control. 2003;26:302–10.
Di Pietro A, Gut-Rella M, Pachlatko JP, Schwinn FJ. Role of antibiotics produced by Chaetomium globosum in biocontrol of Pythium ultimum, a causal agent of damping-off. Phytopathology. 1992;82:131–5.
Dunn-Coleman NS, Smarrelli J, Garrett RH. Nitrate assimilation in eukaryotic cells. Int Rev Cytol. 1984;92:1–50.
Edmundson DA, Nordness ME, Zacharisen MC, Kurup VP, Fink JN. Allergy and “toxic mold syndrome”. Ann Allergy Asthma Immunol. 2005;94:234–9.
Febre N, Silva V, Medeiros EAS, Godoy P, Reyes E, Halker E, Fischman O. Contamination of peritoneal dialysis fluid by filamentous fungi. Rev Iberoam Micol. 1999;16:238–9.
Fogel MR, Douglas DR, Jumper CA, Straus DC. Growth and mycotoxin production by Chaetomium globosum. Mycopathologia. 2007;164:49–56.
Fogel MR, Douglas DR, Jumper CA, Straus DC. Growth and mycotoxin production by Chaetomium globosum is favored in a neutral pH. Int J Mol Sci. 2008;9:2357–65.
Genuis SJ. Clinical medicine and the budding science of indoor mold exposure. Eur J Int Med. 2007;18:516–23.
Goksøyr J. On the effect of chlorate upon the nitrate reduction of plants. I. Experiments with Aspergillus oryzae. Physiol Plant. 1951;4:498–513.
Guarro J, Soler L, Rinaldi MG. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur J Clin Microbiol Infect Dis. 1995;14:613–8.
Hanlin RT. Illustrated genera of the acomycetes, vol. I. St. Paul: APS Press; 1990.
Hattori N, Adachi M, Kaneko T, Shimozuma M, Ichinohe M, Iozumi K. Case report. Onychomycosis due to Chaetomium globosum successfully treated with itraconazole. Mycoses. 2000;43:89–92.
Horner WE, Worthan AG, Morey PR. Air-and dustborne mycoflora in houses free of water damage and fungal growth. Appl Environ Microbiol. 2004;70:6394–400.
Jarvis BB, Miller JD. Mycotoxins as harmful indoor air contaminants. Appl Microbiol Biotechnol. 2005;66:367–72.
Johanning E. Indoor moisture and mold-related health problems. Allerg Immunol. 2004;36:182–5.
Johnston TE. Isolation and characterization of perithecial development mutants in Neurospora. Genetics. 1978;88:27–47.
Köhl J, Bélanger RR, Fokkema NJ. Interaction of four antagonistic fungi with Botrytis aclada in dead onion leaves: a comparative microscopic and ultrastructural study. Phytopathology. 1997;87:634–42.
Korpi A, Pasaneen A, Pasanen P. Volatile compounds originating from mixed microbial cultures on building material under various humidity conditions. Appl Environ Microbiol. 1998;64:2914–9.
Lewis CM, Fincham JRS. Genetics of nitrate reductase in Ustilago maydis. Genetic Res Camb. 1970;16:151–63.
Ohtsubo K, Saito M, Sekito S, Yoshihira K, Natori S. Acute toxic effects of chaetoglobosin A, a new cytochalasan compound produced by Chaetomium globosum. Jpn J Exp Med. 1978;48:105–10.
Piéchard M, Puig J, Pichinoty F, Azoulay E, Le Minor L. Mutation affectant la nitrate-reductase A et d’autre enzymes bactreriennes d’oxydo-reduction. Etude preliminaire. Ann Inst Pasteur. 1967;112:24–37.
Puhalla JE. A visual indicator of heterokaryosis in Fusarium oxysporum from celery. Can J Bot. 1984;62:540–5.
Rakotonirainy MS, Heude E, Lavédrine B. Isolation and attempts of biomolecular characterization of fungal strains associated to foxing on the 19th century book. J Cultural Herit. 2007;8:126–33.
Rao CY, Riggs MA, Chew GL, Muilenberg ML, Thorne PS, Sickle DV, Dunn KH, Brown C. Characterization of airborne molds, endotoxins, and glucans in homes in New Orleans after hurricanes Katrina and Rita. Appl Environ Microbiol. 2007;73:1630–4.
Richter DL, Robinson SC, Beardslee MP, Habarth ML. Differential sensitivity of fungi to lithium chloride in culture media. Mycol Res. 2008;112:717–24.
Rikken GB, Kroon AGM, van Ginkel CG. Transformation of (per)chlorate into chloride by a newly isolated bacterium: reduction and dismutation. Appl Microbiol Biotechnol. 1996;45:420–6.
Rippon JW. Medical mycology. The pathogenic fungi and pathogenic actinomycetes. 3rd ed. Philadelphia: The W.B. Saunders Co.; 1998.
Szczepanowska M, Moomaw WR. Laser stain removal of fungus-induced stains from paper. JAIC. 1994;33:25–32.
Tullio V, Bance G, Allizond B, Roana J, Mandras N, Scalas D, Panzone M, Cervetti O, Valle S, Carlone N, Cuffini AM. Non-dermatophyte moulds as skin and nail foot mycosis agents: Phoma herbarum, Chaetomium globosum and Microascus cinereus. Fungal Biol. 2010;114:345–9.
Van Wijk DJ, Kroon GM, Garttener-Avends CM. Toxicity of chlorate and chlorite to selected species of algae, bacteria and fungi. Ecotoxicol Environ Saf. 1998;40:206–11.
Wijeratne EMK, Turbyville TJ, Fritz A, Whitesell L, Gunatilaka AAL. A new dihydroxanthenone from a plant-associated strain of the fungus Chaetomium globosum demonstrates anticancer activity. Bioorg Med Chem. 2006;14:7917–23.
Wilson SC, Wu C, Andriychuk LA, Martin JM, Brasel TL, Jumper CA, Straus DC. Effect of chlorine dioxide gas on fungi and mycotoxins associated with Sick Building Syndrome. Appl Environ Microbiol. 2005;71:5399–403.
Wright D, Biles C. Environmental and potassium chlorate effects on the growth of Chaetomium globosum. Proc Oklahoma Acad Sci. 2006;86:103.
Young J, Fuego M, Pattison R, Biles CL. Growth and reproduction of Chaetomium globosum on various carbon sources exposed to different light regimes. Proc Oklahoma Acad Sci. 2008;88:53.
Zhdanova NN, Zakharchenko A, Vember VV. Fungi from Chernobyl: mycobiota of the inner regions of the containment structure of the damaged nuclear reactor. Mycol Res. 2000;104:1421–6.
Acknowledgments
This project was supported by the National Center for Research Resources and the National Institute of General Medical Sciences of the National Institutes of Health through Grant Number 8P20GM103447 (OK-INBRE) and the National Institutes of Health-Bridges to the Baccalaureate program at ECU (R25 GM054938-07).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biles, C.L., Wright, D., Fuego, M. et al. Differential Chlorate Inhibition of Chaetomium globosum Germination, Hyphal Growth, and Perithecia Synthesis. Mycopathologia 174, 475–487 (2012). https://doi.org/10.1007/s11046-012-9572-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-012-9572-5