Abstract
This report describes the first isolation of Sporothrix globosa from a Brazilian patient. A 77-year-old woman was examined for sporotrichosis infection. Histopathological examination of skin biopsy revealed chronic granulomatous infiltrate with microabcess. Furthermore, S. schenckii-like yeasts were evident as demonstrated by PAS and Grocott stains. The fungus was identified based on colony morphology on Sabouraud Dextrose Agar slants, Potato Dextrose Agar, and Corn Meal Agar, microscopic morphology on slides cultures, and assimilation of different carbon sources. The species confirmation was made by molecular methodology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sporotrichosis is a subcutaneous mycosis with a global distribution, but the most endemic region is found in Latin America [1–3]. This infection is caused by the dimorphic fungus Sporothrix schenckii, which is associated, in the environment, with plants and soil [4]. Other Sporothrix species, however, have been reported as agents of sporotrichosis such as Sporothrix brasiliensis, Sporothrix globosa, Sporothrix mexicana, and Sporothrix luriei [5–9]. Previous studies revealed the existence of differences in the geographical distribution among the members of the Sporothrix complex, including geographically restricted species such as S. brasiliensis. Conversely, S. globosa is a widespread fungus found up to now in UK, Spain, Italy, China, Japan, USA, India [6, 9] and more recently Mexico, Guatemala, and Colombia [9]. Here, we report a case of lymphocutaneous sporotrichosis due to a non-S. schenckii species, S. globosa in a patient from Rio de Janeiro, an endemic sporotrichosis region in Brazil. This is a recently established species, and to our knowledge, this is the first description of S. globosa in Brazil.
Case Report
A 77-year-old woman, from the Rio de Janeiro metropolitan area (Xerem) presented to Laboratorio de Dermatologia Infecciosa, Instituto de Pesquisa Clínica Evandro Chagas (IPEC), Fundação Oswaldo Cruz for evaluation of a verrucous lesion measuring 3 × 2 cm on her right dorsal hand accompanied of ascending subcutaneous nodules on her arm. There were 12 nodules and some of them were fistulized. The patient reported that the lesions have been developed over a 4-month period. She used to hand plants, vegetable garden and soil in her residence; however, she did not remember the occurrence of local trauma. The only medication used by the patient before medical attending was Captopril 50 mg/day for treatment of high blood pressure.
Skin biopsy was performed on the lesions, and tissue fragments were sent for histopathological and microbiological examination. Direct examination of tissue fragments after KOH clarification of all samples was negative. Hematoxilin and eosin examination revealed chronic granulomatous infiltrate with microabcess. Furthermore, S. schenckii-like yeasts were evident as demonstrated by PAS and Grocott stains. The fungal culture performed at 25°C on Sabouraud Dextrose Agar showed after 7 days filamentous colonies on the agar slants. Microscopic examination of slide cultures demonstrated hyaline septate hypha, with both hyaline and dematiaceous conidia compatible with Sporothrix spp., and the diagnosis of lymphocutaneous sporotrichosis was made. The patient was treated with itraconazole 100 mg/day for 5 months, with regression of all the lesions.
Materials and Methods
Isolation and Phenotypic Characterization of the Fungus
Filamentous fungal colonies grown on Sabouraud Dextrose Agar were visually examined and slide cultures prepared as previously described for Sporothrix identification [10]. In order to study macroscopic features and sporulation [6], the case isolate (IPEC 27135) was subcultured on Potato Dextrose Agar (PDA) plates, and Corn Meal Agar (CMA; BBL TMBecton, Dickinson and Company/Sparks, MD 21152 USA) slants, and incubated at 30°C in the dark. The microscopic features were determined primarily on CMA slide cultures after 10–12 days of incubation at 30°C, and the diameter of the colonies on PDA was measured after 21 days according to the previously described protocol [6]. To check growth at 37°C, the strain was grown on PDA and incubated at 37°C for 3 weeks. Carbohydrate assimilation tests were performed using freshly prepared Yeast Nitrogen Base (YNB) medium [Difco TM Becton, Dickinson and Company/Sparks, MD 21152 USA] and tested for three sugars (dextrose, sucrose, and raffinose) using the technique described previously [6]. Cultures on YNB supplemented with dextrose were used as positive control for growth and YNB without carbohydrates was used as a negative control. Type strain of Sporothrix brasiliensis CBS 120339 (IPEC 16490) characterized by Marimon et al. [6] was also included in this study.
Molecular Identification
Genomic DNA was extracted and purified from S. schenckii yeast phase by phenol/chloroform/isoamyl alcohol method as previously described [11]. For the partial sequencing of the nuclear calmodulin (CAL) gene, we used specific conditions described by Susca et al. [12] with slight modifications. Briefly, the PCR mix consisted of MgCl2 2 mM (Invitrogen, USA), dNTP mix 200 mM (Invitrogen, USA), 2,5 U Taq Gold DNA polymerase (Applied Biosystems, USA), 30 pmol of each primer CL1 (5′-GA(GA)T(AT)CAAGGAGGCCTTCTC-3′), and CL2A (5′-TTTTTGCATCATGAGTTGGAC-3′) [13]. In the PCR, the annealing temperature was altered to 60°C. Automated sequencing was done using the Sequencing Platform at Fundação Oswaldo Cruz—PDTIS/FIOCRUZ, Brazil [14]. Sequences from both DNA strands were generated, edited with the Sequencher ver. 4.6 software package (Genes Codes Corporation, USA), and aligned by means of the Mega version 4.0.2 software. The sequences of our strain were compared by BLAST (Basic Local Alignment Search Tool—NIH) with sequences available from NCBi GenBank (Sporothrix AM 398382/AM 398393/AM 117444/AM 116899/AM 116908). All phylogenetic analyses were performed based on Tamura and coworkers [15], using MEGA vers. 4.0 (http://www.megasoftware.net/). The phylogenetic relationships among isolates were evaluated from tree topologies: (1) the Neighbor-joining (NJ) algorithm [16] was generated using the genetic distances model of Kimura 2-parameter [17] with pair wise deletion (gaps/missing data) and uniform rates among sites; (2) the Maximum parsimony (MP) method [18] was employed to infer trees using the close-neighbor-interchange algorithm [19]; gaps were used as fifth character. A Bootstrap test [20] with 1,000 replicates was conducted for both NJ and MP analyses. Sequence was deposited in GenBank as GU 456632.
Results
Primary Isolation
Pure cultures of identical moulds were found in SDA slants at 30°C. After 7 days of growth the initial colonies of this fungus isolated from clinical material were glabrous, white and, after a few days, a dark pigment was developed in the mycelia. Microscopic observation at this time showed the presence of simple, ovate hyaline and thin-walled conidia in sympodial conidiophores, and a second conidial form of brown, thick-walled dark conidia along the undifferentiated hyphae. At 37°C on Brain Heart Infusion Agar (BHI) (BBL TMBecton, Dickinson and Company/Sparks, MD 21152 USA), moist, glabrous, yeast-like colonies were observed. In the yeast form, the budding yeast cells were hyaline, small, globose, or oval. Based on their micromorphology, this strain was tentatively classified as Sporothrix spp.
Morphological and Physiological Analysis of the Case Isolate
The macroscopic morphologies of Sporothrix spp. from the case isolate, as well strain type S. brasiliensis CBS 120339 were similar. After 3 weeks of incubation at 30°C, colonies on PDA were moist, glabrous to cotton-like surface, white to dark brown. Microscopically these fungi presented thin, septated hyaline hyphae. All the isolates sporulated on CMA after 12 days at 30°C, and developed intercalary or terminal conidial cluster on sympodially conidiophores. The conidia were hyaline or slightly pigmented, usually obovoid. These sessile conidia were globose and measured 2,5 μm long by 2,5 μm wide (Fig. 1a).
The case isolate and the type strain of S. brasiliensis exhibited better growth at 30°C compared to 37°C. The colonies grown on PDA, at the first temperature, achieved a diameter of 34–35 mm after 21 days. At 37°C the isolates showed restricted growth, with 7–13 mm in colony diameter after 21 days (Fig. 1b). Carbohydrate assimilation tests were run in triplicate and presented the following results: both strains assimilated dextrose, and were unable to assimilate raffinose. The Sporothrix isolate (IPEC 27135) also assimilated sucrose, typical phenotypic characteristic of Sporothrix globosa [6].
Molecular Analysis
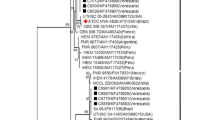
Phenotypic characteristics allowed us to classify this fungus as S. globosa, although this species has shown atypical morphologic profile, with different characteristics among this group [6]. Identity with other Sporothrix strains was confirmed by molecular sequencing data of the calmodulin (CAL) gene. GenBank search revealed that the case isolate (IPEC 27135) showed a 100% match with the CAL sequences of S. globosa (i.e., GenBank accession number AM 116908) with high bootstrap support values sustaining thus the morphological identification by mycological procedures (Figs. 2, 3).
The evolutionary history was inferred using the Neighbor-Joining method among six taxa [16]. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches [20]. All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option). There were a total of 537 positions in the final dataset. Phylogenetic analyses were conducted in MEGA4 [15]. Sequence for strain IPEC 27135 is deposited as GU 456632
Discussion
The first epidemic of sporotrichosis in humans as a result of zoonotic transmission was identified in Rio de Janeiro, Brazil, in 1998, with predominance of women with a median age of 41 who were engaged in domestic activities. The prevalence of sporotrichosis is four times higher among patients in contact with animals, especially cats, irrespective of gender. Although, in the current epidemic of sporotrichosis, contact with sick cats was the main factor associated with transmission of the disease to humans [21], traditional mechanism of transmission by traumatic inoculation with soil or plant-related matter is also occurring [4]. In the present case, the patient denies any trauma, although is possible that patient suffered a small local trauma during her daily occupational activities.
Our phylogenetic analysis has shown a clear separation of S. globosa from the other species that currently include the S. schenckii complex [6]. Apart from genetic character, morphological, and physiological features also indicate the identity of the case isolate (IPEC 27135) as S. globosa, and although in the phenotypic markers proposed by Marimon et al. [6], this species does not present growth at 37°C, our S. globosa isolate presented growth at 37°C, attaining 7 mm of colony diameter. However, the same authors related four exceptions, which exhibit a very restrict growth. Consequently, our results corroborated those data, and suggested that other phenotypic features besides capacity for growth at 37°C, have to be evaluated before the final classification as S. globosa.
Sporothrix species vary in virulence [22, 23]. Recent information from a murine infection model suggests that S. brasiliensis currently causes the highest fungal burdens resulting in the highest morbidity and mortality [24]. S. schenckii caused less damage than S. brasiliensis and S. globosa was avirulent. Interestingly, the conidia of S. globosa isolates did not produce pigment or grow at 37°C. In contrast, the S. globosa isolate described in the present work readily grew at 37°C suggesting that there may be significant variation within this species. We are currently studying additional isolates to assess this hypothesis.
References
Conti-Diaz IA. Epidemiology of sporotrichosis in Latin America. Mycopathologia. 1989;108:113–6.
Kovarik CL, Neyra E, Bustamante B. Evaluation of cats as the source of endemic sporotrichosis in Peru. Med Mycol. 2008;46:53–6.
Pappas PG, Tellez I, Deep AE, Nolasco D, Holgado W, Bustamanate B. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65–70.
Carrada-Bravo T. New observations on the epidemiology and pathogenesis of sporotrichosis. Ann Trop Med Parasitol. 1975;69:267–73.
Marimon R, Gené J, Cano J, Sutton DA, Trilles L, Dos Santos Lazéra M, et al. Molecular phylogeny of Sporothrix schenckii. J Clin Microbiol. 2006;44(9):3251–6.
Marimon R, Cano J, Gené J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45(10):3198–206.
Marimon R, Serena C, Gené J, Cano J, Guarro J. In vitro antifungal susceptibilities of five species of Sporothrix. Antimicrob Agents Chemother. 2008;52(2):732–4.
Marimon R, Gené J, Cano J, Guarro J. Sporothrix luriei: rare fungus from clinical origin. Med Mycol. 2008;46(6):621–5.
Madrid H, Cano J, Gené J, Bonifaz A, Toriello C, Guarro J. Sporothrix globosa, a pathogenic fungus with widespread geographical distribution. Rev Iberoam Micol. 2009;26(3):218–22.
Dixon DM, Salkin IF, Duncan RA, Hurd NJ, Haines JH, Kemna ME, et al. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of sporotrichosis. J Clin Microbiol. 1991;29:1106–13.
Woods JP, Kersulyte D, Goldman WE, Berg DE. Fast DNA isolation from Histoplasma capsulatum: methodology for arbitrary primer polymerase chain reaction-based epidemiological and clinical studies. J Clin Microbiol. 1993;31(2):463–4.
Susca A, Stea G, Mulé G, Perrone G. Polymerase chain reaction (PCR) identification of Aspergillus niger and Aspergillus tubingensis based on the calmodulin gene. Food Addit Contam. 2007;24(10):1154–60.
O’Donnell K, Nirenberg HI, Aoki T, Cigelnik E. A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additional phylogenetically distinct species. Mycoscience. 2000;41:61–78.
Otto TD, Vasconcellos EA, Gomes LHF, Moreira AS, Degrave WM, Mendonça-Lima L, et al. ChromaPipe: a pipeline for analysis, quality control and management for a DNA sequencing facility. Genet Mol Res. 2008;7(3):861–71.
Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–9.
Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molec Biol and Evol. 1987;4:406–25.
Kimura M. A simple method for estimating evolutionary rates of bases substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16(2):111–20.
Eck RV, Dayhoff MO. Atlas of protein sequence and structure. Silver Springs, Maryland: National Biomedical Research Foundation; 1966.
Nei M, Kumar S. Molecular evolution and phylogenetics. Oxford University, NY: Oxford University Press; 2000.
Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–91.
Barros MBL, Schubach AO, Gutierrez-Galhardo MC, Schubach TMP, Reis RS, Conceição MJ, et al. Sporotrichosis with widespread cutaneous lesions: report of 24 cases related to transmission by domestic cats in Rio de Janeiro, Brazil. Int J Dermatol. 2003;42:677–81.
Mesa-Arango AC, Reyes-Montes MR, Pérez-Mejía A, Navarro-Barranco H, Souza V, Zúñiga G, et al. Phenotyping and genotyping of Sporothrix schenckii isolates according to geographic origin and clinical form of sporotrichosis. J Clin Microbiol. 2002;40:3004–11.
Dixon DM, Duncan RA, Hurd NJ. Use of a mouse model to evaluate clinical and environmental isolates of Sporothrix spp. from the largest U.S. epidemic of sporotrichosis. J Clin Microbiol. 1992;30:951–4.
Arrillaga-Moncrieff I, Capilla E, Mayayo E, Marimon R, Mariné M, Gené J, et al. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15(7):651–5.
Acknowledgments
Financial support for this work was provided by FAPERJ (Grant Proc. E-26/111.619/2008). R. M. Z. O. is in part supported by CNPq 350338/2000-0. Automated sequencing was done using the Genomic Platform-DNA Sequencing Platform at Fundação Oswaldo Cruz—PDTIS/FIOCRUZ (RPT01A), Brazil
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Oliveira, M.M.E., de Almeida-Paes, R., de Medeiros Muniz, M. et al. Sporotrichosis Caused By Sporothrix globosa in Rio De Janeiro, Brazil: Case Report. Mycopathologia 169, 359–363 (2010). https://doi.org/10.1007/s11046-010-9276-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-010-9276-7