Phase and structural transformations in structural steels are studied by the methods of dilatometric analysis and light, scanning and transmission microscopy. Thermokinetic diagrams of decomposition of supercooled austenite are plotted. Principal possibility of formation of a structure of lower carbide-free bainite by delayed continuous cooling is demonstrated for steels of two alloying systems, namely, Kh3G3MFS and KhN3MFS containing 0.25 – 0.30 and 0.40 – 0.45% C. Principal approaches to obtaining a chemical composition of lower carbide-free bainite in structural steels are formulated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The task of formation of α + γ structure in sparingly alloyed iron-base alloys is not new. In the 1950s a Soviet scientist L. M. Pevzner spoke of elevation of the “structural strength of steels due to formation of lower bainite, especially in silicon-containing steels, when the transformation yields a mixture of an α -phase and carbon-enriched austenite without precipitation of carbides” [1].
In the 1980s the problem interested Bojarski and Bold [2]. In Russia the methods of formation of a structure of carbide-free bainite (CFB) and elevation of properties of steels with such structure was studied by a group of scientists of the Institute for the Physics of Metals of the Ural Branch of the Russian Academy of Sciences, which included Academicians V. D. Sadovskii and V. M. Schastlivtsev and Yu. M. and A. Yu. Kaletins [3]. Work [4] performed at the time showed that the factor determining the level of crack resistance in silicon steels with structure of carbide-free bainite was stability of retained austenite.
Today, steels with CFB are studied in foreign countries by groups of scientists headed by Caballero [5, 6] and Bhadeshia [7, 8]. In Russia research in this direction is lead by V. M. Schastlivtsev, Yu. M. Kaletin, A. Yu. Kaletin et al [9–11].
However, the principles for design of the composition of alloys with α + γ structure have not been formulated yet, especially where delayed continuous cooling of large-section parts and preforms is concerned. The factors providing efficient control of stability of the obtained retained austenite have not been determined either and, as a consequence, the principles of control of the level of crack resistance of alloys with such a structure.
In addition, we have grounds to expect that alloys with α + γ structure as compared to the α-alloys treated for the same strength level, should possess a higher resistance to specific kinds of fracture, for example, to stress corrosion cracking or hydrogen embrittlement.
Analyzing reference data [12] and our own results of plotting of thermokinetic diagrams of decomposition of supercooled austenite we established that the low stability of austenite in the range of pearlitic decomposition makes it impossible to obtain a CFB structure in low-alloy steels with silicon by continuous cooling. This is connected with the fact that any, even very low decrease in the rate of cooling of hypoeutectoid steels (for example, 30KhGSA and 60S2A) leads inevitably to development of a pearlitic transformation that yields excess ferrite. In hypereutectoid steel 9KhS a bainitic structure can be obtained as a result of continuous cooling only in the range of cooling rates v cool = 4 – 0.4 K/sec. At lower cooling rates the pearlitic transformation occurs with preliminary precipitation of a carbide. A CFB structure can be obtained in low-alloy silicon steels only in small sections (up to 30 – 35 mm) after a special heat treatment that consists of isothermal quenching in tank furnaces.
Thus, the first necessary condition of formation of a structure of low carbide-free bainite (LCFB) under delayed continuous cooling of large-section parts and preforms is enhancement of stability of supercooled austenite in the range of pearlitic transformation.
The second condition of formation of LCFB is high stability of austenite in the bainitic range. Here we should make some reservations.
-
1.
To obtain a LCFB structure in a wide range of cooling rates the stability of austenite in the bainitic region should be though high but lower than in the pearlitic region. In other words, the pearlitic region should not cover the region of bainitic transformation or the transformation intensity in the region of pearlitic transformation should be so low that the proportion of the formed excess structural components (ferrite and/or pearlite) should be negligibly low.
-
2.
Most researchers assume that the interval of 350 – 450°C is the boundary between the domains of existence of upper and lower bainite and 400°C is the average boundary [13–16]. Therefore, the temperature of the start of bainitic transformation should not exceed 400°C in order to guarantee the formation of LCFB.
The principles of design of chemical composition for providing substantial enhancement of the stability of supercooled austenite both in pearlitic and bainitic ranges and thus implementing formation of a structure of lath martensite under slow cooling of low-carbon martensitic steels with elevated strength have been formulated earlier in [17].
The main principle states that the solid solution should contain carbide-forming elements with progressively increasing susceptibility to formation of carbides and the concentration of the elements should decrease in a specific proportion upon growth of this susceptibility [17].
The principles mentioned have been used to develop chemical compositions of low-carbon steels 10Kh3G3MF, 10Kh3G3MFT and 10Kh3G3MFS [18]. The steels were melted and pressure treated. Results of a systematic study of these steels are presented in [19].
The data of Table 1, which presents the critical points for steels 10Kh3G3MF, 10Kh3G3MFT and 10Kh3G3MFS, rereflect high stability of austenite in the regions of pearlitic and bainitic transformations. The temperature of the start of bainitic transformation in these steels is at a level of 400 – 450°C.
Thus, the principle of systematic alloying developed by us 10 years ago for formation of a structure of lath martensite by slow cooling of low-carbon austenite [17, 19] has turned out to be quite useful for the development of principles of formation LCFB structures, because it provides observation of the first two conditions.
A substantial disadvantage of the developed compositions from the standpoint of formation of LCFB structure in them is a very narrow temperature range of bainitic transformation. For example, in steel 10Kh3G3MFS it is only 23°C in the range of 398 – 375°C (Table 1). To widen the temperature range of bainitic transformation we should lower the temperature of the end of bainitic transformation B f , because the temperature of its start B s should not exceed 400°C due to the appearance of upper bainite in the structure. Therefore, we decided to increase the content of carbon in the designed steels to 0.45% in order to lower the temperature of the start of martensitic transformation and the temperature range of the bainitic transformation.
In this connection, the third important condition for obtaining LCFB is correction of the carbon content aimed at lowering the temperature of the start of bainitic transformation and widening the bainitic range.
Finally, the fourth condition for obtaining LCFB is obligatory alloying of the designed steel with silicon, because the later (in addition to aluminum) delays the process of precipitation of cementite-type carbides and thus provides formation of carbide-free bainite.
The aim of the present work was to check experimentally the possibility of formation of a structure of lower carbidefree bainite by slow cooling of steels of two alloying systems, namely, Kh3G3MFS and KhN3MFS.
Methods of Study
To check the mentioned principles experimentally we melted four heats based on composition Kh3G3MFS and KhN3MFS containing 0.25 – 0.30 and 0.40 – 045% carbon. Each range of carbon content corresponded to two ranges of silicon content, namely, 1.0 – 1.5% and 2.5 – 3.0%.
The chemical compositions of the tested heats are presented in Table 2. For better cutting all the steels were tempered for 3 h at 650°C before making specimens.
The dilatometric studies were performed for cylindrical test pieces with a size of ∅ 3 × 10 mm with the help of a Linseis L78 RITA dilatometer using a regime that included heating to 1000°C, 15-min hold and cooling at a rate of 30.0 – 0.03 K/sec. Then we plotted the thermokinetic diagrams of decomposition of the supercooled austenite. The metallographic study was performed for laps prepared from dilatometric specimens. The specimens were pressed into a thermoset plastic with the help of a CitoPress-10 Struers mill. Then they were ground, polished at a load of 30 N in a Tegramin-30 Struers mill, and etched in a 4% solution of nitric acid in ethyl alcohol. The metallographic analysis was performed with the help of an Olympus GX-51 microscope at a magnification of × 500 – 1000; the electron microscope study was performed using a Phenom World G2 ProX scanning electron microscope (SEM) at an accelerating voltage of 15 kV and a magnification of × 1500 – 15,000.
The fine structure was studied with the help of a JEM-200CX transmission electron microscope (TEM) at an accelerating voltage of 160 kV. The foils were prepared by etching specimens in a phosphoric-chromic electrolyte. The proportion of the structural components was evaluated using the SIAMS-700 processing system.
The standard mechanical characteristics (σr , σ0.2 , δ, ψ) were determined for specimens with functional part 5 mm in diameter according to the GOST 1497–84 Standard using an Instron-SATEC 300 LX testing machine. The impact toughness (KCV ) was determined for specimens of type 11 of GOST 9454–78 using a MK-30 impact pendulum. We determined the arithmetic mean of each characteristic after testing 3 – 4 specimens in each case. The level of static crack resistance was evaluated in terms of the ultimate crack resistance exhibited in tests for three-point bending in accordance with the requirements of GOST 25.506–85 for specimens 10 × 11 × 55 mm in size. The relative length of a crack λ = 0.5.
The x-ray diffraction analysis was performed in a DRON-3M device in K α iron radiation. The relative fraction of retained austenite was determined in terms of the ratio of the intensities of x-ray lines (110)α and (111)γ.
Results and Discussion
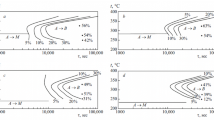
The studies showed that martensitic transformation in steel 25Kh3G3MFS (M s = 285°C) occurred in the rage of cooling rates 10 – 0.3 K/sec. At lower cooling rates (0.3 – 0.03 K/sec) at 285 – 315°C we detected a small region of bainitic transformation. No region of pearlitic transformation was detected in steel 25Kh3G3MFS in the studied range of cooling rates (Fig. 1 a).
The resistance of the supercooled austenite of steel 25Kh3G3MFS2 that contained more silicon than steel 25Kh3G3MFS differed substantially (Fig. 1 b). Martensitic transformation occurred at v cool = 30 – 0.1 K/sec; M s = 275°C. Bainitic transformation was detected only at the rates of 0.05 and 0.03 K/sec (B s = 380 – 390°C). We may conclude that growth in the silicon content in steel 25Kh3G3MFS was accompanied by elevation of the stability of austenite in the bainitic range. At the same time, at v cool = 0.05 and 0.03 K/sec the dilatometric study showed occurrence of pearlitic transformation at 620 – 730°C. The metallographic analysis showed the presence of a considerable content of a ferritecarbide mixture in the structure, namely, 36 and 58% after cooling at 0.05 and 0.03 K/sec, respectively. Thus, the increase in the silicon content lowered the stability of the austenite in the range of pearlitic transformation.
In steel 45Kh3G3MFS (Fig. 1 c) martensitic transformation occurred only after cooling at v cool = 10 and 1.5 K/sec, while at v cool = 0.8 K/sec we observed starting of bainitic transformation (B s = 250 – 275°C). In addition, at the lowest rate of 0.03 K/sec we detected formation of some pearlite (8 – 10%).
In steel 45Kh3G3MFS2 (Fig. 1 d) only martensitic transformation was observed under cooling at a rate no lower than 0.3 K/sec. At v cool = 0.1 K/ec we detected both pearlitic and bainitic transformations; the proportion of pearlite was 22%. Cooing at a still lower rate (0.05 and 0.03 K/sec) yielded 44 and 63% pearlite, respectively.
Thus, general decrease in the stability of supercooled austenite is observed in steels of type Kh3G3MFS upon growth in the content of carbon from 0.30 to 0.45% at preserved tendencies caused by growth in the silicon content (increase in the stability of austenite in the bainitic range and its decrease in the range of pearlitic decomposition), i.e., the whole of the diagram is shifted to the left.
In steel 25KhN3MFS only martensitic transformation (M s = 370°C) is observed at cooling rates no lower than 3 K/sec (Fig. 1 e). At lower rates and up to 0.03 K/sec bainitic transformation develops. Note that at v cool = 3 – 0.3 K/sec bainitic transformation starts below 400°C, and the bainite formed is of a chiefly lower nature. At lower cooling rates B s = 400 – 500 K/sec, and the formed bainite is upper one. At the lowest cooling rates v cool = 0.05 – 0.3 K/sec a small fraction of excess ferrite (5 – 8%) forms at a temperature of 600 – 700°C.
Growth in the content of silicon in steel 25KhN3MFS2 produces the same effect as in steel 25Kh3G3MFS, i.e., the stability of the austenite increases in the bainitic range and decreases in the pearlitic range. The minimum rate of occurrence of only martensitic transformation decreases from 3 to 1.5 K/sec, and the minimum rate of formation of excess ferrite and occurrence of pearlitic decomposition increases from 0.05 to 0.3 K/sec (Fig. 1 e and f). The proportion of excess phases increases too, i.e., at v cool = 0.2, 0.05 and 0.03 K/sec the total content of ferrite and pearlite in the structure is 12, 71 and 100% respectively. Increase in the silicon content is also accompanied by widening of the temperature range of precipitation of excess phases; in steel 25KhN2MFS the temperature range of formation of excess ferrite is 600 – 700°C, whereas in steel 25Kh3G3MFS2 its upper boundary is 800°C at the lowest cooling rate.
In steel 45Kh3G3MFS martensitic transformation (M s = 195 – 200°C) is observed upon cooling at a rate of about 0.1 K/sec (Fig. 1 g ). At v cool = 0.05 and 0.03 K/sec pearlitic transformation is detected in the temperature range of 550 – 650°C and bainitic transformation is detected at 220 – 410°C. The pearlite fraction is relatively low. For example, at v cool = 0.03 K/sec it reaches 17% while at v cool = 0.03 K/sec it is 35%.
Growth in the silicon content in steel 45Kh3G3MFS2 lowers somewhat the stability of the austenite in the bainitic range; a well defined region of bainitic transformation is detectable after cooling in the range of 250 – 350°C at a rate of 0.1 K/sec (Fig. 1 h). The location of the pearlitic region is virtually not affected by growth in the silicon content. However, as in all the previous cases it increases the fraction of the pearlite component at this cooling rate by almost a factor of 2. For example, after cooling of steel 45Kh3G3MFS2 at a rate of 0.05 and 0.03 K/sec the fraction of pearlite in the metal is 30 and 63% respectively.
Thus, in all the cases growth in the silicon content from 1.5 – 1.7% to 2.5 – 2.7% causes lowering of the stability of supercooled austenite in the pearlitic range and elevation of this stability in the bainitic range. Such variation of the stability of supercooled austenite with growth of the silicon content results in formation of a great amount of pearlitic component. In this connection, the steels containing 2.5 – 3.0% Si are not promising from the standpoint of formation of LCFB structure under slow continuous cooling, and we will exclude them from further consideration.
Growth in the carbon content from 0.25 – 0.30% to 0.40 – 0.45% in Cr – Mn steels causes general decrease in the stability of the austenite; in Cr – Ni steels it elevates the stability, especially in the region of bainitic transformation.
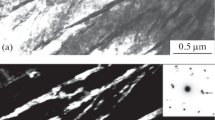
The metallographic study performed with the help of SEM and TEM has shown that the fraction of bainitic structure obtained under cooling at a rate of 0.3 – 0.03 K/sec in steel 25Kh3G3MFS is very low and does not exceed 10 – 15% (Fig. 2 b) due to the small temperature range of the bainitic transformation, and the main structural component is lath martensite (Fig. 2 a). Aclose picture is observed for steel 45Kh3G3MFS.
A LCFB structure (Fig. 2 c; Fig. 3 a – c) is formed in steel 25KhN3MFS cooled at a rate of 0.3 K/sec (B s = 400 – 410°C). The LCFB structure of this steel is represented by alternating plates of bainitic ferrite and retained austenite arranged in packets. The transverse size of the ferrite plates is 200 – 800 nm; that of the retained austenite is 50 – 150 nm. The dislocation density is high both in the ferrite and in the retained austenite.
Microstructure (TEM) of steels 25KhN3MFS (a – d ) and 45Kh3G3MFS (e – h ): a – c) v cool = 0.3 K/sec [a) light background, b) dark background, c) microdiffraction]; d ) v cool = 0.05 K/sec (light background); e – g ) v cool = 0.05 K/sec [e) light background, f) dark background, g) microdiffraction]; h) v cool= 0.3 K/sec (light background); c, g ) the arrows point at austenite reflections.
Cooling of steel 25KhN3MFS at a rate of 0.05 K/sec produces an UCFB structure (Fig. 2 d and Fig. 3 d), because the temperature of the start of bainitic decomposition attains 490 – 500°C (Fig. 1 e). The morphology of the UCFB structure differs substantially from that of the LCFB structure. The UCFB contains regions of an irregular shape with angular boundaries. The fineness of the UCFB is much lower than that of the LCFB; the regions of bainitic ferrite and retained austenite in it may reach 5 – 7 μm (Fig. 2 d).
Thus, we have used steel 25KhN3MFS as an example to show that the temperature of 400°C is a boundary between the domains of existence of upper and lower bainite.
Steel 45KhN3MFS exhibits a quite high stability of supercooled austenite; a martensitic structure is detected in it after cooling in the range of 0.3 – 0.1 K/sec (Fig. 2 e and 3 h ). After cooling at a rate of 0.05 K/sec the steel has a structure with parameters close to those of the structure of steel 25KhN3MFS after cooling at 0.3 K/sec. A substantial difference is the presence of a fraction of cooled martensite with retained austenite about equal to that of LCFB. Thus, the structure of steel 45KhN3MFS after cooling at a rate of 0.05 K/sec is rather complex; extended packets of LCFB with alternating thin lath crystals of ferrite and retained austenite formed at higher temperatures have neighbor regions of dispersed martensite (a mixture of lath and plate martensite) and “block” retained austenite (Fig. 2 f).
Table 3 presents characteristics of mechanical properties of the studied steels and the proportions of retained austenite after cooling at different rates. It is obvious that the gradual replacement of martensite with carbide-free bainite, which occurs in them upon decrease in the cooling rate is accompanied by growth of the fraction of retained austenite, and the strength characteristics decrease. In the steels based on KhN3MFS increase in the fraction of CFB structure (lowering of the cooling rate) produces elevation of the impact toughness and static crack resistance. In the steels based on Kh3G3MFS the degradation of strength observed upon lowering of the cooling rate is accompanied by decease in the impact toughness and static crack resistance. In all probability, this is connected with the appearance of a very low (no higher than 2 – 3%) fraction of a ferrite-carbide mixture located over grain boundaries of the former austenite. The reduced level of ductility and impact toughness of all the steels may be explained by insufficiently high metallurgical quality of the test heats.
Work [19] presents the results of simulation of variation of temperature in the center of different-section billets upon their cooling in still air in horizontal position. These data reflect the possibility of formation of this or that structure under delayed continuous cooling. Analyzing the data of [20] and the data of the thermokinetic diagrams plotted in the present work we estimated the sizes of preforms where a LCFB structure could be formed by cooling in still air. It turned out that cooling of dilatometric specimens (3 mm in diameter and 10 mm long) at a rate of 0.3, 0.1, 0.05 and 0.03 K/sec was about similar to cooling of the central parts of preforms 50, 80, 200, and 400 mm in diameter, respectively, in still air.
Analysis of the thermokinetic curves (Fig. 1) shows that a LCFB structure combined with lath martensite can be obtained in steels 25Kh and 45Kh3G3MFS by air cooling for sections from 80 to 400 mm in size. In steel 25KhN3MFS a LCFB structure is formed in preforms up to 50 mm in diameter; the structure represented by UCFB and 5 – 8% ferrite can be formed in preforms 200 – 400 mm in diameter. In steel 45KhN3MFS a structure of LCFB, martensite and a small fraction of ferrite-carbide mixture is formed in preforms with a section of from 80 – 100 to 200 mm due to air cooling.
We plan to study the regular features in formation of LCFB structure in the process of isothermal treatment and to estimate the level of mechanical properties (especially of the crack resistance) in steels Kh3G3MFS and KhN3MFS with various contents of carbon after continuous cooling and after isothermal treatment.
Conclusions
1. We have shown principal possibility of forming a structure of lower carbide-free bainite (LCFB) in steels of type Kh3G3MFS and KhN3MFS with 0.30 – 0.45% C under the conditions of continuous delayed cooling and formulated the principal approaches to designing the chemical composition of structural steels, which provide formation of LCFB structure under such conditions.
2. Air cooling of steels 25Kh3G3MFS and 45Kh3G3MFS may provide a structure of LCFB + lath martensite in sections 80 – 400 mm in size. In steel 25KhN3MFS a LCFB structure forms in preforms with a diameter of up to 50 mm and a structure of UCFB + 5 – 8% ferrite is formed in preforms 200 – 400 mm in diameter. In steel 45KhN3MFS a structure of LCFB + martensite + some ferrite-carbide mixture forms in sections from 80 – 100 to 200 mm in size.
3. Steel 25KhN3MFS has been used to show once more that the boundary between the domains of existence of UCFB and LCFB is a temperature of about 400°C; LCFB forms at the temperature of the start of bainitic transformation B s < 400°C and UCFB forms at B s > 400°C.
4. Increase in the silicon content of the steels from 1.5 – 1.7% to 2.7 – 3.0% causes decrease in the stability of supercooled austenite in the pearlitic range and increases it in the range of bainitic transformation, which is not promising from the standpoint of formation of LCFB structure in the process of delayed continuous cooling of these steels.
The work has been performed with financial support of the Ministry of Education and Science of the Russian Federation within implementation of Regulation 218 “Development of Cooperation between Russian Higher Educational Institutions and Industrial Enterprises,” Agreement No. 02.G25.31.0068 between the Ministry of Education and Science and the “Motovilikha Plants” Company, and Agreement No. 2013/050 between the “Motovilikha Plants” Company and the Perm National Research Polytechnic University.
References
L. M. Pevzner, in: Metal Science and Heat Treatment [in Russian], Mashgiz, Moscow (1955), pp. 74 – 106.
Z. Bojarski and T. Bold, “Structure and properties of carbidefree-bainite,” Acta Metall., 22(10), 1223 – 1234 (1974).
Yu. M. Kaletin, A. G. Ryzhkov, and A. Yu Kaletin, “Alloying and heat treatment of steels with bainitic structure,” Metalloved. Term. Obrab. Met., No. 10, 13 – 17 (1987).
M. N. Georgiev, A. Yu. Kaletin, Yu. N. Simonov, and V. M. Schastlivtsev, “Effect of stability of retained austenite on crack resistance of structural steel,” Fiz. Met. Metalloved., No. 1, 113 – 212 (1990).
F. G. Caballero, C. Garcia-Mateo, M. J. Santofimia, et al., “New experimental evidence on the incomplete transformation phenomenon in steel,” Acta Mater., 57, 8 – 17 (2009).
F. G. Caballero, M. K. Miller, S. S. Babu, C. Garcia-Mateo, “Atomic scale observations of bainite transformation in a high carbon high silicon steel,” Acta Mater., 55, 381 – 390 (2007).
F. G. Caballero and H. K. D. Bhadeshia, “Very strong bainite,” Current Opinion Solid State Mater. Sci., No. 8, 251 – 257 (2004).
H.-S. Yang and H. K. D. H. Bhadeshia, “Designing low carbon, low temperature bainite,” Mater. Sci. Technol., 24(3), 335 – 342(2008).
V. M. Schastlivtsev, Yu. V. Kaletina, E. A. Fokina, and A. Yu. Kaletin, “About the role of retained austenite in the structure of alloy steels and effect of external impacts on it,” Fiz. Met. Metalloved., 115(9), 962 – 976 (2014).
A. Yu. Kaletin, A. G. Ryzhov, and Yu. V. Kaletina, “Elevation of the impact toughness of structural steels due to formation of carbide-free bainite,” Fiz. Met. Metalloved., 116(1), 114 – 120(2015).
A. Yu. Kaletin and Yu. V. Kaletina, “Evolution of the structure and properties of silicon steels under austenite-bainite phase transformation,” Fiz. Tverd. Tela, 57(1), 56 – 62 (2015).
A. A. Popov and L. E. Popova, Isothermal and Thermokinetic Diagrams of Decomposition of Supercooled Austenite [in Russian], Metallurgiya, Moscow (1965), 497 p.
Yu. M. Lakhin, Metal Science and Heat Treatment of Metals [in Russian], Metallurgiya, Moscow (1983), 359 p.
M. A. Smirnov, V. M. Schastlivtsev, L. G. Zhuravlev, Fundamentals of Heat Treatment of Steel [in Russian], Ekaterinburg, UrO RAN (1999), 496 p.
I. I. Novikov, The Theory of Heat Treatment [in Russian], Metallurgiya, Moscow (1986), 480 p.
I. I. Novikov, S. I. Grachev, and Yu. G. Veksler, Special Steels [in Russian], Metallurgiya, Moscow (1985), 408 p.
Yu. N. Simonov, “Conditions of formation of a structure of lath martensite under delayed cooling of low-carbon austenite,” Fiz. Met. Metalloved., 97(5), 77 – 81 (2004).
Yu. N. Simonov, D. O. Panov, M. Yu. Simonov, A. V. Kasatkin, and D. P. Poduzov, “Low-carbon alloy steel, RF Patent 2477333 C22 C38/38,” Byull. Izobr. Polezn. Modeli, No. 7 (2013).
Yu. N. Simonov, M. Yu. Simonov, D. P. Poduzov, et al., “Transformations, structure and properties of systematically alloyed low-carbon nickel-free steels,” Metalloved. Term. Obrab. Met., No. 11, 4 – 11 (2012).
S. V. Lekomtsev, D. O. Panov, M. Yu. Simonov, and I. N. Shardakov, “Methods of forming low carbide-free bainite for special kinds of steel,” Nauch.-Tekh. Vestn. Povolzh., No. 6, 204 – 209 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 7, pp. 20, 28, July, 2015
Rights and permissions
About this article
Cite this article
Simonov, Y.N., Panov, O.D., Simonov, M.Y. et al. Principles of Design of the Chemical Composition of Steels for Forming a Structure of Lower Carbide-Free Bainite Under Delayed Cooling. Met Sci Heat Treat 57, 386–394 (2015). https://doi.org/10.1007/s11041-015-9894-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-015-9894-2