The phases obtained in copper aluminum bronze alloy (Cu – 10 wt.% Al – 2 wt.% Fe) cast into a permanent die were investigated. The parameters examined were the preheating temperatures of the die and the graphite coating thickness. The phases α and γ2 were detected as well as the metastable phases β′ and γ′. The intermetallics of the system Fe – Al were obtained in various stoichiometric compositions. The different cooling rates of the casting resulted in two mechanisms of transformation to α grains out of the unstable β phase, one being nucleation and growth producing needle-shaped α grains, the other exhibiting a massive transformation to spherical α grains. These two mechanisms determine the changes in the size of the α grains as a result of changes in the cooling rate in its various ranges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cooling at equilibrium of the system 90% Cu – 10% Al from the liquid state results in α and β phases. The eutectic reaction takes place at 1037°C and 8.5% Al. At 565°C and 11.8% Al the β phase decomposes to the γ2 phase by eutectoid decomposition [1].
During cooling in nonequilibrium conditions the martensitic phase β′ replaces the β phase, and in addition the αphase is obtained by solid-state transformation. There are phase diagrams that combine the metastable phases with the martensitic temperature of the transition β– β′at about 520°C [2]. Adding 4% Fe causes only a small change in the phase diagram but contributes to the refinement of the structure [1].
High cooling rates produce the needle-like structure of the αphase. Nucleation starts at the β-phase grain boundaries and around the iron particles. Another possibility is the creation of a massive structure of the α phase, which takes place at high cooling rates without segregation during solidification and in very specific alloy structures [3]. Such a specific structure may, for example, be the alloy Cu – 39% Zn or Cu – 10% Al. It can be deduced from the phase diagram that with such compositions and a temperature of about 500°C, α and β (or β′) phases may exist side by side. If the β-phase is cooled sufficiently rapidly, so that the precipitation of the α-phase is suppressed, the β-phase transforms to the α-phase of the same composition. The only change is in the crystalline structure. The driving force is thermal activation, so that the massive α-phase quantity is seen to grow through a maximum as the cooling rate increases.
At yet higher cooling rates α-phase nucleation still takes place, but the mobility of the interface is insufficient for the growth of the α-phase, and no massive transformation ensues.
This paper reports on an investigation into the influence of the casting parameters on the phase transformation mechanisms as a function of the cooling rates characteristic of the process.
Experimental Procedure
The experiments were carried out in a permanent die made of nodular iron (DIN 1693 GGG60) and coated with a layer of colloidal graphite, Neolube, made by Huron Industries, Inc. The mould plate was 20 mm thick, and five Cromel-Alumel thermocouples were inserted in it at a distance of 2 mm from the casting mould interface. The casting, in the form of a block 50 mm wide by 100 mm height and 15 mm thick, was made of CuAl10Fe2-C EN1982:1998 copper-aluminum alloy. In a number of experiments the temperature of the casting was measured in the course of solidification, for which purpose a thermocouple had been introduced into the mould space before the cast, while its exact final location was found by cutting through the casting.
Temperatures were measured every second, the readings being fed into a computer for analyzing the temperature field and calculating the heat flow to the mould.
The temperature field in the die was calculated with the aid of the inverse solution of the heat conductance equation based on the measurement of the temperature close to the surface of the casting. Knowing the temperature distribution in the die at any time allows the calculation of the heat flow into the die as a function of time. The source of the heat is the casting, so that it may be assumed that the heat flow into the die equals the heat flow out of the casting. The temperature distribution in the casting is obtained by solving the one-dimensional heat conduction equation, account being taken of the emission of latent heat in the solidification range and of the dependence of the specific heat and the heat conduction on the temperature together with the boundary conditions determined before. The temperature field in the casting was used for the calculation of the cooling rate in the solid state.
All the metallographic specimens were taken from the center of the casting, normal to the die wall. The metallurgical test and examinations comprised optical metallography, SEM, micro analyses with the aid of EDS, x-ray diffraction and quantitative determination of the phases.
The relative amount of the α-phase (X α) was determined by quantitative metallography; in addition, the number of intersections (N s) was counted along a line of given length L. The grain size is thus equal to LX α/N α. This procedure was repeated at least five times and the mean value was used for the further analysis.
The phases of the Cu – Al system were identified with the aid of x-ray diffraction on a solid specimen, and those of the Fe – Al system with the aid of a powder chemically prepared by the following procedures. Chips were dissolved in nitric acid, producing a sediment of the intermetallic phase Fe – Al. This was rinsed with water and the result dried, leaving the desired powder [6].
Results
The rates of solidification and cooling of the casting in the permanent die are influenced by the temperature of the die and the thickness of the graphite coating on the die walls. It was found that these parameters also affect the microstructure of the casting made of Cu – 10 wt.% Al – 2 wt.% Fe in the as-cast state. In the course of the experiment the casting is allowed to cool in the die for 2 min and is then exposed to air cooling. The characteristics of the microstructure and of the phases detected will be detailed below.
Metallography
From the phase diagram of the Cu – Al alloy, it is seen [2] that at 10% Al a solid β-phase is formed first, and that about 930°C the α phase begins to precipitate from the solid β-phase. The growth of the α-phase is thus dependent on the rate of the heat extraction in the solid state.
Water quenching the alloy from the liquid state demonstrates the nucleation of the α-phase at the β-phase grain boundaries and around the iron particles and its inability to grow due to the rapid cooling (Fig. 1). A drop in the cooling rate in the die enables the α-phase to be obtained in two different morphologies.
Casting into a cold (about 150°C) or a hot (about 400°C) mould coated with graphite 0.01 mm thick led to the formation of mainly needle-shaped α grains (Figs. 2 and 3). Casting into a die of median temperature (about 280°C) with the maximum cooling rate [5] resulted in the formation of spherical α particles (Fig. 4).
Casting conditions including a die coated with a thick (0.10 mm) graphite coating led to the formation of exclusively needle-shaped α grains attended by emphasized β grain boundaries, especially in the zone close to the casting die interface (Fig. 5). With the graphite thickness stated above the needle-shaped α particles were obtained at any preheating temperature of the die. It can be seen that the thick graphite coating — the dominant factor in determining a high cooling rate of the casting — is also reflected in the microstructure.
Identifying the Phases
The different phases were identified with the aid of x-ray diffraction (copper radiation. The principal phases of the Cu – Al system are the equilibrium α phase and small amounts of γ2 and the metastable β′phase. The metastable γ′phase appeared to be a function of the cooling rates, which created conditions of microsegregation during the solidification of the casting. A hot (about 400°C) die, which slows down the rate of solidification, causes the γ′phase to appear (Fig. 6a ), whereas a colder (225°C) die speeds up the rate of solidification, and γ′phase does not form (Fig. 6b ).
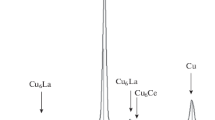
The presence of iron in the alloy leads to the formation of intermetallics of the system Fe – Al. Owing to their low concentration (4 wt.%) no x-ray diffraction can be obtained when they are in the matrix, and they are identified after the dissolution of the matrix and the preparation of the powder. The results of the diffraction show that the precipitates are Al13Fe4, Fe3Al, Al5Fe2, and the Fe(α).
The iron particles that did not interact with the aluminum are spherical in shape, while the Fe3Al precipitates are arranged in grape-like structures (Fig. 7). The precipitates in the SEM pictures were identified by determining their composition with the aid of EDS.
Discussion
The cooling rates ranged on an average between 238 and 404°C/min in different casting conditions, and the phases α, γ2, β′and γ′were obtained. A connection was found between the cooling rate of the casting and the α grain size and shape.
A comparison of Fig. 4 (high cooling rate) with Figs. 2 and 3 (relatively low cooling rates) shows that, in Fig. 4, the majority of the α grains are spherical, whereas in Figs. 2 and 3 the grains are needle-shaped. Needle-shaped α grains are formed, as a rule, by a nucleation and growth mechanism, while the spherical grains are created by massive transformation [3]. In addition, Fig. 8 presents the α grain size and the cooling rates as functions of the preheating temperature of the die.
When casting into an uncoated die or one with a coating up to 0.01 mm thick (Fig. 8a ), the α grain grows as the cooling rate rises. After the cooling rate and the αgrain have reached a maximum and the cooling rate begins to drop, the α grain shrinks to a minimum (Zone I, Fig. 8a ). From this point onward the cooling rate continues to drop, but the α grain grows again (Zone II, Fig. 8a).
It follows from this that the growth of the a phase in Zone I does not obey the nucleation and growth mechanism but rather that of massive transformation characterized by large and spherical α grains, as can be seen in Fig. 4.
In Zone II solidification is slower because of the hot die and resulted in segregation of the γ′phase (Fig. 6). This in turn, caused the formation of the α phase by the nucleation and growth mechanism and not by the massive transformation mechanism. Therefore, a drop in the cooling rate of the solid causes an increase in the α grain size, and the α phase has the needle-like shape as well (Fig. 3).
At even higher cooling rates, as encountered in metallic dies with graphite coating 0.10 mm thick, no massive transformation takes place, the α grains are needle-like, and with rising cooling rate the α grain shrinks, as seen in Fig. 8b . The reason for the absence of massive transformation (in Figs. 1 and 5) at such high cooling rates is that the alloy rapidly cools to such low temperature that the mobility of the interface α– β is insufficient to permit the growth of βinto the unstable β.
Conclusions
-
1.
The phases obtained by casting aluminum bronze into a permanent die are αand and the metastable phases β′and γ′. The phases γ2 and γ′are created locally because of microsegregation in the casting.
-
2.
At high or low cooling rates the α phase precipitates by a process of nucleation and growth to needle-like grains. At median cooling rates the phase α is obtained by a process of massive transformation, the grains being spherical in shape and of large sizes. The size increases with rising cooling rates. Segregation of the γ′phase impedes the massive transformation, so that the nucleation and growth mechanism prevails in the creation of the α phase although, in the absence of the γ′phase, massive transformation would occur.
-
3.
Coating the die with a thick layer of graphite increases the cooling rate and leads to a needle-like structure that emphasizes the β phase. In these conditions there is not massive transformation.
-
4.
The iron and aluminum intermetallics formed are of the compositions Al13Fe4, Fe3 Al, and Al5Fe2. In addition, there are spherical α iron particles that did not interact with the aluminum.
References
I. Cenoz and J. Fernandez, Rev. Met. Madrid, 43, 272 – 283 (2007).
V. Callcut, Metallurgy of Copper and Copper Alloys, Aluminium Bronze, Part 1, The Copper Page Innovations, The Copper Development Association Inc., USA (2002), pp. 3 – 6.
C. M. Friend, Scr. Metall., 23, 1.817 – 1.820 (1989).
W. S. Li, Z. P. Wang, Y. Lu, Y. H. Jin, L. H. Yuan, and F. Wang, Wear, 261, 155 – 163 (2006).
M. Kaplan and A. K. Yildiz, Mater. Lett., 57, 4.402 – 4.411 (2003).
A. Al-Hashem andW. Riad, Mater. Caracter., 48, 37 – 41 (2002).
M. Eddahbi and O. A. Ruano, Rev. Met. Madrid, 41, 251 – 257 (2005).
A. Monsalve and R. Morales, Rev. Met. Madrid, 40, 431 – 435 (2004).
E. Van Der Heide, E. D. Stam, H. Giraud, G. Lovato, N. Akdut, F. Clarysse, P. Caenen, and E. I. Heikillä, Wear, 261, 68 – 71 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 6, pp. 10 – 14, June, 2011.
Rights and permissions
About this article
Cite this article
Cenoz, I., Gutierrez, M. Phase transformations in Cu – Al alloy. Met Sci Heat Treat 53, 265–269 (2011). https://doi.org/10.1007/s11041-011-9380-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-011-9380-4