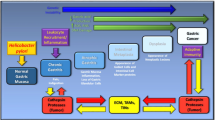
Abstract
Helicobacter pylori (H. pylori) is a common pathogen that infects more than half of the world’s population. Its infection can not only lead to a variety of gastrointestinal diseases, such as chronic gastritis and gastric cancer (GC) but also be associated with many extra-gastrointestinal diseases. Exosomes, as a new intercellular information transmission medium, can carry biological signal molecules such as microRNAs (miRNAs) to regulate a variety of cellular physiological activities and are involved in multiple cancer processes. In this article, we provide a systematic review on the role of exosomal miRNAs in H. pylori-associated GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
GC is a common malignant tumor in the world with high incidence and mortality rates. H. pylori infection is recognized as the most serious risk factor for GC. It is estimated that more than 50% of the occurrence of GC is associated with H. pylori infection. Multiple virulence factors, such as cagA and vacA, interact with different cellular proteins to regulate the host inflammatory response and lead to the occurrence of GC as the long-term consequence of H. pylori infection [1]. At present, gastroscopic pathology is still the gold standard for the diagnosis of GC, but invasive shortcomings limit its use as a means of GC screening. Some markers of GC, such as CEA and CA19-9, are also used in the diagnosis of GC, but lack enough specificity and sensitivity. Most GC patients are already in the middle or late stages when they seek treatment, therefore, early detection and timely treatment of GC have become the focus of clinical treatment of GC.
To date, more than 2500 human-specific miRNAs (data from the miRBase, Release 22.1) have been identified, and their dysregulation was associated with tumor cell proliferation, apoptosis, cell cycle, metastasis, and invasion [2]. Especially, some aberrantly expressed miRNAs are involved in the key pathophysiology of GC and showed potentially valuable biomarkers for GC diagnosis, prognosis, and disease surveillance. Furthermore, exosomes, a novel intercellular information transmission medium, can shuttle miRNAs to the recipient cells and affect a range of cellular physiological functions. Recent research has shown that exosomal miRNAs may play a significant role in the occurrence and development of GC, drug resistance, and diagnosis of GC [3]. This review focused on summarizing the structure and biological functions of exosomes, the role of exosomes miRNAs in H. pylori-associated GC, and providing new insights for understanding the pathogenic mechanism of GC.
H. pylori
H. pylori infection
H. pylori is a Gram-negative spiral-shaped microaerobic bacteria. Statistically, more than 50% of people worldwide are infected with this pathogen. H. pylori infection is a major risk factor for peptic ulcer, GC, and gastric mucosa lymphoma, and it has been recognized as a class I carcinogen [1]. H. pylori diversity is linked to H. pylori virulence factors vacA and cagA, as well as inflammation and immune response after infection. Here, we briefly described the mechanism of cagA and vacA on GC.
CagA is a highly immunogenic protein encoded by the cag pathogenicity island (cagPAI) and delivered into the attached gastric epithelial cell by the type IV secretion system (T4SS). The cytoplasmic translocation cagA interacts with numerous proteins in phosphorylation-dependent and independent manners, leading to changes in multiple intracellular pathways [4]. For example, cagA maintains multidirectional differentiation and self-renewal of cancer stem cells by activating the Wnt/β-catenin signaling pathway in GC cells.
VacA is a channel-forming toxin encoded by a vacA gene and presents marked differences in vacA toxin activity based on variations in vacA amino acid sequences, levels of vacA transcription, and secretion. VacA accumulation can induce cytoplasmic vacuolation, disruption of endocytic, mitochondrial perturbations, autophagy, or even cell death in gastric epithelial cells. VacA also can directly destroy the integrity of the epithelial cell monolayer, enhance the ability of carcinogens to enter the gastric mucosa, promote the invasiveness and spreading ability of malignant tumors, and increase the risk of disease [1, 4]. Moreover, vacA can inhibit T cell proliferation, suppress Th1 and Th17 responses, mediate cell apoptosis, and allow tumor cells to evade immune surveillance, ultimately leading to GC.
MiRNAs associated with H. pylori infection
MiRNAs are endogenous non-coding RNAs that can modulate gene expression via directly binding to the 3’ untranslated region (3’UTR) of their target gene and consequently degrade the mRNAs or suppress the protein expression. Most miRNAs are characterized by high sequence conservation, temporal and tissue specificity, and they perform a variety of biological functions as oncogenes or tumor suppressor genes during cell growth, development, metastasis, and invasion [5]. Sun et al. found that miR-29a-3p promoted cell migration by directly targeting the A20 gene in H. pylori-infected human gastric epithelial cells [6]. Yang et al. discovered that H. pylori (CagA +) infection increased the expression of miR-223-3p, and promoted GC cell proliferation and migration by directly targeting ARID1A [7]. Liu et al. discovered that H. pylori infection up-regulated the expression of miR-146a in gastric epithelial cells and gastric mucosal tissues in an NF-κB-dependent manner, and the miR-146a upregulation played a potential role in the negative feedback loop by targeting IRAK1 and TRAF6, thereby modulating the H. pylori-induced immune response [8]. Xu et al. showed that miR-1915 was down-regulated in H. pylori-infected GC tissues and cell lines, and it inhibited the growth and metastasis of GC cells by targeting RAGE [9]. Li et al. found that miR-1298-5p was decreased under H. pylori infection, its down-regulation not only inhibits autophagy but also affects P38 MAPK by targeting MAP2K6, leading to the promotion of cell proliferation, migration, and invasion [10]. Rossi et al. discovered that H. pylori could regulate the expression of miR-19a and miR-34a, and promote early apoptosis of cells by TNFR2 [11]. The latest study demonstrated lncRNA NEAT1 promotes tumorigenesis in H. pylori-associated GC by sponging miR-30a to regulate the COX-2/BCL9 pathway [12].
H. pylori is not only considered a key cause of GC but also has a sublinear correlation with lymphatic and distant metastasis of GC. Saito et al. showed that C-MYB-induced miR-17 and miR-20a play a leading role in the CagA-dependent P21 signaling pathway, and promote the epithelial-mesenchymal transition (EMT) of GC [13]. Shi et al. showed that CagA-induced miR-543 can inhibit autophagy by targeting SIRT1, leading to the increase of EMT expression and promoting cell migration and invasion [14]. Huang et al. found that H. pylori could down-regulate the expression of miR-134, miR-134 inhibited the occurrence of EMT in SGC-7901 cells by targeting FOXM1 [15]. The above-mentioned findings show that H. pylori can control a variety of miRNA targets and plays an essential role in a variety of GC-related signaling pathways, providing a new idea for further understanding the pathogenesis of H. pylori-related gastric diseases.
Exosomal
Biological characteristics of exosomes
Exosomes are cell-derived extracellular vesicles with diameters of 30–150 nm. Its formation involves multiple processes such as endocytosis, content sorting, and transport. (Fig. 1) First, under the action of the Golgi apparatus, the membrane depresses inward to form early endosomes. Then, the early endosomes mature into the late endosomes, and the late endosomes envelop the proteins, nucleic acids, and lipids to form multiple intraluminal vesicles (ILVs). Eventually, ILVs evolved into multi-vesicle bodies (MVBs). After MVBs fuse with the membrane, the luminal vesicles inside them sag again, and form granules by budding and released into the extracellular environment, which are called exosomes [16, 17]. After exosomes are formed, they can not only be distributed around secretory cells but also be transported to other parts in a variety of ways. They are distributed in various body fluids such as blood, urine, and cerebrospinal fluid, and are more abundant in the tumor microenvironment.
The process of exosomes formation ([16] modification of Fu et al. Molecular Cancer (2019) 18:41)
Tubulin, actin, a variety of Rab proteins, TSG101, members of the four-transmembrane region protein superfamily, MHC-I and HSP70 are involved in exosome biogenesis, Rab protein plays an important role in the formation of exosomes. While Rab27a primarily controls the rearrangement of the airway cytoskeleton during the fusion of polyvesicle corpuscles and plasma membrane, Rab27b primarily controls MVBs transport to the plasma membrane [17]. However, their functions in exosome biogenesis need to be further explored. The loss or gain of Rab protein function may interfere with intracellular autophagy and lysosomal pathways, then have an indirect impact on exosome biogenesis. Many studies have found that exosomes are involved in a variety of diseases, especially cancer, and have been found to transfer bioactive substances between tumor cells stromal cells, fibroblasts endothelial cells, and immune cells, mediating communication in the entire tumor microenvironment [24, 25].
Mechanism of miRNAs sorting and transmission in exosomes
The role of miRNAs in cancer has received extensive attention, but there are few studies on the sorting and delivery mechanism of miRNAs in exosomes. Professor Sanchez Madrid led a team that analyzed the mechanism of exosome sorting and miRNA loading in T lymphocytes [18]. In this study, they found that hnRNPA2B1 in exosomes plays an important role in exosomal transport miRNAs, hnRNPA2B1 binds to miR-198 by SUMOylation, and this binding effect localizes hnRNPA2B1-miR-198 complex to exosomes and participates in extracellular transport. Some researchers have successfully isolated Ago2 from exosomes and proved that Ago2 can protect exosomes from RNase degradation during miRNA delivery. After Ago2 binds to miRNAs, it enters exosomes through the KRAS-MEK-ERK signaling pathway, and finally plays a role outside the cell [19]. In addition, membrane proteins involved in exosome biogenesis, such as caveolin-1 and nSMase2, can also be involved in the sorting and loading of miRNAs. For example, cancer cells bind nSMase2 to miR-210 and load it onto exosomes to promote increased endothelial cell migration and capillary formation in vitro [20]. This study provides a new idea for the use of exosome transport target miRNAs.
Exosomal miRNAs-mediated cell communication
Exosomes loaded with small molecules are important in cell-to-cell communication. Exosomes can deliver specific miRNAs, and other non-coding RNAs to distant tissues or cells to play a regulatory role. Exosomes play a significant role in cell-to-cell or intracellular communication in three stages. The first stage is ligands on exosomes' membranes combine with receptors on target cell membranes to deliver intercellular information. The second stage is the secret body release of intracellular substances on the target cell surface receptors, complete information transfer to produce a biological effect. The third stage is the fusion of exosomes with target cells to release various promoters and nucleic acids contained in them to achieve the function of information transport.
With the deepening of research, it is increasingly recognized that miRNAs contained in exosomes may become special markers for the diagnosis and treatment of some diseases. To study the cellular communication of miRNAs mediated by exosome transport, researchers used lentivirus to construct an exosome of fluorescently labeled bone marrow-derived macrophages (BMDM) and treat vascular epithelial cells (ECs) with these exosomes. It was found that miRNAs in some BMDM-derived exosomes were also significantly up-regulated in ECs. By constructing special lentiviruses, researchers ensured that miRNAs in exosomes could retain their gene regulatory functions. RNA-seq analysis also proved that the target genes of miRNAs in ECs were indeed regulated by miRNAs [21]. In addition, exosomes produced by endothelial cells can also promote angiogenesis in vivo by delivering miRNAs. These data suggest that cells can use exosomes to transport miRNAs and play a regulatory role in entering other cells by endocytosis.
Exosomal miRNAs and H. pylori infection
Exosomes created by H. pylori infection can indicate alterations in bioactive molecules such as miRNAs, and proteins, which play essential roles in gastric and extra-gastric disorders. Wang et al. found that exosomes derived from H. pylori-infected macrophages, carrying miR-155 were internalized by macrophages and regulated the expression of various proinflammatory mediators and inflammation-related proteins in macrophages, including the expressions of TNF-α, IL-6, IL-23, CD40, CD63, CD81 and MHC-I were up-regulated, while MyD88, NF-κB was down-regulated, suggesting that exo-miR-155 may act as a novel negative regulator to modulate the inflammatory response and modulate the immune response of H. pylori-infected macrophages [22]. Li et al.found that miR-25 was elevated in GES-1-derived exosomes of H. pylori-infected gastric mucosal cells and plasma exosomes of patients, and miR-25 regulated NF-κB signaling pathway by targeting KLF2, leading to IL-6, MCP-1, VCAM-1, ICAM-1 expression increases, which promotes vascular endothelial cell injury. In conclusion, H. pylori-induced miRNAs in exosomes can mediate immunomodulatory and other important physiological and pathological processes, thus regulating the occurrence and development of gastrointestinal diseases and extra-gastric diseases [23].
Exosomal miRNAs in GC
Regulating the microenvironment of GC
The tumor microenvironment is a key factor affecting tumor growth and metastasis, which can provide nutritional and material basis. Exosome transport miRNAs can affect the microenvironment of tumor growth and participate in the process of tumor genesis and development. Cancer-associated fibroblasts (CAFs) are important components of tumor stroma. Tumor-derived exosomes can be taken up by CAFs to alter their biological activity and reprogram energy metabolism. Exo-miR-27a derived from GC can transform fibroblasts into CAFs and plays an important role in the microenvironment of GC [24]. Exo-miR-139 derived from CAFs can inhibit the progression and metastasis of GC by downregulation of MMP11 in the tumor microenvironment [25]. Recent research has demonstrated that tumor-derived exosomal miRNAs have immunosuppressive properties as well, and they can help tumor cells communicate with their microenvironment by using immune escape, thereby mediating tumorigenesis. For instance, the GC-cell-derived exo-miR-135b-5p can inhibit Vγ9Vδ2 T cell function by targeting the SP1 pathway, decrease Vγ9Vδ2 T cell viability, trigger cell apoptosis, and decrease the production of cytotoxic cytokines IFN-γ and TNF-α [26].
The occurrence of mesoderm-mesenchymal transition (MMT) of peritoneal mesothelial cells (PMCs) can provide a good environment for metastatic cancer. The study showed that exo-miR-106a is involved in the adhesion, invasion, and metastasis of tumor cells by targeting Smad7 and regulating the TGF-β signaling pathway [27]. Zhu et al. further confirmed that exo-miR-106a can also target TIMP2 to activate the TGF-β pathway to induce MMT and accelerate the degeneration of the extracellular matrix, thereby destroying the mesothelial barrier and promoting peritoneal dissemination of GC [28]. Another study found that exo-miR-21-5p induces MMT of PMCs and promotes cancer dissemination by directly targeting Smad7 [29]. These studies have revealed a new mechanism of GC proliferation and metastasis. Exosomal miRNAs can regulate the immune microenvironment and thus affect the development of tumors. The related discussion has become the most attractive field for the study of exosome functions.
Regulating the growth, invasion, metastasis and angiogenesis of GC
Tumor-derived exosomes have a limited impact on angiogenesis, invasion, metastasis, and tumor growth. Exosomes can regulate receptor cells to play a corresponding role by transporting specific miRNAs. Tang et al. successfully identified four exosomal miRNAs that are involved in the growth and metastasis of the disease, they also discovered that up-regulating miR-3149, miR-6727, and miR-32 while down-regulating miR-4741 could inhibit the growth, migration, and invasion of GC cells [30]. Exo-miR-1290 can enhance the proliferation and invasion ability of GC cells by reducing the expression of the target gene NKD1 [31]. Exo-miR-1228 derived from bone marrow mesenchymal stem cells is highly expressed in GC patients and inhibits the occurrence and development of GC by targeting MMP-14 [32]. Exo-miR-122-5p can inhibit the proliferation and metastasis of GC cells by targeting GIT1 [33]. M2 macrophage-derived exo-miR-487a isolated from GC tissue can promote the proliferation of GC cells by targeting TIA1 [34]. Shi et al. found that exo-miR-155-5p promoted the proliferation and migration of AGS cells by targeting TP53INP1 [35]. Exo-miR-196a-1 secreted by highly invasive GC cells promotes the invasion of less invasive GC cells by targeting SFRP1 [36]. Exo-miR-423-5p can promote the growth and metastasis of GC by inhibiting the expression of the SUFU gene [37]. Exo-miR-130a promotes angiogenesis and tumor invasion by inhibiting the expression of C-MYB in human umbilical vein endothelial cells [38]. Exo-miR-135b, derived from GC cells, promotes peritumor angiogenesis by inhibiting the expression of FOXO1 in endothelial cells [39]. These findings suggest that the study of exosomal miRNAs and their target pathways may contribute to the development of new diagnostic and therapeutic methods for GC.
Regulating of the drug resistance of GC
Exosomes produced by cancer cells have been shown to carry specific miRNAs into cells via receptor endocytosis, promote the internalization of coated medications, inhibit the binding of anticancer medications to target proteins on the surface of cancer cells, and contribute to the formation of chemotherapeutic drug resistance. (Fig. 2) At present, platinum-based chemotherapy is the most commonly used chemotherapy standard for GC, but in the process of long-term drug chemotherapy, GC patients will appear drug-resistant, leading to the failure of chemotherapy. Exo-miR-21 plays a role in the drug resistance of GC and can reduce the sensitivity of tumor cells to drugs. Exosomes derived from tumor-associated macrophages (TAMs) can directly transport miR-21 from M2-type TAMs to GC cells. It can inhibit cell apoptosis and enhance the resistance of GC cells to cisplatin (DDP) by regulating the PTEN/PI3K/AKT signaling pathway [40]. Studies have detected PKH67-labeled M2 macrophage-derived exo-miR-588 from SGC7901 cells, which contributes to the DDP resistance of GC cells by partially targeting CYLD [41]. Exo-miR-769-5p, according to Jing et al., inhibits the downstream caspase pathway by concentrating on CASP9, which encourages the degradation of p53 via the ubiquitin–proteasome pathway and results in the DDP resistance in GC [42]. Paclitaxel is an antitumor drug with a taxane diterpene structure, which can play a therapeutic role in a variety of malignant tumors, however, its clinical application is limited due to its highly hydrophobic nature and dose dependence. It has recently been reported that paclitaxel can be delivered using exosomal miRNAs to address this flaw. For example, exo-miR-155-5p causes malignant transformation and drug-resistant phenotype in MGC-803 cells by directly targeting GATA3 and TP53INP1 [43].
The DYNLT1/Caspase-3/Caspase-9 signaling pathway is a newly discovered signaling pathway that can regulate the development of GC and the formation of drug resistance. Exo-miR-15b-3p, derived from BGC-823 cells, can be transferred into SGC-7901, GES-1 cells and regulates cell apoptosis by targeting DYNLT1 [44]. Exo-miR-501 can reduce the expression of BLID, inactivate the Caspase-9/Caspase-3 pathway and inhibit apoptosis. In addition, it can also promote AKT phosphorylation and promote adriamycin resistance in GC [45]. Exo-miR-106a-5p and miR-421, which are increased in GC, can regulate TFAP2E methylation, and induce GC cells to be resistant to 5-FU via target gene E2F1, mTOR, and STAT3 [46]. These results imply that exosomal miRNAs derived from drug-resistant cells can potentially predict antitumor chemotherapy therapeutically, improve the efficacy of antitumor chemotherapy, and offer a new treatment option for GC.
The diagnosis and prognosis of GC
The related bioactive substances contained in exosomes can reflect the type and survival state of cells. Exosomes can reflect the type and stage of life of the source cells through the related bioactive substances they contain. Almost all types of bodily fluids can be used to detect exosomes, and the molecular characteristics of tumor exosomes partially reflect the phenotype of the tumor from which they are derived. Exosomes carry tumor-specific miRNAs that can be used as tumor diagnostic markers [3]. Studies showed that exo-miR-92b-3p, let-7 g-5p, miR-146b-5p, and miR-9-5p were significantly correlated with early GC, and the combination of exosomal miRNAs and CEA was more effective at diagnosing early GC than a single exosomal miRNAs marker [47]. In a bioinformatics analysis of exosome miRNAs profiles, Wang et al. found that the expressions of miR-10401-3p, miR-1255b-5p, and miR-6736-5p were significantly down-regulated in GC patients, which may be used as diagnostic markers for GC [48].
Exosomal miRNAs can also be used to evaluate the clinical risk or efficacy of GC, as well as to determine the prognosis. For example, exo-miR-23b and serum exo-miR-451 play important roles in predicting the recurrence and prognosis of GC, which are closely related to tumor size, degree of invasion, lymph node metastasis, liver metastasis, and TNM stage, in addition, low exo-miR-23b suggested poor prognosis [49, 50]. Exo-miR-21 and exo-miR-1225-5p are overexpressed in the peritoneum of GC, which may be used as biomarkers of peritoneal recurrence after radical resection of GC for the early diagnosis [51]. According to Lu et al., there were differences in the expression levels of serum exo-miR-92a-3p between GC patients and healthy controls, and miR-92a-3p levels were low in GC patients, which was closely related to lymph node metastasis and the stage of tumor lymph node metastasis in GC patients [52]. In conclusion, the findings of the above studies provide a new direction for GC diagnosis and lay a foundation for the future application of exosomal miRNAs in the clinical detection of GC.
The therapeutic response marker of GC
Exosomes are characterized by small size, uniform distribution, good biological adaptability, and wide distribution, so they are suitable for being level drug carriers or biological factor carriers, showing potential application prospects in GC-targeted therapy [53]. As the main immune cells in the tumor microenvironment, TAMs have become a new therapeutic target and prognostic indicator in personalized therapy of malignant tumors. Macrophage-derived exosomes can be used as carriers to transfer miR-21 inhibitors to GC cells, reduce cell migration and induce apoptosis [54], it is suggested that exosomes are a good system for delivering drugs to target cells to treat diseases. This study provides valuable references for exploring new strategies of TAMs in the clinical diagnosis and treatment of GC. Studies have also shown that high-dose PPIs can inhibit the release of exosomes from GC cells, exosome-encapsulated miRNA can regulate GC cells and their microenvironment, enhance the apoptosis-inducing effect of anti-tumor drugs, inhibit cell migration, and play a role in GC invasion by regulating HIF-1a-FoxO1 axis. These results suggest that PPIs inhibit the malignant behavior of GC through exosomes to a certain extent [55].
Exosomes could become a novel transporter and be used to cure cancer by transporting anticancer medicines or miRNAs. The zebrafish model proved that exosomes could deliver anticancer drugs across the blood–brain barrier, and fluorescence showed that the drugs effectively inhibited the proliferation and growth of cancer cells, suggesting that exosomes derived from brain epithelial cells could be used to deliver chemotherapy drugs for the treatment of brain cancer [56]. Wang et al. prepared exosomes containing anti-miR-214 and delivered them to SGC-7901 cells with DDP resistance, which could reverse the DDP resistance of SGC-7901 cells, and is expected to provide a new regimen for the treatment of DDP-resistant GC [57]. Jiang et al. found that exo-miR-107 could significantly enhance the sensitivity of drug-resistant GC cells to chemotherapy drugs by inhibiting the HMGA2/mTOR/ P-gp pathway [58].
In addition to in vitro studies, the therapeutic effects of exosomal miRNAs have also been confirmed in vivo. In some studies, exo-miR-210 was injected intravesical in a mouse model of transient middle cerebral artery occlusion (MCAO), and it was found that the lesion area of the ischemic brain was repaired, indicating that exo-miR-210 is beneficial to the repair of brain tissue after cerebral ischemia and provides an angiogenic agent for the treatment of ischemic stroke [59]. In conclusion, exosome miRNAs are engaged in the occurrence and progression of GC, alter the microenvironment of GC, and play a significant role in drug resistance and treatment. In the future detection and treatment of GC, tailored regulation of exosome miRNAs may give a new avenue for prevention and treatment.
Conclusion
Tumor-derived exosomes have dual effects on tumor inhibition or promotion, which may be the result of the complex interaction among exosomes, cells, and environmental factors, and are closely related to the degree of tumor progression and immune status of the body. Exosomes not only carry pathological marker miRNAs derived from cells but also the active molecules in them have direct pharmacodynamic effects, that is, exosomes themselves can be used as carriers to transport drugs, small molecules, or biological therapy/gene therapy agents to specific lesion sites. At the same time, it also has the potential to be modified, processed, and transformed.
To date, H. pylori infection is still the most significant risk factor involved in the onset and progression of GC. H. pylori-induced outside secrete body can take advantage of the lipid bilayer's high physical and chemical stability and biocompatibility, and through signal transduction and the effect of membrane fusion, the function of miRNAs to receptor cells, not only can regulate tumor cell proliferation and apoptosis but can also regulate tumor cell growth microenvironment. However, due to a large number of miRNAs and the complex regulatory network identified, the interaction between miRNA and its target requires specific miRNAs to be identified for treatment, which brings certain difficulties to clinical treatment. Moreover, the molecular mechanism of exosome secretion and function is still not completely clear. Therefore, in the future, we should focus on identifying specific miRNAs and studying the specific mechanism of action of exosomal miRNAs to determine their role in GC diagnosis and prevention.
Data availability
N/A.
References
FitzGerald R, Smith SM (2021) An overview of helicobacter pylori infection[J]. Methods Mol Biol 2283:1–14
Liu X, Ma R, Yi B et al (2021) MicroRNAs are involved in the development and progression of gastric cancer[J]. Acta Pharmacol Sin 42(7):1018–1026
Ingenito F, Roscigno G, Affinito A et al (2019) The role of Exo-miRNAs in cancer: a focus on therapeutic and diagnostic applications[J]. Int J Mol Sci 20(19):4687
Tegtmeyer N, Neddermann M, Asche CI et al (2017) Subversion of host kinases: a key network in cellular signaling hijacked by Helicobacter pylori CagA[J]. Mol Microbiol 105(3):358–372
Selbach M, Schwanhäusser B, Thierfelder N et al (2008) Widespread changes in protein synthesis induced by microRNAs[J]. Nature 455(7209):58–63
Sun F, Ni Y, Zhu H et al (2018) microRNA-29a-3p, up-regulated in human gastric cells and tissues with H.Pylori infection, promotes the migration of GES-1 Cells via A20-mediated EMT pathway[J]. Cell Physiol Biochem 51(3):1250–1263
Yang F, Xu Y, Liu C et al (2018) NF-kappaB/miR-223-3p/ARID1A axis is involved in Helicobacter pylori CagA-induced gastric carcinogenesis and progression[J]. Cell Death Dis 9(1):12
Liu Z, Xiao B, Tang B et al (2010) Up-regulated microRNA-146a negatively modulate Helicobacter pylori-induced inflammatory response in human gastric epithelial cells[J]. Microbes Infect 12(11):854–863
Xu XC, Zhang WB, Li CX et al (2019) Up-Regulation of MiR-1915 inhibits proliferation, invasion, and migration of helicobacter pylori-infected gastric cancer cells via targeting RAGE[J]. Yonsei Med J 60(1):38–47
Li X, Zhu M, Zhao G et al (2022) MiR-1298-5p level downregulation induced by Helicobacter pylori infection inhibits autophagy and promotes gastric cancer development by targeting MAP2K6[J]. Cell Signal 93:110286
Rossi A, Da SMF, Biselli JM et al (2022) Downregulation of TNFR2 decreases survival gene expression, promotes apoptosis and affects the cell cycle of gastric cancer cells[J]. World J Gastroenterol 28(24):2689–2704
Rao X, Liu X, Liu N, et al. Long noncoding RNA NEAT1 promotes tumorigenesis in H. pylori gastric cancer by sponging miR-30a to regulate COX-2/BCL9 pathway[J]. Helicobacter, 2021,26(6):e12847.
Saito Y, Murata-Kamiya N, Hirayama T et al (2010) Conversion of Helicobacter pylori CagA from senescence inducer to oncogenic driver through polarity-dependent regulation of p21[J]. J Exp Med 207(10):2157–2174
Shi Y, Yang Z, Zhang T et al (2019) SIRT1-targeted miR-543 autophagy inhibition and epithelial-mesenchymal transition promotion in Helicobacter pylori CagA-associated gastric cancer[J]. Cell Death Dis 10(9):625
Huang L, Wang ZY, Pan DD (2019) Penicillin-binding protein 1A mutation-positive Helicobacter pylori promotes epithelial-mesenchymal transition in gastric cancer via the suppression of microRNA-134[J]. Int J Oncol 54(3):916–928
Fu M, Gu J, Jiang P et al (2019) Exosomes in gastric cancer: roles, mechanisms, and applications[J]. Mol Cancer 18(1):41
Zhang J, Li S, Li L et al (2015) Exosome and exosomal microRNA: trafficking, sorting, and function[J]. Genomics Proteomics Bioinformatics 13(1):17–24
Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F et al (2013) Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs[J]. Nat Commun 4:2980
McKenzie AJ, Hoshino D, Hong NH et al (2016) KRAS-MEK signaling controls Ago2 sorting into exosomes[J]. Cell Rep 15(5):978–987
Kosaka N, Iguchi H, Hagiwara K et al (2013) Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis[J]. J Biol Chem 288(15):10849–10859
Squadrito ML, Baer C, Burdet F et al (2014) Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells[J]. Cell Rep 8(5):1432–1446
Wang J, Deng Z, Wang Z et al (2016) MicroRNA-155 in exosomes secreted from helicobacter pylori infection macrophages immunomodulates inflammatory response[J]. Am J Transl Res 8(9):3700–3709
Li N, Liu SF, Dong K et al (2019) Exosome-transmitted miR-25 induced by H. pylori promotes vascular endothelial cell injury by targeting KLF2[J]. Front Cell Infect Microbiol 9:366
Wang J, Guan X, Zhang Y et al (2018) Exosomal miR-27a derived from gastric cancer cells regulates the transformation of fibroblasts into cancer-associated fibroblasts[J]. Cell Physiol Biochem 49(3):869–883
Xu G, Zhang B, Ye J et al (2019) Exosomal miRNA-139 in cancer-associated fibroblasts inhibits gastric cancer progression by repressing MMP11 expression[J]. Int J Biol Sci 15(11):2320–2329
Li J, Sun L, Chen Y et al (2022) Gastric cancer-derived exosomal miR-135b-5p impairs the function of Vgamma9Vdelta2 T cells by targeting specificity protein 1[J]. Cancer Immunol Immunother 71(2):311–325
Zhu M, Zhang N, He S et al (2020) Exosomal miR-106a derived from gastric cancer promotes peritoneal metastasis via direct regulation of Smad7[J]. Cell Cycle 19(10):1200–1221
Zhu M, Zhang N, Ma J et al (2022) Integration of exosomal miR-106a and mesothelial cells facilitates gastric cancer peritoneal dissemination[J]. Cell Signal 91:110230
Li Q, Li B, Li Q et al (2018) Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition[J]. Cell Death Dis 9(9):854
Tang G, Wang J, Dong W et al (2022) Exosomal miRNA expression profiling and the roles of exosomal miR-4741, miR-32, miR-3149, and miR-6727 on gastric cancer progression[J]. Biomed Res Int 2022:1263812
Huang J, Shen M, Yan M et al (2019) Exosome-mediated transfer of miR-1290 promotes cell proliferation and invasion in gastric cancer via NKD1[J]. Acta Biochim Biophys Sin (Shanghai) 51(9):900–907
Chang L, Gao H, Wang L et al (2021) Exosomes derived from miR-1228 overexpressing bone marrow-mesenchymal stem cells promote growth of gastric cancer cells[J]. Aging (Albany NY) 13(8):11808–11821
Jiao Y, Zhang L, Li J et al (2021) Exosomal miR-122-5p inhibits tumorigenicity of gastric cancer by downregulating GIT1[J]. Int J Biol Markers 36(1):36–46
Yang X, Cai S, Shu Y et al (2021) Exosomal miR-487a derived from m2 macrophage promotes the progression of gastric cancer[J]. Cell Cycle 20(4):434–444
Shi SS, Zhang HP, Yang CQ et al (2020) Exosomal miR-155-5p promotes proliferation and migration of gastric cancer cells by inhibiting TP53INP1 expression[J]. Pathol Res Pract 216(6):152986
Feng C, She J, Chen X et al (2019) Exosomal miR-196a-1 promotes gastric cancer cell invasion and metastasis by targeting SFRP1[J]. Nanomedicine (Lond) 14(19):2579–2593
Yang H, Fu H, Wang B et al (2018) Exosomal miR-423-5p targets SUFU to promote cancer growth and metastasis and serves as a novel marker for gastric cancer[J]. Mol Carcinog 57(9):1223–1236
Yang H, Zhang H, Ge S et al (2018) Exosome-derived miR-130a activates angiogenesis in gastric cancer by targeting C-MYB in vascular endothelial cells[J]. Mol Ther 26(10):2466–2475
Bai M, Li J, Yang H et al (2019) miR-135b delivered by gastric tumor exosomes inhibits FOXO1 expression in endothelial cells and promotes angiogenesis[J]. Mol Ther 27(10):1772–1783
Zheng P, Chen L, Yuan X et al (2017) Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells[J]. J Exp Clin Cancer Res 36(1):53
Cui HY, Rong JS, Chen J et al (2021) Exosomal microRNA-588 from M2 polarized macrophages contributes to cisplatin resistance of gastric cancer cells[J]. World J Gastroenterol 27(36):6079–6092
Jing X, Xie M, Ding K et al (2022) Exosome-transmitted miR-769-5p confers cisplatin resistance and progression in gastric cancer by targeting CASP9 and promoting the ubiquitination degradation of p53[J]. Clin Transl Med 12(5):e780
Wang M, Qiu R, Yu S et al (2019) Paclitaxel-resistant gastric cancer MGC-803 cells promote epithelial-to-mesenchymal transition and chemoresistance in paclitaxel-sensitive cells via exosomal delivery of miR-155-5p[J]. Int J Oncol 54(1):326–338
Wei S, Peng L, Yang J et al (2020) Exosomal transfer of miR-15b-3p enhances tumorigenesis and malignant transformation through the DYNLT1/Caspase-3/Caspase-9 signaling pathway in gastric cancer[J]. J Exp Clin Cancer Res 39(1):32
Liu X, Lu Y, Xu Y et al (2019) Exosomal transfer of miR-501 confers doxorubicin resistance and tumorigenesis via targeting of BLID in gastric cancer[J]. Cancer Lett 459:122–134
Jingyue S, Xiao W, Juanmin Z et al (2019) TFAP2E methylation promotes 5-fluorouracil resistance via exosomal miR-106a-5p and miR-421 in gastric cancer MGC-803 cells[J]. Mol Med Rep 20(1):323–331
Tang S, Cheng J, Yao Y et al (2020) Combination of four serum exosomal MiRNAs as novel diagnostic biomarkers for early-stage gastric cancer[J]. Front Genet 11:237
Wang JF, Jiang YM, Zhan WH et al (2022) Screening of serum exosomal miRNAs as diagnostic biomarkers for gastric cancer using small RNA sequencing[J]. J Oncol 2022:5346563
Kumata Y, Iinuma H, Suzuki Y et al (2018) Xosome-encapsulated microRNA-23b as a minimally invasive liquid biomarker for the prediction of recurrence and prognosis of gastric cancer patients in each tumor stage [J]. Oncol Rep 40(1):319–330
Liu F, Bu Z, Zhao F et al (2018) Increased T-helper 17 cell differentiation mediated by exosome-mediated microRNA-451 redistribution in gastric cancer infiltrated T cells[J]. Cancer Sci 109(1):65–73
Tokuhisa M, Ichikawa Y, Kosaka N et al (2015) Exosomal miRNAs from peritoneum lavage fluid as potential prognostic biomarkers of peritoneal metastasis in gastric cancer[J]. PLoS ONE 10(7):e130472
Lu X, Lu J, Wang S et al (2021) Circulating serum exosomal miR-92a-3p as a novel biomarker for early diagnosis of gastric cancer[J]. Future Oncol 17(8):907–919
Ha D, Yang N, Nadithe V (2016) Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges[J]. Acta Pharm Sin B 6(4):287–296
Wang JJ, Wang ZY, Chen R et al (2015) Macrophage-secreted Exosomes Delivering miRNA-21 Inhibitor can Regulate BGC-823 Cell Proliferation[J]. Asian Pac J Cancer Prev 16(10):4203–4209
Guan XW, Zhao F, Wang JY et al (2017) Tumor microenvironment interruption: a novel anti-cancer mechanism of Proton-pump inhibitor in gastric cancer by suppressing the release of microRNA-carrying exosomes[J]. Am J Cancer Res 7(9):1913–1925
Yang T, Martin P, Fogarty B et al (2015) Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio[J]. Pharm Res 32(6):2003–2014
Wang X, Zhang H, Bai M et al (2018) Exosomes serve as nanoparticles to deliver Anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer[J]. Mol Ther 26(3):774–783
Jiang L, Zhang Y, Guo L et al (2021) Exosomal microRNA-107 reverses chemotherapeutic drug resistance of gastric cancer cells through HMGA2/mTOR/P-gp pathway[J]. BMC Cancer 21(1):1290
Zhang H, Wu J, Wu J et al (2019) Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice[J]. J Nanobiotechnology 17(1):29
Funding
This work was supported by the National Natural Science Foundation of China (No. 82102398), Shandong Provincial Natural Science Foundation (ZR2020QC073 and ZR2021MH409), and the 2019 Technology Development Project of Shandong Medicine and Health Science (2019WS230).
Author information
Authors and Affiliations
Contributions
Y.T. established the original concept and design. X.X., S.S., D.G., Z.W., L.G., and P.L. conducted literature retrieval. X.X., S.S., and Y.T. wrote the manuscript, designed the figures, and made critical revisions to the manuscript. D.G., L.G., Z.W., and P.L. helped with the discussion and corrected the text. All authors read and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
N/A.
Consent to participate and consent for publication
WE shall commit that this manuscript is original. Neither whole nor part of the texts has been published in any other journal in any form, and all listed authors agree to enclose the original manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, XH., Shao, SL., Guo, D. et al. Roles of microRNAs and exosomes in Helicobacter pylori associated gastric cancer. Mol Biol Rep 50, 889–897 (2023). https://doi.org/10.1007/s11033-022-08073-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08073-x