Abstract
Background:
Coronary artery disease (CAD) is a complex disease that is influenced by environmental and genetic factors. Lipid levels are regarded as a major risk factor for CAD, and epigenetic mechanisms might be involved in the regulation of CAD development. This study was designed to investigate the association between the DNA methylation status of 8 lipid metabolism-related genes and the risk of CAD in the Chinese Han population.
Methods:
A total of 260 individuals were sampled in this study, including 120 CAD cases and 140 normal healthy controls. DNA methylation status was tested via targeted bisulfite sequencing.
Results:
The results indicated a significant association between hypomethylation of the APOC3, CETP and APOC1 gene promoters and the risk of CAD. Individuals with higher methylation levels of the APOA5 and LIPC gene promoters had increased risks for CAD. In addition, ANGPTL4 methylation level was significantly associated with CAD in males but not females. There were no significant differences in the methylation levels of the APOB and PCSK9 gene promoters between CAD patients and controls.
Conclusions
The methylation status of the APOC3, APOA5, LIPC, CETP and APOC1 gene promoters may be associated with the development of CAD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease (CAD) is a common chronic inflammatory disease that has been recognized as the leading cause of death worldwide [1]. In China, it is estimated that 700,000 people died from CAD every year [2]. Blood lipid levels, including triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and serum total cholesterol (TC) levels, have been identified as important independent risk factors for CAD [3]. Recent advances have started to reveal the genetic architecture of CAD and have shown that genetic variants and epigenetic regulation of lipid metabolism-related genes also contribute to CAD etiology [4,5,6].
DNA methylation is a biological process in which gene expression is regulated by the recruitment of proteins involved in gene repression or by inhibition of the binding of transcription factors to DNA without changing the DNA sequence. Aberrant promoter hypermethylation and hypomethylation may be associated with risks of various diseases, including cardiovascular, cancer and metabolic diseases [7,8,9]. Several previous studies have revealed that the methylation signatures of critical genes may play a role in CAD development [4,5,6].
In this study, we aimed to investigate the association of the methylation status of 8 lipid metabolism-related genes (ANGPTL4, APOC3, APOA5, APOB, LIPC, CETP, PCSK9 and APOC1) with the risk of CAD development in the Chinese Han population.
Materials and methods
Study population.
The participants in this study were recruited from Shanghai Renji Hospital between 2018 and 2020. A total of 120 CAD patients (88 male and 32 female) and 140 non-CAD controls (93 male and 47 female) were included in this study. The criterion for CAD diagnosis was at least one of the major segments of the coronary arteries (right coronary, left circumflex, or left anterior descending artery) with at least 50% organic stenosis based on coronary angiography. All unaffected controls were determined to be free of CAD. All participants were genetically unrelated Chinese Han individuals from Shanghai. This study was approved by the Medical Ethics Committee of Renji Hospital affiliated with the Shanghai Jiaotong University School of Medicine and compliant with the principles set forth by the Declaration of Helsinki. Written informed consent was obtained from all subjects. Blood samples (5 ml) were collected from the participants into EDTA tubes and then stored at − 80 °C for further use.
DNA extraction, bisulfite conversion and targeted bisulfite sequencing.
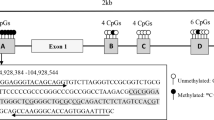
Genomic DNA was extracted from whole blood with a Tiangen DNA extraction kit (Tiangen Ltd., Beijing, China) according to the manufacturer’s instructions. DNA quality and concentration were analyzed using electrophoresis and a NanoDrop spectrophotometer (NanoDrop Technologies, Houston, TX, USA). Bisulfite conversion of 200 ng genomic DNA was performed by the EZ DNA Methylation-Gold Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s standard protocol. For each gene, PCR primers were designed specifically for bisulfite-converted DNA using MethPrimer [10]. PCR primers were synthesized by Shanghai Free Biotechnology Co., Ltd. (Shanghai, China). Multiplex PCR of target CpG regions was performed, and the products were sequenced with Illumina NovaSeq sequencing instruments (Novogene, Beijing, China). A mean sequencing depth of > 500X was achieved for all samples. CpG sites were named according to their relative distance (in bp) to the transcriptional start site (TSS) (with negative distances upstream from the TSS). The methylation level of each CpG site was calculated as the percentage of the methylated cytosines over the total tested cytosines. The average methylation level was calculated using the methylation levels of all measured CpG sites within the gene.
Statistical analysis
Statistical analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA, USA). The correlation between DNA methylation and CAD was assessed using an independent sample t test and expressed as the mean ± standard deviation (SD). Both the average gene methylation data and the methylation data for the individual CpG loci were analyzed. Stratified analyses based on sex were carried out. A P value < 0.05 was considered statistically significant.
Results
A total of 120 CAD patients and 140 healthy controls were recruited for this study. Targeted bisulfite sequencing was used to assess a total of 98 CpG sites in 8 lipid metabolism-related gene (ANGPTL4, APOC3, APOA5, APOB, LIPC, CETP, PCSK9 and APOC1) promoters. The methylation levels of each CpG site were compared between patients and healthy controls (Fig. 1). The methylation levels of 14 sites were significantly higher in the case group than in the control group, while the methylation levels of 37 sites were significantly lower in the case group than in the control group (supplementary Table 1).
The mean methylation level of each gene was calculated (Table 1). As shown in Table 1, significantly decreased methylation levels of the APOC3, CETP and APOC1 genes were observed in the case group compared with the control group. Most CpG sites were hypomethylated in APOC3 (5 of 5), CETP (9 of 10) and APOC1 (11 of 12). Significantly increased methylation levels of the APOA5 and LIPC genes were observed in the case group compared with the control group. For LIPC, 3 of 4 CpG sites were hypermethylated. For APOA5, 2 CpG sites were hypomethylated, and 2 CpG sites were hypermethylated. However, no significant correlations between methylation level and CAD were observed in ANGPTL4, APOB or PCSK9.
Discussion
CAD is a complex disease that is influenced by environmental, biochemical, and genetic risk factors. Lipoprotein metabolism disorder is a causal risk factor for cardiovascular diseases in the general population. DNA methylation, the most widely studied epigenetic mechanism, plays an important role in the etiology of human disease. Recent studies revealed that DNA methylation changes in gene promoters might be implicated in the development of CAD. In this study, we investigated the methylation status of a subset of lipid metabolism-related genes in CAD patients and control subjects in the Chinese Han population via targeted bisulfite sequencing.
The results support the hypothesis that epigenetic changes within lipid metabolism-related genes might account for blood lipid profile variability and could be a molecular mechanism explaining the pathogenesis of CAD. Different methylation statuses of the APOC3, APOA5, LIPC, CETP and APOC1 gene promoters were observed between CAD patients and healthy controls. DNA methylation could serve as a biomarker for predicting the risk of CAD [11,12,13].
APOC3 encodes a protein component of TG-rich lipoproteins (TRLs) and plays a role in promoting the hepatic secretion of TRLs. APOC3 is an inhibitor of lipoprotein lipase (LPL) enzyme activity and prevents TRL clearance [14, 15]. Loss-of-function APOC3 mutations were associated with low plasma TG levels and reduced risk of cardiovascular disease [16, 17]. Genetic variation in the promoter region of the APOC3 gene was associated with increased risks of hypertriglyceridemia, metabolic syndrome and CAD [18,19,20]. Overexpression of the APOC3 gene in transgenic animals induces severe hypertriglyceridemia, while APOC3 gene deletion results in hypotriglyceridemia [21,22,23,24]. In this study, the APOC3 gene was hypomethylated in CAD patients. The methylation level of CpG-119 in the APOC3 gene promoter was significantly lower in the CAD group than in the control group (13.5% vs. 24.5%) and might lead to higher gene expression.
The APOC1 gene encodes a member of the apolipoprotein C1 family and resides within the APOE/APOC1/APOC2 gene cluster. This gene is predominantly expressed in the liver, lung, skin, spleen, adipose tissue, and brain [25]. APOC1 plays an important role in high-density lipoprotein (HDL) and very-low-density lipoprotein (VLDL) metabolism. APOC1 is a very potent and highly selective inhibitor of cholesteryl ester transfer protein (CETP) in plasma [26, 27]. Transgenic analysis revealed that increased expression of APOC1 inhibits the hepatic uptake of lipoproteins and results in combined hyperlipidemia [28,29,30]. In this study, 12 CpG sites in APOC1 were analyzed, and 11 CpG sites from − 38 to + 164 were hypomethylated in CAD patients. The methylation level of CpG-50 was not significantly different between the case and control groups.
CETP mediates the transfer of cholesteryl ester from HDL to other lipoproteins and promotes the formation of TG-rich and CE-poor HDL particles. Genetic variation in CETP has been reported to be associated with HDL-C levels [28,29,30,31,32,33. CETP deficiency was associated with slow progression of CAD, high HDL-C level, low HDL-TG levels and a larger HDL particle size [34, 35]. CETP inhibitors effectively reduce LDL-C levels and increase HDL-C levels and may be effective in reducing atherosclerosis and cardiovascular events [36]. In this study, 9 of 10 CpG sites from − 94 to + 140 in the CETP gene were hypomethylated in CAD patients. The methylation level of CpG + 269 did not differ significantly between the case and control groups.
LIPC encodes hepatic triglyceride lipase, which participates in the hydrolysis of TGs and phospholipids and is mainly expressed in and secreted from the liver. Variants in the promoter region of LIPC were reported to be correlated with high HDL-C levels [37, 38]. It has been reported that in familial hypercholesterolemia, subjects with a previous history of CAD had higher LIPC DNA methylation levels than those without a CAD history [39]. Our study revealed that 3 of 4 CpG sites from − 40 to + 44 in the LIPC gene were hypermethylated in CAD patients. The methylation level of CpG + 131 did not differ significantly between the case and control groups.
The APOA5 gene plays an important role in the regulation of blood triglyceride levels and is regarded as a major risk factor for coronary heart disease. Genome-wide methylation analysis revealed that APOA5 was hypomethylated in children with obesity [40]. APOA5 hypomethylation is also involved in aortic valve stenosis (AVS) [41]. Genetic variation in the APOA5 gene was associated with the levels of plasma lipids and an increased risk of cardiovascular disease [42, 43]. The mean methylation level of the APOA5 gene was relatively high in CAD patients compared with the control group. However, CpG − 50 and CpG + 32 were hypermethylated, while CpG − 343 and CpG − 186 were hypomethylated.
DNA methylation affects gene expression and regulates lipid metabolism. However, the cause of methylation variation is still poorly understood. DNA methylation signature was previously shown to be partially inherited. The methylation pattern can be affected by nearby single nucleotide polymorphisms (SNPs) [44, 45]. It has been revealed that the interaction of genetic and epigenetic variation contributes to the development of complex diseases [46]. In addition to genetic variants, environmental and lifestyle modifications are also considered to be potential causes of DNA methylation diversity [47,48,49]. Previous reports showed that a high-fat diet introduced DNA methylation changes in skeletal muscle and subcutaneous adipose tissue. Consumption of a high-fat diet is also associated with an increased risk of metabolic diseases [50, 51]. Hahn found that dietary restriction remodels DNA methylation patterns and gene expression, particularly of genes involved in lipid metabolism [52]. In addition, physical activity, smoking and drinking also induce DNA methylation variations [53,54,55,56].
In conclusion, targeted bisulfite sequencing was used to assess the methylation status of 8 lipid metabolism-related candidate genes in patients diagnosed with CAD and control subjects without CAD. We revealed that the methylation levels of the APOC3, CETP and APOC1 gene promoters were lower in the CAD group than in the control group. The methylation levels of the APOA5 and LIPC gene promoters were higher in the CAD group than in the control group. Our findings support the hypothesis that DNA methylation of lipid-related genes plays a role in the development of CAD and provide some new insight for the prevention and treatment of CAD. However, there are some limitations to this study. The expression levels of the target genes were not investigated, so we could not determine whether promoter methylation affects gene expression. Moreover, variations in the gene regions and DNA methylation might have dual effects on disease development. Further study of a larger sample with expression and genotyping data is needed to confirm our results.
References
Roger VL, Go AS, Lloyd-Jones DM et al (2011) Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123(4):e18–e209
Wang F, Xu CQ, He Q et al (2011) Genome-wide association identifies a susceptibility locus for coronary artery disease in the Chinese Han population. Nat Genet 43(4):345–349
Foody J, Yong H, Ji L et al (2013) Unique and Varied Contributions of Traditional CVD Risk Factors: A Systematic Literature Review of CAD Risk Factors in China. Clin Med Insights Cardiol. ; 2013(7):59–86
Duan L, Liu C, Hu J et al (2018) Epigenetic mechanisms in coronary artery disease: The current state and prospects. Trends Cardiovasc Med 28(5):311–319
Pjanic M, Miller CL, Wirka R et al (2016) Genetics and Genomics of Coronary Artery Disease. Curr Cardiol Rep 18(10):102
Musunuru K, Kathiresan S (2019) Genetics of Common, Complex Coronary Artery Disease. Cell 177(1):132–145
Ghaznavi H, Mahmoodi K, Soltanpour MS (2018) A preliminary study of the association between the ABCA1 gene promoter DNA methylation and coronary artery disease risk. Mol Biology Res Commun 7(2):59–65
Su J, Li J, Yu Q et al (2019) Association of PON1 gene promoter DNA methylation with the risk of Clopidogrel resistance in patients with coronary artery disease. J Clin Lab Anal 33(5):e22867
Zhuang J, Peng W, Li H et al (2012) Methylation of p15INK4b and Expression of ANRIL on Chromosome 9p21 Are Associated with Coronary Artery Disease. PLoS ONE 7(10):e47193
Li LC, Dahiya R, Methprimer (2002) Designing Primers for Methylation PCRs. Bioinformatics 18(11):1427–1431
Agha G et al “Blood Leukocyte DNA Methylation Predicts Risk of Future Myocardial Infarction and Coronary Heart Disease.“ Circulation140.8(2019):645–657
Zhao X et al (2022) “F2RL3 Methylation in the Peripheral Blood as a Potential Marker for the Detection of Coronary Heart Disease: A Case-Control Study.“ Front Genet. 24:833923
Xia Y, Brewer A, Bell JT “DNA methylation signatures of incident coronary heart disease: findings from epigenome-wide association studies.“Clinical Epigenetics13.1(2021):1–16
Taskinen MR, Packard CJ, Boren J (2019) Emerging Evidence that ApoC-III Inhibitors Provide Novel Options to Reduce the Residual CVD. Curr Atheroscler Rep 21(8):27
Akoumianakis I, Zvintzou E, Kypreos K et al (2021) ANGPTL3 and Apolipoprotein C-III as Novel Lipid-Lowering Targets. Curr Atheroscler Rep 23(5):20
Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG et al (2014) Loss-of-Function Mutations in APOC3 and Risk of Ischemic Vascular Disease. N Engl J Med 3711(1):32–41
Crosby J, Peloso GM, Auer PL et al (2014) Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 371(1):22–31
Li WW, Dammerman MM, Smith J et al (1996) Common genetic variation in the promoter of the human apo CIII gene abolishes regulation by insulin and may contribute to hypertriglyceridemia. J Clin Invest 96(6):2601–2605
Pollex RL, Ban MR, Young TK et al (2007) Association between the – 455T > C promoter polymorphism of the APOC3 gene and the metabolic syndrome in a multi-ethnic sample. BMC Med Genet 8(80):1471–2350
Olivieri O, Stranieri C, Bassi A et al (2002) ApoC-III gene polymorphisms and risk of coronary artery disease[J]. J Lipid Res 43(9):1450–1457
Jong MC, Rensen PC, Dahlmans VE et al (2001) Apolipoprotein C-III deficiency accelerates triglyceride hydrolysis by lipoprotein lipase in wild-type and apoE knockout mice. J Lipid Res 42(10):1578–1585
Yan H, Niimi M, Matsuhisa F et al (2020) Apolipoprotein CIII Deficiency Protects Against Atherosclerosis in Knockout Rabbits.Arteriosclerosis Thrombosis and Vascular Biology. ; 40(9)
Ding Y, Wang Y, Hong Z et al (2011) Hypertriglyceridemia and delayed clearance of fat load in transgenic rabbits expressing human apolipoprotein CIII. Transgenic Res 20(4):867
Ito Y, Azrolan N, O’Connell A et al (1990) Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science 249(4970):790–793
Lauer SJ, Walker D, Elshourbagy NA et al (1988) Two copies of the human apolipoprotein C-I gene are linked closely to the apolipoprotein E gene. J Biol Chem 263(15):7277–7286
Gautier T, Masson D, Jong MC et al (2002) Apolipoprotein CI Deficiency Markedly Augments Plasma Lipoprotein Changes Mediated by Human Cholesteryl Ester Transfer Protein (CETP) in CETP Transgenic/ApoCI-knocked Out Mice. J Biol Chem 277(35):31354
Gautier T, Masson D, de Barros JP et al (2000) Human Apolipoprotein C-I Accounts for the Ability of Plasma High Density Lipoproteins to Inhibit the Cholesteryl Ester Transfer Protein Activity. J Biol Chem 275(48):37504–37509
Shachter NS, Ebara T, Ramakrishnan R et al (1996) Combined hyperlipidemia in transgenic mice overexpressing human apolipoprotein Cl. J Clin Invest 98(3):846–855
Jong MC, Dahlmans VE, Gorp P et al (1996) In the absence of the low density lipoprotein receptor, human apolipoprotein C1 overexpression in transgenic mice inhibits the hepatic uptake of very low density lipoproteins via a receptor-associated protein-sensitive pathway. J Clin Investig 98(10):2259–2267
Berbee JF, Hoogt CC, Sundararaman D et al (2005) Severe hypertriglyceridemia in human APOC1 transgenic mice is caused by apoC-I-induced inhibition of LPL. J Lipid Res 46(2):297–306
Takahashi K, Jiang XC, Sakai N et al (1993) A missense mutation in the cholesteryl ester transfer protein gene with possible dominant effects on plasma high density lipoproteins. J Clin Invest 92(4):2060–2064
Arai T, Yamashita S, Sakai N et al (1996) A novel nonsense mutation (G181X) in the human cholesteryl ester transfer protein gene in Japanese hyperalphalipoproteinemic subjects. J Lipid Res 37(10):2145
Boekholdt SM, Thompson JF (2003) Natural genetic variation as a tool in understanding the role of CETP in lipid levels and disease. J Lipid Res 44(6):1080–1093
Inazu A, Jiang XC, Haraki T et al (1994) Genetic cholesteryl ester transfer protein deficiency caused by two prevalent mutations as a major determinant of increased levels of high density lipoprotein cholesterol. J Clin Invest 94(5):1872–1882
Arai T, Tsukada T, Murase T et al (2000) Particle size analysis of high density lipoproteins in patients with genetic cholesteryl ester transfer protein deficiency. Clin Chim Acta 301(1–2):103–117
Mabuchi H, Nohara A, Inazu A (2014) Cholesteryl Ester Transfer Protein (CETP) Deficiency and CETP Inhibitors. Molecules & Cells 37(11):777–784
Aaron I, Sayed-Tabatabaei FA, jajou OT et al (2004) The – 514 C->T hepatic lipase promoter region polymorphism and plasma lipids: a meta-analysis.Journal of Clinical Endocrinology & Metabolism. (8):3858–3863
Hodoglugil U, Williamson DW, Mahley RW (2010) Polymorphisms in the hepatic lipase gene affect plasma HDL-cholesterol levels in a Turkish population. J Lipid Res 51(2):422
Guay SP, Brisson D, Lamarche B et al (2014) Epipolymorphisms within lipoprotein genes contribute independently to plasma lipid levels in familial hypercholesterolemia[J]. Epigenetics 9(5):718–729
Li Y, Zhou Y, Zhu L et al (2018) Genome-wide analysis reveals that altered methylation in specific CpG loci is associated with childhood obesity. J Cell Biochem 119(9):7490–7497
Radhakrishna U, Albayrak S, Alpay-Savasan Z et al (2016) Genome-Wide DNA Methylation Analysis and Epigenetic Variations Associated with Congenital Aortic Valve Stenosis (AVS). PLoS ONE 11(5):e0154010
Zhao T, Zhao J (2010) Association of the apolipoprotein A5 gene – 1131 T > C polymorphism with fasting blood lipids: A meta-analysis in 37859 subjects. BMC Med Genet 11:120
Wang J, Ban MR, Kennedy BA et al (2008) APOA5 genetic variants are markers for classic hyperlipoproteinemia phenotypes and hypertriglyceridemia. Nat Clin Pract Cardiovasc Med 5(11):730–737
Kerkel K et al (2008) “Genomic surveys by methylation-sensitive SNP analysis identify sequence-dependent allele-specific DNA methylation. " Nat Genet 40:904
Lu T et al “Whole-genome bisulfite sequencing in systemic sclerosis provides novel targets to understand disease pathogenesis.“BMC Medical Genomics12.1(2019):1–12
Dayeh TA et al “Identification of CpG-SNPs associated with type 2 diabetes and differential DNA methylation in human pancreatic islets.“ Diabetologia 56.5(2013):1036–1046
Zhou D et al “High Fat Diet and Exercise Lead to a Disrupted and Pathogenic DNA Methylome in Mouse Liver.“ Epigenetics12.1(2016):00–00
Martin EM, Fry RC (2018). “Environmental Influences on the Epigenome: Exposure- Associated DNA Methylation in Human Populations.“ Annual Review of Public Health39.1
Suderman M et al (2014) “Childhood abuse is associated with methylation of multiple loci in adult DNA.“ BMC Medical Genomics,7,1(2014-03-11) 7.1:13
Gillberg L et al “PPARGC1A DNA methylation in subcutaneous adipose tissue in low birth weight subjects–impact of 5 days of high-fat overfeeding.“ Metabolism-clinical & Experimental63.2(2014):263–271
Jacobsen SC et al (2012) Effects of short-term high-fat overfeeding on genome-wide DNA methylation in the skeletal muscle of healthy young men. Diabetologia 55:3341–3349
Hahn O et al (2017) Dietary restriction protects from age-associated DNA methylation and induces epigenetic reprogramming of lipid metabolism. Genome Biol 18(1):56
Romain, Barrès et al (2012) “Acute exercise remodels promoter methylation in human skeletal muscle. " Cell Metabolism 153:405–411
Rönn T et al (2013) A Six Months Exercise Intervention Influences the Genome-wide DNA Methylation Pattern in Human Adipose Tissue. PLoS Genet 9:e1003572
Dogan MV et al “The effect of smoking on DNA methylation of peripheral blood mononuclear cells from African American women.“ BMC Genomics,15,1(2014-02-22) 15.1(2014):151
Philibert RA et al (2014) “A pilot examination of the genome-wide DNA methylation signatures of subjects entering and exiting short-term alcohol dependence treatment programs. " Epigenetics 9 9:1212–1219
Acknowledgements
This project was funded by the Shanghai Science and Technology Development Foundation (SY20221RUE01). We also thank Hu Liu from Shanghai Lehao Bio-Science Company for his technical support in gene sequencing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, W., Wang, Y., Huang, R. et al. Association of lipid metabolism-related gene promoter methylation with risk of coronary artery disease. Mol Biol Rep 49, 9373–9378 (2022). https://doi.org/10.1007/s11033-022-07789-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07789-0