Abstract
Despite major advances, breast cancer (BC) is the most commonly diagnosed carcinoma and remains a deadly disease among women worldwide. Many researchers point toward an important role of an epithelial to mesenchymal transition (EMT) in BC development and promoting metastasis. Here, will be discussed that how functional changes of transcription factors, signaling pathways, and microRNAs (miRNA) in BC promote EMT. A thorough understanding the EMT biology can be important to determine reversing the process and design treatment approaches. There are frequent debates as to whether EMT is really relevant to BC in vivo, in which due to the intrinsic heterogeneity and tumor microenvironment. Nevertheless, given the importance of EMT in cancer progression and metastasis, the implementation of therapies against cancer-associated EMT will continue to help us develop and test potential treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most prevalent malignancy among women worldwide, and its incidence is 11.7% among all types of cancers. BC is very heterogeneous and based on the presence or absence of the hormone receptors epithelial-mesenchymal transition including progesterone receptor (PR), estrogen receptor (ER), and ERBB2 receptor or human epidermal growth factor receptor type 2 (HER2), BC is classified into three clinical subtypes: HER2 positive (HER2+), hormone-receptor (HR)-positive (HR+; ER+, PR+/– and HER2–), and triple-negative (TN; ER–, PR– and HER2–) [1]. The treatment strategy for this cancer is selected based on different factors such as morphology, size and grade of tumor, metastases, and the expression of ER, PR, and HER2 [2]. Metastasis is the main cause of breast cancer-related mortality, which is modulated by different factors such as epithelial to mesenchymal transition (EMT) [3]. Thus, understanding the molecular mechanisms of metastasis and EMT in this cancer is critical.
Metastatic cascade can be recognized as an inefficient and deadly process that is highly complex. The importance of metastatic cascade has been under numerous investigations, in the hope of decreasing metastatic progression and prolonging survival. The first step in cancer metastasis is the migration of epithelial cancer cells away from the primary tumors and penetrate the circulation blood. Invasion-metastasis cascade is an extremely versatile process that is regulated at several levels, whereby tumor cell adhesion is eliminated and cell motility is enhanced. The key process underlying tumor cell individualization and achievement of migratory and invasive capacities frequently occurs within the framework of EMT [4].
MicroRNAs (miRNAs) are a class of non-coding single-stranded RNAs (ssRNAs), which are small (approximately 18–24 nucleotides) and evolutionarily conserved. MiRNAs play important roles in regulating gene expression post-transcriptionally, which functions through repressing target mRNA translation or increasing the degradation of mRNA. Emerging evidence has demonstrated that deregulation of miRNAs is correlated with differentiation, apoptosis, metastasis, and invasion in various types of cancers. MiRNA microarray analysis has reported that multiple miRNAs contribute to tumor growth, chemoresistance, and metastasis in breast cancer. Accumulating evidence has demonstrated that miRNAs are correlated with BC. MiRNAs function as oncogenes (onco-miRNAs) and tumor suppressors.
In the past few years, many miRNAs have been identified to regulate multiple steps of the EMT process [5]. Given these attributes, EMT–related miRNAs are known to play a crucial role during tumorigenesis, and can be considered as potential biomarkers or therapeutic targets for BC. This review will summarize the existing literature regarding the contribution of EMT during metastasis with the main focus on miRNA regulation in BC.
Overview of EMT
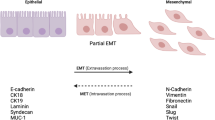
During the EMT process, molecular reprogramming and phenotypic alteration occur, and polarized immotile epithelial cells change to motile mesenchymal cells. This fundamental cellular process contributes to tumor-initiating potential, invasion, metastasis, and chemoresistance. In EMT the expression of several proteins decrease, which are responsible for enhancing cell-cell contacts such as E-cadherin and γ-catenin. The expression of mesenchymal markers also increases such as N-cadherin, vimentin, and fibronectin [6].
Metastasis, the process by which tumor cells migrated from the original site to other parts of the body, causes roughly 90% of carcinoma-related deaths. An early essential step of the metastatic cascade is a phenotypic switch termed EMT. EMT is a basic developmental program wherein epithelial cells (ECs) undergo many transcriptional and morphological transformations and acquire mesenchymal characteristics, that includes reinforced migratory capability, increased apoptosis, and elevated extracellular matrix (ECM) production (Fig. 1) [7, 8].
EMT signaling pathways and core regulators
EMT is regulated by several signaling pathways and growth factors according to the cellular contexts. Epidermal growth factor (EGF) and transforming growth factor (TGF) pathway have been widely investigated and shown that they contributed to angiogenesis progression [9]. Other mechanisms are also identified to drive EMT in BC including hepatocyte growth factor (HGF), fibroblast growth factor (FGF) [10, 11], insulin-like growth factor (IGF) platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), Stem cell factor (SCF)/c-kit signaling pathway, bone morphogenetic proteins (BMPs), autocrine motility factor (AMF), Wnt/β-catenin/LEF-1, hedgehog proteins, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), Notch, interleukin-like EMT inducer (ILEI), stress including hypoxia, inflammatory cytokine IL-6, leptin, cyclooxygenase-2/prostaglandin E2 (COX-2/PGE2), and ECM proteins [12]. Given that stimulation majority of signal transduction pathways can reinforce EMT and invasion processes. Following the multiple EMT-inducing signals and their downstream signaling effectors, the expression of EMT-activating transcriptional factors (EMT-TFs) is upregulated to coordinate this process. The major EMT‐TFs include the Snail (Snail1, Snail2, Snail3) families of zinc finger transcription factors, TWIST (TWIST1 and TWIST2) basic helix-loop-helix protein (bHLH) family members, and also zinc finger E-box binding homeobox 1/2 (ZEB 1/2) [13, 14]. In the EMT process, downregulation of E-cadherin, which is an essential event in invasive/metastatic potential, is closely associated with loss of cell-cell adhesion junctions and gain of ability in motility and metastasis [15, 16]. The coordinated activity of diverse EMT-TFs can lead to reducing E-cadherin gene expression.
All of directly or indirectly repressors involved in tumor metastasis and EMT through a signaling network comprising signal transducer and activator of transcription 3 (STAT3), PI3K-AKT-mTOR signaling pathways, Ras/mitogen-activated protein kinase (MAPK) pathway, Wnt/β-catenin pathway, and NF-κB pathway, along with the final result of the reduction of E-cadherin and induction of metastatic proteins, like vimentin, fibronectin, N-cadherin, matrix metalloproteinase, etc. Also, changes in E-cadherin expression level are the result of a series of epigenetic modifications, for example, hypermethylation in the promoter region leads to the reduction of E-cadherin, associated with EMT phenotype in BC [17].
Cell junctions, cell adhesion, and the extracellular matrix
Ectopic expression of EMT-TFs leads to repression of genes encoding junction proteins, whereby ECs lose apical-basal polarity and cell junctions including adherens junctions, desmosomes, and tight junctions [8]. Since cellular adhesion is involved in cell signaling, differentiation, growth, inflammation, as well survival, so, alteration of these molecules affects multiple downstream pathways and might enhance cancer metastasis and EMT [18]. For instance, following the downregulation of E-cadherin expression, EMT up-regulates N-cadherin, which reduces the adhesion strengths and enhances motile and invasive capabilities [19, 20]. Likewise, upregulation of another EMT marker vimentin, as a significant regulator of cell motility, was associated with an increase in the receptor tyrosine kinase Axl which enhances breast epithelial cell metastatogenic capacity [21]. Additionally, several cell surface proteins and nonepithelial cadherin that are essential for migration and invasiveness are increased. Another study showed that the integrity of occludin and the tight junction is involved in the cancer progression when it was shown that the loss of occludin expression was significantly related to in the development of tumors [22]. The regulation of mesenchymal migration modes facilitates the formation and extension of new membrane protrusions and mesenchymal cell migration. Increased cellular protrusion and secretion of matrix metalloproteinases (MMPs) finally facilitate degradation of ECM, migration, and invasive cell behavior of cancer cells [23]. The crosstalk complexities between different downstream effectors displayed that developmental EMT is a process that in addition to involvement in cell adhesion and cytoskeleton organization, provides a range of many expression profiles of markers. Selection of these molecular markers and related activity changes in different signals increase the process of EMT.
EMT-related miRNAs
MiRNAs post-transcriptionally control tumor suppressor genes and oncogenes through targeting the complementary sequences of the 3´untranslated region (3’UTRs) of specific mRNAs, often resulting in gene silencing [24, 25]. Firstly, miRNAs are expressed as larger primary miRNAs (or pri-miR), which are processed in the nucleus by the Drosha-DGCR8 complex to liberate the precursor-miRNA (or pre-miR) structure. The pre-miRNA is subsequently transported to the cytoplasm during a process mediated via exportin-5 (EXP-5). MiRNA duplexes (approximately 22-nt) are excised from imperfectly pre-miRNA by Dicer. A single strand of miRNA is loaded in the RNA-induced silencing complex, to bind to complementary target mRNA and trigger repression of corresponding protein expression, although the underlying mechanism is not yet been fully understood [26]. Hence, alterations of balance between mRNA–miRNA interactions may result in changes in the physiological status of the tissues and initiate pathological conditions [27].
Accumulating evidence has demonstrated that dysregulation of miRNAs is involved in human disease pathogenesis. Studies have also corroborated that miRNAs are highly correlated with tumor progression including BC [28, 29].
Almost 50% of human miRNA genes are frequently located on cancer-associated genomic regions (CAGRs) or fragile sites. Numerous miRNAs are known to regulate several well-known cancer-associated genes, and act as oncogene or tumor suppressor miRNAs [24].
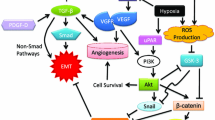
Additionally, several miRNAs have been implicated in direct regulation of the EMT process [30]. Multiple miRNAs have been identified to regulate EMT. The regulatory miRNA mechanisms are very complex and their function is extremely under the influence of cellular functions. Clearly, this heterogeneity in studies makes a comparison of outcomes difficult. Furthermore, RNA studies are still poorly understood and several obstacles were identified in the interpretation of studies analyzing. Concerning EMT, miR-200 family, and miR-205, as key regulators of EMT, are able to maintain epithelial differentiation [31].By contrast, MiR-21 involves in TGF-β-induced EMT and was one of the earliest detected cancer-promoting ‘OncomiRs’ [32]. Examples of miRNAs that impinge on the EMT-TFs regulation in another cancer cell is described in Fig. 2. Recently, various feedback loops among miRNAs and core EMT-TFs have been outlined in vivo or in vitro EMT-based studies. A study by Slabáková et al. showed that miR-34a is involved in different feedbacks including EMT regulators Snail, ZEB1, and AXL [33].
MiR-200 plays a role in modulating the epithelial to mesenchymal transition (EMT) in breast cancer (BC) by interfering with phosphoglucose isomerase/autocrine motility factor (PGI/AMF), as an activator of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). Loss of PGI/AMF expression decreases the activity of NF-κB. Moreover, the expression of PGI/AMF correlates with zinc finger E-box binding homeobox 1/2 (ZEB 1/2) activity and finally EMT
EMT-related miRNAs in BC
Breast carcinoma is a heterogeneous malignant disease, involving a diversity modification of gene expression and structure. Shahabi et al. in 2021 reported that let-7d and miR-185 suppressed EMT by targeting Rab25 expression in BC and supposed to be a new apportunity for this cancer [34]. Upregulation of miR-129-5p remarkably can enhance E-cadherin and inhibit the expression of vimentin and N-cadherin in MCF-7/doxorubicin-treated cells [35, 36]. MiR-129-5p decreased IC50 of doxorubicin, paclitaxel, and vincristine in these cells. In MCF-7 cells, miR-129-5p expression level decreased by SOX4 and EZH2, that function as an epigenetic modification-silencing gene and a master control gene of EMT, respectively [37]. ZEB1 and ZNF217, which were suppressed by miR-200c, were recognized as a transcriptional activator of TGF-β and can promote the trastuzumab sensitivity and decrease migration and invasion in BC. Reportedly, ZEB1 can suppress miR-200c, therefore, ZNF217 might contribute to miR-200c inhibition through TGF-β/ZEB1 signaling [38].
Several other reports have described that altered expression of miR-10b, -206, -335, -373, and − 520c promoted the late stages of malignant BC via impacting in tumor cell EMT, migration, invasion, angiogenesis, etc. [39]. A recent study in 2020 assessed the association of miR-93 with the EMT process and vasculogenic mimicry in triple-negative breast cancer (TNBC). Their results showed that knockdown of miR93 leads to an increase in the expression levels of Ecadherin and occludin as EMT-related genes and decreases the expression levels of vimentin and Ncadherin. Furthermore, the microtubuleforming potency of cells decreased after miR-93 knockdown [40]. Moreover, a recent study reported that up-regulation of miR-33b leads to inhibiting EMT, invasion, and metastasis through the alternation of E-cadherin, β-catenin, and vimentin expression levels [41].
Several examples of EMT-associated miRNAs will be explained in Table 1.
MiRNAs, EMT-related TFs, and signaling pathways in BC.
Many miRNAs can directly or indirectly target EMT-TFs in BC. Several examples of these regulatory networks are described below. Based on the study of Ahmad et al. [42], miR-200 members (miR-200a/b/c) could involve in the regulation of EMT in BC by interfering with phosphoglucose isomerase/autocrine motility factor (PGI/AMF), as an activator of NF-κB signaling. Loss of PGI/AMF expression reduced the activity of NF-κB signaling (about 50%) and its downstream genes MMP-2 and uPAR in MDA-MB-231 and BT549 cells. In addition, the expression of PGI/AMF correlates with ZEB1/2 activity. MiR-200 members are required to inhibit ZEB1/2 by negative regulation of PGI/AMF (Fig. 2). In BC, increased miR-103/107 levels are related to metastasis potential and EMT. They affect the in vitro directional cell migration and invasive ability via indirectly decreasing miR-200 expression and also facilitate a metastatic cascade of otherwise non-metastatic BC cell lines under in vivo conditions [43]. Another study in 2020 reported that miR-4472 promoted EMT and metastasis in malignant BC through downregulation of repulsive guidance molecule A (RGMA) as an EMT suppressor [44]. Moreover, a recent study in 2021, reported that increased miR-614 expression and repression of TAPT1 and Miro1 can regulate the EMT in BC [45]. Bo lei et al. reported an important role of miR-615-3p in the progression of EMT in BC via the TGF-B signaling pathway [46]. Based on Bracken’s group finding, an EMT network inclusive of the negative feedback loops have been identified among pro-mesenchymal TFs (driven by Snail, ZEB, Wnt, and members of the Notch family) and epithelial-enforcing miRNAs (members of the miR- 200, -203 and 34 families). These reciprocal repressions not only can reinforce the activation of the EMT program but also control the balance of epithelial versus mesenchymal phenotype of the cells [47].
In metastatic BC cells, EMT-TF TWIST could activate transcription of the miR-10b gene and induce its expression, and thereby preventing translation of its target (homeobox D10 mRNA), which was inversely related to pro-metastatic gene, RHOC [48].
On the other hand, as shown by Nairismägi et al. [49], miR-145a-5p, miR-151-5p as well as a combination of miR-145a-5p plus miR-151-5p and miR-151-5p plus miR-337-3p remarkably suppressed the translation of TWIST1. Among the different miRNA functions in cancer, identifying their multiple interactions with oncogenes and cancer signaling pathways could lead to exploring new therapeutic opportunities.
A large number of studies signified that overexpression of the epidermal growth factor receptor (EGFR) impinging on EMT and BC development [50]. Several miRNAs have been known to associate with the EGFR pathway as repressors which are mentioned in Table 1.
Crosstalk between miRNAs and microenvironmental factors
The tumor microenvironment (TME) plays a pivotal role in forming cancer or non-cancer cell phenotypes. It significantly contributes to cancer initiation, development, metastasis, therapeutic response, and clinical outcome [51]. TME comprises different types of stromal cells such as cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), leukocytes, endothelial cells, regulatory T cells (Tregs), and immune cells in addition to extracellular components including growth factors, cytokines, hormones, ECM, etc., which are within the tumor or in the surrounding of the tumor and nourished by a vascular network and the extracellular matrix [52]. Cytokines, hormones, and growth factors can transmit signals between cancer cells and the TME, which lead to the transformation through EMT and metastasis [53, 54] (Fig. 3).
Epithelial to mesenchymal transition (EMT), which can be modulated by microRNAs (miRNAs), comprises cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), leukocytes, endothelial cells, regulatory T cells (Tregs), growth factors, cytokines, hormones, extracellular matrix (ECM), etc., tumor microenvironment (TME) represents a key factor for tumor heterogeneity maintenance, tumor initiation, progression, metastasis, and therapeutic resistance, and miRNA can provide a supportive environment surrounding the tumor
Accumulating evidence has also demonstrated that a plethora of EMT-promoting microenvironmental factors consists of pro-inflammatory cytokines, hypoxia conditions, and ECM components. It has been well noticed that miRNAs can be affected by EMT-related cytokines [55]. These findings were supported by the study of Keklikoglou et al. [56], who reported that NF-kB mediated inflammation and EMT process due to reduction in miR-520/373 family. Moreover, a regulatory loop between miR-448/NF-kB was described in MCF-7 BC cells [57]. Hypoxia and/or HIF-1 orchestrates metastasis and EMT phenotype via the coordinated regulation of diverse EMT-TFs (e.g., TWIST and Snail) [58]. A study by Azimi et al. [59] reported that the existence of a reactive oxygen species (ROS)/EMT axis in which hypoxia-induced ROS mediate N-cadherin and SERPINE1 expression in BC cells. Numerous studies also exhibited that alterations of CpG are associated with dysregulation of miRNAs expression in cancers such as BC [60]. Overall, the cross-regulation among EMT-TF/miRNA and oncogenes/miRNA was briefly described. Nevertheless, despite the interaction loops between the expression pattern of miRNAs and EMT regulators, it is usually unclear whether miRNA expression alteration leads to EMT, or it is the EMT pathway that causes dysregulation of miRNA.
MiRNAs and cell architecture components in BC
Various miRNAs are described to control EMT-TFs and cellular architecture components including BC Fils-Aime et al. [61] discovered that the downregulation of tumor suppressor miR-584 is a prerequisite for the TGF-β-induce cell migration of BC cells. They recognized that TGF-β reduces the expression of miR-584, and leads to rearrangement of the actin cytoskeleton, and migration of BC. MiR-23b, the main regulator of cytoskeletal remodeling, modulates cell architecture and reinforces in vivo invasion of tumor cells during BC progression. Overexpression of miR-23b abolished EMT phenotype and increased the E-cadherin level [62].MiR-9 was also one of the upregulated miRNAs in BC tissues and cancer cells. MiR-9 has been shown to be able to inhibit E-cadherin, and thereby elevates BC cell motility and invasion capacity. Transcriptional repression of E-cadherin by miR-9, leads to the activation of the Wnt/β-catenin pathway, which can increase the level of gene encoding VEGF and angiogenesis [63]. MiR-150-5p is believed to be an oncogene miRNA and shown to be upregulated in all BC cell lines. Loss of miR‑150‑5p expression impeded the abilities of cell migration and invasion by suppressing the level of mesenchymal markers (i.e. N-cadherin, vimentin, and β‑catenin) [64]. MiR-190 is downregulated in BC clinical specimens and correlates with better survival in BC patients. It is directly controlled by ZEB1 and makes a negative feedback loop with TGF-β/SMAD2 signaling in human BC. Forced the expression of miR-190 also reversed the TGF-β‐mediated EMT progression, and BC metastasis via SMAD2 inhibition both in vitro and in vivo [65].
MiR-138 is another miRNA, that is downregulated in BC tissues compared to adjacent normal tissue. By comparing expressing miR-138 in MCF-7 and MDA-MB-231 cell lines, miR-138 has been signified to be able to increase and decrease vimentin and E-cadherin expression levels, respectively. By functioning as a feedback loop, miR-138 affects the EMT phenotype, and thereby modulates tumorigenesis, lymph node metastasis in BC cells [66]. All these findings validate the ability of miRNAs to regulate EMT in tumorigenesis, through the targeting of cell architecture components. It is worth noting that some miRNAs targeted multiple EMT/MET components in BC. For example, ectopic expression of miR-221/222 family significantly increases EMT in luminal cells by negatively targeting Notch3 and E-cadherin in luminal cells, whereas the miR-221/222 repression promoted MET in basal-like BC cells [67].
The potential value of EMT-related miRNAs in BC treatment
Functional genomics research has enabled pathway-based therapeutic strategies for cancer management. Despite significant progress in finding signaling networks in tumor cells, effective treatment choices are scarce. The discovery that a miRNA could be involved in the different cancer pathways, makes them highly promising therapeutic candidates in cancer [68].
Prior studies offered that high expression of miRs − 24 [69], -125 [70] and − 214 [71] were correlated with low survival. In contrast, the expression level of miR − 206 and − 145 [72], -155 [73] and − 375 was positively related to a better prognosis. Given the relevance of miRNAs and EMT in the development of BC, two emerging questions are how miRNAs control EMT cascade, and another important question is whether miRNA-based therapeutic approaches in BC can be reliable treatment options in oncology.
EMT-related miRNAs, and therapy resistance
Several studies in this regard have focused on the association between dysregulation expression of miRNAs in EMT regulation and treatment failure of BC. For instance, in human BC cells, epigenetic silencing of miR-200 members is related to drug-resistant phenotypes and EMT features. More precisely, miR-200-mediated upregulation of E-cadherin was directly related to repression of ZEB1 expression as well as indirectly association in the enhancement of acetylation of histone H3 at the promoter of E-cadherin in both mesenchymal MDA-MB-231 and BT-549 BC cells [74]. In another study, miR-375 re-expression has been reported to be linked with restored tamoxifen sensitivity and inhibited the EMT phenotype in TamR BC cells [75]. In addition, several miRNAs have been contributed to the cytokine-mediated BC chemoresistance due to the activation of the EMT phenotype. MiR-30c regulates drug resistance of BC by negatively regulation EMT-related cytokines including vimentin, twinfilin 1, stress fiber F-actin, interleukin-11 (IL-11), and IL-6 [76]. Moreover, in 2020 Hong Li et al. evaluate the effect of miR1297 on BC cell EMT and proliferation and its molecular mechanisms related to fatty acid 2-hydroxylase (FA2H). FA2H is a hydroxy fatty acid enzyme, which can increase the sensitivity of cancer cells to chemotherapy drugs. In this study, researchers proposed miR-1297/FA2H as a novel potential therapeutic agent [77]. As mentioned before, lots of miRNAs such as miR-137, miR-34a, miR-142-3p, miR-129, miR-205, and miR-99a play essential roles on CSCs (cancer stem cells) maintenance and so they can serve as novel tools for therapeutic purposes via modulation of breast CSCs [78]. Wang et al. in 2020 reported that miR-1976 downregulated in TNBC and promoted EMT and CSCs maintenance. They also proposed that this downregulation is associated with a low survival rate in BC patients [79]. These studies undoubtedly provide essential data about the association between the acquisition of EMT and maintenance of stem-like characteristics in BC patients. These studies undoubtedly provide essential data about the association between EMT and stem-like phenotype in BC patients. However, as reviewed in the current manuscript, the majority of studies are cell-based studies, whereas there is only limited evidence of reporting on in vivo experiments.
TGF-β suppression, miR-200c, and silencing ZEB1 or ZNF217 can inhibit metastasis and trastuzumab resistance in BC. Thus, the interaction between miR-200c/ZEB1 and miR-200c/ZNF217/TGF-β/ZEB1 can inhibit the metastasis and trastuzumab resistance of cancer cells, suggesting that EMT might be involved in the molecular induction of the malignant behaviors of breast cancers [80]. Because of the inhibitory characteristic of EMT, miR-708-3p is also recognized as a tumor-suppressor miRNA in BC. Moreover, miR-708-3p can be proposed as a proper option for overcoming chemoresistance and metastasis in BC [81]. The miR-106b-25 cluster comprises miR-25, miR-93, and miR-106b, which target a transcriptional stimulator of E-cadherin such as EP300 and up-regulated in doxorubicin-resistant. With this cluster, a single miRNA upregulation may lead to that target cells achieving the EMT phenotype, besides proliferative capability in doxorubicin therapy [82]. It seems EMT may be significantly associated with poor prognosis and enhanced chemoresistance in BC, whereas miRNAs can modulate EMT. Therefore, miRNAs can be suggested as a useful therapeutic approach for overcoming chemoresistance in BC.
Challenge and limitation
Suppression of EMT is a crucial therapeutic strategy, which may have a serious effect on disease outcomes. As reviewed by Malek et al. [83], there are 3 different strategies to inhibit the EMT process via (a) EMT extracellular inducers, (b) EMT-related TFs, and (c) the EMT downstream effectorsDifferent studies have noted that miRNAs have an established role in regulating EMT through directly or indirectly targeting families of EMT-TFs or affecting the integrity of the epithelial architecture during EMT development. Other studies have also stated a close relation between dysregulated miRNA and cancer progression. Therapeutic strategies include up-regulation or downregulation of specific miRNAs since dysregulation of miRNA expression has been observed in several cancers. MiRNA-based therapy approach effectively employs synthetic antisense oligonucleotides (also known as anti-miRNAs) to block OncomiR, or synthetic miRNA mimics to reactivate tumor-suppressive miRNAs. However, due to major challenges like off-target multiple gene regulation, obstacles of in vivo miRNA delivery, and pharmacokinetics, clinical research on miRNA-based therapeutic strategies for cancer patients is still in the early stage. EMT suppressing or reversing can effectively cause a significant adverse event in favor of MET as well tumor colonization at metastatic sites through circulating cancer cells [84]. Additionally, molecular heterogeneity of cancer and plasticity of EMT phenotype indicates the need for a multimodal treatment strategy that is not only focused on one EMT-target gene. For instance, the combination of chemotherapy and anti-miRs and or miRs replacement strategies were widely studied both in-vivo and in-vitro experiments. Roscigno et al. have displayed that inhibition of miR-24 using transfection of an anti-miR-24 oligonucleotide, might be a potentially effective approach for combating resistance to cisplatin and survival in BC cells that harbored EMT phenotype [69]. Furthermore, Plantamura et al. reported that miR-205 expression level is significantly correlated with aggressiveness of BC and it is decreasing from a less aggressive subtype to a more aggressive one. They also propose that this affects drug response and patient survival [85]. Overall, further research about EMT-regulating miRNAs in various cancer models is needed to more translate present knowledge to the clinical context.
Future directions
Currently, cancer treatments based on miRNAs have attracted more attention because in this approach the genes, which are not been manipulated previously, can be targeted. Moreover, miRNAs are remarkably flexible for regulating a wide range of genes. However, due to challenges in the stability and delivery of miRNAs, a longer time is required for getting better results. Development in biological science, biochemistry, and nanotechnology can develop the available knowledge on miRNA-based therapies. A combination of miRNAs and alongside medicines such as small molecules or antibodies may provide superior outcomes for cancer patients. Using novel biotechnology techniques for working on miRNAs, scientists may present promising approaches for RNA-based therapeutics to overcome cancer.
Conclusions
EMT can enhance the migratory potential and invasiveness of BC cells, however, the interpretation of regulatory networks in which miRNAs control the EMT process is still not fully understood. Moreover, the function of many miRNAs and pathways is still unknown. Although the miRNA-mediated EMT process has been known as a promising target to treat cancer, they still have many issues hindering their reliability of the clinical application. Conversely, a better understanding of regulatory mechanisms of miRNAs, new therapeutic approaches targeting miRNAs must be identified, which will establish a more effective means for treating BC patients particularly in combination with chemo or radiation therapies.
Availability of data and material:
Not applicable.
Code Availability
Not applicable.
References
Lakhani SR, Ellis IO, Schnitt S, Tan PH, van de Vijver M (2012)WHO Classification of Tumours of the Breast
Lee S-y, Seo JH (2018) Current strategies of endocrine therapy in elderly patients with breast cancer. BioMed research international 2018
Paget S (1889) The distribution of secondary growths in cancer of the breast. The Lancet 133:571–573
Elisha Y, Kalchenko V, Kuznetsov Y, Geiger B (2018) Dual role of E-cadherin in the regulation of invasive collective migration of mammary carcinoma cells. Sci Rep 8:4986
Wang J-y, Chen L-j (2019) The role of miRNAs in the invasion and metastasis of cervical cancer. Biosci Rep 39:BSR20181377
Zaravinos A (2015) The regulatory role of microRNAs in EMT and cancer. Journal of oncology 2015
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Investig 119:1420–1428
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 15:178
Grände M, Franzen Ã, Karlsson J-O, Ericson LE, Heldin N-E, Nilsson M (2002) Transforming growth factor-β and epidermal growth factor synergistically stimulate epithelial to mesenchymal transition (EMT) through a MEK-dependent mechanism in primary cultured pig thyrocytes. J Cell Sci 115:4227–4236
Lee JM, Dedhar S, Kalluri R, Thompson EW (2006) The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell biol 172:973–981
Acevedo VD, Gangula RD, Freeman KW et al (2007) Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer Cell 12:559–571
Jena MK, Janjanam J (2018) Role of extracellular matrix in breast cancer development: a brief update. F1000Res. 7: 274
Gonzalez DM, Medici D (2014) Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 23:7(344)
Lu W, Kang Y (2019) Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell 49:361–374
Pećina-Šlaus N (2003) Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int 3:17
Guarino M, Rubino B, Ballabio G (2007) The role of epithelial-mesenchymal transition in cancer pathology. Pathology 39:305–318
Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S (1995) Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proceedings of the National Academy of Sciences 92: 7416–7419
Sousa B, Pereira J, Paredes J (2019) The Crosstalk Between Cell Adhesion and Cancer Metabolism. International journal of molecular sciences 20: 1933
Serrano-Gomez SJ, Maziveyi M, Alahari SK (2016) Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer 15:18
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. cell 139: 871–890
Lian N, Wang W, Li L, Elefteriou F, Yang X (2009) Vimentin inhibits ATF4-mediated osteocalcin transcription and osteoblast differentiation. J Biol Chem 284:30518–30525
Kaufhold S, Bonavida B (2014) Central role of Snail1 in the regulation of EMT and resistance in cancer: a target for therapeutic intervention. J Experimental Clin Cancer Res 33:62
Leong HS, Robertson AE, Stoletov K et al (2014) Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep 8:1558–1570
Shahabi A, Naghili B, Ansarin K, Zarghami N (2019) The relationship between microRNAs and Rab family GTPases in human cancers. J Cell Physiol 234:12341–12352
Ha M, Kim VN (2014) Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15:509
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Paul P, Chakraborty A, Sarkar D et al (2018) Interplay between miRNAs and human diseases. J Cell Physiol 233:2007–2018
Tan W, Liu B, Qu S, Liang G, Luo W, Gong C (2018) MicroRNAs and cancer: Key paradigms in molecular therapy. Oncol Lett 15:2735–2742
Lo P-K, Wolfson B, Zhou X, Duru N, Gernapudi R, Zhou Q (2015) Noncoding RNAs in breast cancer. Brief Funct Genomics 15:200–221
Bullock MD, Sayan AE, Packham GK, Mirnezami AH (2012) MicroRNAs: critical regulators of epithelial to mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in cancer progression. Biol Cell 104:3–12
Gregory PA, Bert AG, Paterson EL et al (2008) The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10:593
Davis BN, Hilyard AC, Lagna G, Hata A (2008) SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454:56
Slabáková E, Culig Z, Remšík J, Souček K (2017) Alternative mechanisms of miR-34a regulation in cancer. Cell Death Dis 8:e3100
Shahabi A, Naghili B, Ansarin K, Montazeri M, Dadashpour M, Zarghami N (2021) Let-7d and miR-185 Impede Epithelial-Mesenchymal Transition by Downregulating Rab25 in Breast Cancer. Asian Pac J Cancer Prevention: APJCP 22:305
Mallini P (2015) Epithelial-to-mesenchymal transition: What is the impact on breast cancer stem cells and drug resistance. Cancer Treat Rev 40(3):341–8
Saxena M, Stephens MA, Pathak H, Rangarajan A (2011) Transcription factors that mediate epithelial–mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis 2:e179–e179
Luan Q, Zhang B, Li X, Guo M (2016) MiR-129-5p is downregulated in breast cancer cells partly due to promoter H3K27m3 modification and regulates epithelial-mesenchymal transition and multi-drug resistance. Eur Rev Med Pharmacol Sci 20:4257–4265
Burk U, Schubert J, Wellner U et al (2008) A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 9:582–589
Tang J, Ahmad A, Sarkar FH (2012) The role of microRNAs in breast cancer migration, invasion and metastasis. Int J Mol Sci 13:13414–13437
An G, Lu F, Huang S et al (2020) Effects of miR–93 on epithelial–to–mesenchymal transition and vasculogenic mimicry in triple–negative breast cancer cells. Mol Med Rep 23:1–1
Pattanayak B, Garrido-Cano I, Adam-Artigues A et al (2020) MicroRNA-33b Suppresses Epithelial–Mesenchymal Transition Repressing the MYC–EZH2 Pathway in HER2 + Breast Carcinoma. Front Oncol 10:1661
Ahmad A, Aboukameel A, Kong D et al (2011) Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res 71:3400–3409
Martello G, Rosato A, Ferrari F et al (2010) A MicroRNA targeting dicer for metastasis control. Cell 141:1195–1207
Li Y, Wang Y-W, Chen X et al (2020) MicroRNA-4472 promotes tumor proliferation and aggressiveness in breast cancer by targeting RGMA and inducing EMT. Clin Breast Cancer 20:e113–e126
Dang TT, McIntosh AT, Morales JC, Pearson GW (2021) miR614 Expression Enhances Breast Cancer Cell Motility. Int J Mol Sci 22:112
Lei B, Wang D, Zhang M, Deng Y, Jiang H, Li Y (2020) miR-615-3p promotes the epithelial-mesenchymal transition and metastasis of breast cancer by targeting PICK1/TGFBRI axis. J Experimental Clin Cancer Res 39:1–14
Bracken CP, Khew-Goodall Y, Goodall GJ (2015) Network-based approaches to understand the roles of miR-200 and other microRNAs in cancer. Cancer Res 75:2594–2599
Ma L, Teruya-Feldstein J, Weinberg RA (2007) Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449:682
Nairismägi M-L, Füchtbauer A, Labouriau R, Bramsen JB, Füchtbauer, E-M (2013) The proto-oncogene TWIST1 is regulated by microRNAs. PLoS ONE 8:e66070
Ali R, Wendt MK (2017) The paradoxical functions of EGFR during breast cancer progression. Signal Transduct Target therapy 2:16042
Wu T, Dai Y (2017) Tumor microenvironment and therapeutic response. Cancer Lett 387:61–68
Li I, Nabet BY (2019) Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer 18:1–10
Hinshaw DC, Shevde LA (2019) The tumor microenvironment innately modulates cancer progression. Cancer Res 79:4557–4566
Yekula A, Yekula A, Muralidharan K, Kang K, Carter BS, Balaj L (2020) Extracellular vesicles in glioblastoma tumor microenvironment. Front Immunol 10:3137
Bhaumik D, Patil CK, Campisi J (2009) MicroRNAs: an important player in maintaining a balance between inflammation and tumor suppression. Oncogene 18:27(42):5643–7
Keklikoglou I, Koerner C, Schmidt C et al (2012) MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-κB and TGF-β signaling pathways. Oncogene 31:4150
Li Q, Chen Z, Cao X et al (2011) Involvement of NF-κB/miR-448 regulatory feedback loop in chemotherapy-induced epithelial–mesenchymal transition of breast cancer cells. Cell Death Differ 18:16
Lundgren K, Nordenskjöld B, Landberg G (2009) Hypoxia, Snail and incomplete epithelial–mesenchymal transition in breast cancer. Br J Cancer 101:1769
Azimi I, Petersen RM, Thompson EW, Roberts-Thomson SJ, Monteith GR (2017) Hypoxia-induced reactive oxygen species mediate N-cadherin and SERPINE1 expression, EGFR signalling and motility in MDA-MB-468 breast cancer cells. Sci Rep 7:15140
Oltra SS, Peña-Chilet M, Vidal-Tomas V et al (2018) Methylation deregulation of miRNA promoters identifies miR124-2 as a survival biomarker in Breast Cancer in very young women. Sci Rep 8:14373
Fils-Aimé N, Dai M, Guo J et al (2013) MicroRNA-584 and the protein phosphatase and actin regulator 1 (PHACTR1), a new signaling route through which transforming growth factor-β mediates the migration and actin dynamics of breast cancer cells. J Biol Chem 288:11807–11823
Pellegrino L, Krell J, Roca-Alonso L, Stebbing J, Castellano L (2013) MicroRNA-23b regulates cellular architecture and impairs motogenic and invasive phenotypes during cancer progression. Bioarchitecture 3:119–124
Ma L, Young J, Prabhala H et al (2010) miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 12:247
Lu Q, Guo Z, Qian H (2019) Role of microRNA–150–5p/SRCIN1 axis in the progression of breast cancer. Experimental and therapeutic medicine 17:2221–2229
Yu Y, Luo W, Yang Z-J et al (2018) miR-190 suppresses breast cancer metastasis by regulation of TGF-β-induced epithelial–mesenchymal transition. Mol Cancer 17:70
Zhang J, Liu D, Feng Z et al (2016) MicroRNA-138 modulates metastasis and EMT in breast cancer cells by targeting vimentin. Biomed Pharmacother 77:135–141
Liang Y-K, Lin H-Y, Dou X-W et al (2018) MiR-221/222 promote epithelial-mesenchymal transition by targeting Notch3 in breast cancer cell lines. NPJ breast cancer 4:20
Si W, Shen J, Zheng H, Fan W (2019) The role and mechanisms of action of microRNAs in cancer drug resistance. Clin epigenetics 11:25
Roscigno G, Puoti I, Giordano I et al (2017) MiR-24 induces chemotherapy resistance and hypoxic advantage in breast cancer. Oncotarget 8:19507
Zhou M, Liu Z, Zhao Y et al (2010) MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J Biol Chem 285:21496–21507
Kalniete D, Nakazawa-Miklaševiča M, Štrumfa I et al (2015) High expression of miR-214 is associated with a worse disease-specific survival of the triple-negative breast cancer patients. Hereditary cancer in clinical practice 13:7
Quan Y, Huang X, Quan X (2018) Expression of miRNA–206 and miRNA–145 in breast cancer and correlation with prognosis. Oncol Lett 16:6638–6642
Mattiske S, Suetani RJ, Neilsen PM, Callen DF (2012) The oncogenic role of miR-155 in breast cancer. Cancer Epidemiol Prev Biomarkers 21:1236–1243
Tryndyak VP, Beland FA, Pogribny IP (2010) E-cadherin transcriptional down‐regulation by epigenetic and microRNA‐200 family alterations is related to mesenchymal and drug‐resistant phenotypes in human breast cancer cells. Int J Cancer 126:2575–2583
Ward A, Balwierz A, Zhang JD et al (2013) Re-expression of microRNA-375 reverses both tamoxifen resistance and accompanying EMT-like properties in breast cancer. Oncogene 32:1173
Hu W, Tan C, He Y, Zhang G, Xu Y, Tang J (2018) Functional miRNAs in breast cancer drug resistance. OncoTargets and therapy 11:1529
Li H, Lian B, Liu Y, Chai D, Li J (2020) MicroRNA–1297 downregulation inhibits breast cancer cell epithelial–mesenchymal transition and proliferation in a FA2H–dependent manner. Oncol Lett 20:1–1
Niu T, Zhang W, Xiao W (2021) MicroRNA regulation of cancer stem cells in the pathogenesis of breast cancer. Cancer Cell Int 21:1–14
Wang J, Li M, Han X et al (2020) MiR-1976 knockdown promotes epithelial–mesenchymal transition and cancer stem cell properties inducing triple-negative breast cancer metastasis. Cell Death Dis 11:1–12
Bai WD, Ye XM, Zhang MY et al (2014) MiR-200c suppresses TGF‐β signaling and counteracts trastuzumab resistance and metastasis by targeting ZNF217 and ZEB1 in breast cancer. Int J Cancer 135:1356–1368
Lee JW, Guan W, Han S, Hong DK, Kim LS, Kim H (2018) Micro RNA-708‐3p mediates metastasis and chemoresistance through inhibition of epithelial‐to‐mesenchymal transition in breast cancer. Cancer Sci 109:1404–1413
Zhou Y, Hu Y, Yang M et al (2014) The miR-106b∼ 25 cluster promotes bypass of doxorubicin-induced senescence and increase in motility and invasion by targeting the E-cadherin transcriptional activator EP300. Cell Death & Differentiation 21:462–474
Malek R, Wang H, Taparra K, Tran PT (2017) Therapeutic targeting of epithelial plasticity programs: focus on the epithelial-mesenchymal transition. Cells Tissues Organs 203:114–127
Ocaña OH, Córcoles R, Fabra Á et al (2012) Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 22:709–724
Plantamura I, Cataldo A, Cosentino G, Iorio MV (2021) miR-205 in Breast Cancer: State of the Art. Int J Mol Sci 22:27
Gwak JM, Kim HJ, Kim EJ et al (2014) MicroRNA-9 is associated with epithelial-mesenchymal transition, breast cancer stem cell phenotype, and tumor progression in breast cancer. Breast Cancer Res Treat 147:39–49
Ma L (2010) Role of miR-10b in breast cancer metastasis. Breast Cancer Res 12:210
Patel N, Garikapati KR, Makani VKK et al (2018) Regulating BMI1 expression via miRNAs promote Mesenchymal to Epithelial Transition (MET) and sensitizes breast cancer cell to chemotherapeutic drug. PLoS ONE 13:e0190245
Cheng C-W, Wang H-W, Chang C-W et al (2012) MicroRNA-30a inhibits cell migration and invasion by downregulating vimentin expression and is a potential prognostic marker in breast cancer. Breast Cancer Res Treat 134:1081–1093
Kim NH, Kim HS, Li X-Y et al (2011) A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial–mesenchymal transition. J Cell biol 195:417–433
Siemens H, Jackstadt R, Hünten S et al (2011) miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle 10:4256–4271
Yu Y, Zhao Y, Sun X-H et al (2015) Down-regulation of miR-129-5p via the Twist1-Snail feedback loop stimulates the epithelial-mesenchymal transition and is associated with poor prognosis in breast cancer. Oncotarget 6:34423
Jiang D, Zhou B, Xiong Y, Cai H (2019) miR-135 regulated breast cancer proliferation and epithelial-mesenchymal transition acts by the Wnt/β-catenin signaling pathway. Int J Mol Med 43:1623–1634
Yan M, Li X, Tong D et al (2016) miR-136 suppresses tumor invasion and metastasis by targeting RASAL2 in triple-negative breast cancer. Oncol Rep 36:65–71
Li W, Zhai L, Zhao C, Lv S (2015) miR-153 inhibits epithelial–mesenchymal transition by targeting metadherin in human breast cancer. Breast Cancer Res Treat 150:501–509
Yu J, Xie F, Bao X, Chen W, Xu Q (2014) miR-300 inhibits epithelial to mesenchymal transition and metastasis by targeting Twist in human epithelial cancer. Mol Cancer 13:121
Drasin DJ, Guarnieri AL, Neelakantan D et al (2015) TWIST1-induced miR-424 reversibly drives mesenchymal programming while inhibiting tumor initiation. Cancer Res 75:1908–1921
Wu Z, Li X, Cai X, Huang C, Zheng M (2016) miR-497 inhibits epithelial mesenchymal transition in breast carcinoma by targeting Slug. Tumor Biology 37:7939–7950
Gao J, Yu SR, Yuan Y et al (2019) MicroRNA-590‐5p functions as a tumor suppressor in breast cancer conferring inhibitory effects on cell migration, invasion, and epithelial–mesenchymal transition by downregulating the Wnt–β‐catenin signaling pathway. J Cell Physiol 234:1827–1841
Li L-Z, Zhang CZ, Liu L-L et al (2013) miR-720 inhibits tumor invasion and migration in breast cancer by targeting TWIST1. Carcinogenesis 35:469–478
Acknowledgements
None.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
All authors contributed equally in designing the project, literature searching, drafting, and critically revising the manuscript.
Corresponding author
Ethics declarations
Funding
No funds, grants, or other support was received.
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics approval:
Not applicable.
Consent to participate:
Not applicable.
Consent for publication:
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Javdani, H., Mollaei, H., Karimi, F. et al. Review article epithelial to mesenchymal transition‑associated microRNAs in breast cancer. Mol Biol Rep 49, 9963–9973 (2022). https://doi.org/10.1007/s11033-022-07553-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07553-4