Abstract
Mounting evidences have shown that nicotinamide adenine dinucleotide phosphate oxidase-2 (Nox-2) pathway modifies glutamic-acid decarboxylase-67 (GAD67) (GABAergic enzyme) and cholinergic systems via oxidative-nitrergic mechanisms in schizophrenia pathology. Rutin, a neuroactive antioxidant compound, with proven neuroprotective property has been shown to reduce schizophrenic-like behavior in mice. This study sought to investigate the mechanisms of action of the psychopharmacological activity of rutin in the preventive and reversal effects of ketamine-induced schizophrenic-like behavior, oxidative-nitrergic stress, cholinergic and GABAergic derangements in mice. In the preventive treatment, male mice were given rutin (0.1, 0.2 and 0.4 mg/kg) or risperidone (0.5 mg/kg) orally for 14 days prior to ketamine (20 mg/kg, i.p.) treatment from the 8 to 14th day. However, in the reversal treatment, ketamine was given for 14 days prior to rutin and risperidone. Behavioral (open-field, social-interaction and Y-maze tests), biochemical (oxidative/nitrergic stress markers, acetylcholinesterase activity), immunohistochemical (GAD67, Nox-2) and neuronal cell deaths in the striatum, prefrontal cortex, and hippocampus were evaluated. Ketamine-induced behavioral impairments were prevented and reversed by rutin. Exposure of mice to ketamine increased malondialdehyde, nitrite contents, acetylcholinesterase activity, neuronal cell death and Nox-2 expressions in the striatum, prefrontal cortex and hippocampus. Conversely, these derangements were prevented and reversed by rutin. The decreased glutathione levels due to ketamine were marked increased by rutin. Rutin only prevented ketamine-induced decrease in GAD67 expression in the striatal-hippocampal region. Altogether, the study showed that the prevention and reversal treatments of mice with rutin attenuated ketamine-induced schizophrenic-like behaviors via reduction of Nox-2 expression, oxidative/nitrergic stresses, acetylcholinesterase activity, and increased GAD67 enzyme.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is a major psychiatric disease that affects approximately 1% of the world population [1, 2], and characterized by psychotic symptoms such as delusions, hallucinations, social withdrawal, and cognitive impairments [3]. Although the pathogenesis of schizophrenia remains elusive and multifaceted, the dysregulation of neurochemicals such as dopamine, serotonin and glutamate are well established [4,5,6].
Accumulating evidence from clinical, genetic and epidemiologic studies supports the neurodevelopmental origin of schizophrenia and identified GABA-related abnormalities in the disease process [7, 8]. Hyperactivity of dopaminergic and glutamatergic systems have been partly attributed to decreased GABAergic neurotransmissions in cortical and subcortical brains regions [5, 7, 8]. Anatomical and functional studies have reported the role of prefrontal-cortical and hippocampal GABAergic systems in the regulation of excitatory neurotransmissions via strong interconnectivity with subcortical brain regions, such as striatum [9]. Moreso, hypofunctional glutamate system and/or a deficit of GABA are known to disrupt the excitatory balance of the striatum, resulting in the dopaminergic hyperactivity. Thus, dysregulation of cortical and subcortical GABAergic systems serves as a prominent marker in both human and rodents in neuropsychiatric disorder like schizophrenia [5]. Indeed, decreased glutamic acid decarboxylase isoform-67 (GAD67), the rate-limiting enzyme for GABA synthesis correlates with altered GABAergic neurotransmission and behavioral hyperactivity that typifies schizophrenic disorder [7]. Consistent with this hypothesis, postmortem investigations and animal studies have repeatedly found reduced levels of mRNA GAD67 and GABA plasma membrane transporter 1 (GAT-1) in the striatum, prefrontal cortex and hippocampus of schizophrenic patients [7]. Also, decreased GAD67 enzyme activity is believed to contribute significantly to reduced gamma band frequencies in the medial part of the prefrontal cortex, and cornu ammonis (CA1) 1 and 3 of the hippocampus, as well as impaired dorsolateral prefrontal cortical- and hippocampal-dependent cognitive performance seen in schizophrenic patients [7, 8, 10].
Similarly, the roles of oxidative, nitrergic aberrations and inflammation have been widely reported in schizophrenic patients [5, 11]. Indeed, previous studies have shown that repeated exposure to ketamine, a popular NMDA receptor antagonist, to adult rodents induces oxidative stress through a depolarization induced production of cytokines and up-regulation of the superoxide producing enzyme, NADPH oxidase-2 (Nox-2) [5, 6, 12, 13]. Accumulating evidence have also shown that up-regulation of Nox-2 combined with oxidative stress causes degeneration of GABA-related proteins such as GAD67 [13] via mechanisms related to decreased neurotrophic factors [10]. Thus, down-regulation of Nox-2 pathway and oxidative stress with enhancement of GABAergic system-dependent GAD67 activity by neuroprotective compounds is regarded as a viable strategy to improving schizophrenic conditions [10]. Moreover, adjunctive use of benzodiazepine-like drugs is now being reported to improve the subfields of cortical brain areas of GABAergic system and significant beneficial effects have been demonstrated in schizophrenia conditions [10, 14]. Hence, drugs such benzodiazepines and GABAergic agonists which increase cortical and subcortical GABAergic transmissions are currently being sought to mitigate glutamatergic-mediated behavioral hyperactivity and downstream Nox-2-induced oxidative stress [15].
Rutin (3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside), is a citrus flavonoid glycoside that is widespread in many fruits and vegetables such as ruta graveolens, passion flower, buckwheat, orange, lemon and apple [16]. Previous studies have shown that rutin exhibits strong anti-oxidant, anti-inflammatory [17], anti-diabetic [18], anti-cancer [19], and anti-microbial [20] activities. Importantly, rutin has also demonstrated neuroprotective activity in neurological diseases characterized of oxidative stress, neuroinflammation and neurochemical defects [17]. Rutin attenuated streptozotocin-induced hippocampal damage by down-regulating interleukin-8, cyclooxygenase-2, inducible nitric oxide synthase and nuclear factor-kappa-B [17]. In addition, rutin demonstrates central nervous depressant activity [21], anti-convulsant [22] and anxiolytic [23] effects, via GABAA-mediated enhancement of GABAergic activity in rats cerebral cortical synaptosomal membranes [24]. In addition, previous studies have reported that rutin exerted anti-psychotic-like effect [25], and also prevented haloperidol-induced tardive dyskinesia and neurochemical derangements [26]. These findings raises the exciting possibility that rutin could be a beneficial compound as an effective and safer neuroleptic compound. In this light, we sought to investigate the effects of rutin on ketamine-induced schizophrenic-like behavior, oxidative stress, cholinergic deficit and GAD67-dependent GABAergic alterations in the preventive and reversal protocols in mice.
Materials and methods
Laboratory animals
Six weeks old Adult male Swiss mice weighing 20–25 g were handled and maintained four per plastic cage (42 × 30 × 27 cm) in standard laboratory conditions comprising of a 12-h light/dark cycles and 24 ± 2 °C temperature. They had free access to pelleted feed (Livestock Feeds, Ikeja) and water. The experimental procedures adopted were in compliance with the ethical approval obtained from Health Research Ethics Committee of the College of Medicine, University of Lagos, Nigeria (CMUL/HREC/01/19/481) which is in line with the United States National Institutes of Health Guidelines for Care and Use of Laboratory Animals in Biomedical Research.
Drug and treatments
Rutin (RUT) and risperidone (RIS) were purchased from Sigma-Aldrich, St. Louis, MO, USA and dissolved in saline before and administered per os (p.o.). Ketamine hydrochloride (KET) purchased from Rotex Medica, Germany was diluted with saline and given intraperitoneally (i.p.). The doses of KET (20 mg/kg, i.p.) [10], RUT (0.1, 0.2 and 0.4 mg/kg, p.o.) [25], RIS (0.5 mg/kg, p.o.) and vehicle control group (saline) (SAL, 10 mL/kg, p.o.) [10] used in this study were chosen based on previous studies and preliminary findings in our laboratory.
Experimental design
The preventive and reversal effects of RUT on KET-induced schizophrenia-like behaviors and neurochemical damages were assessed as previously described [6, 10] (Scheme 1). The experimental design consists of two set of experiments and forty two (42) male mice were used in each experiment. In the first experiment, the preventive study, mice were randomized into 6 experimental groups (n = 10/group). Groups 1 and 2 received saline (10 mL/kg, p.o.), and served as normal control and negative control respectively. Groups 3–5 received RUT (0.1, 0.2 and 0.4 mg/kg, p.o.), while group 6 was administered RIS (0.5 mg/kg, p.o.) and served as positive control respectively, for 14 days. Between the 8th and 14th day of treatment, the animals in groups 2–6 additionally received a daily dose of 20 mg/kg, i.p. of KET or vehicle 30 min after RUT or RIS administrations respectively. In the second experiment (reversal protocol) (n = 10/group), KET (20 mg/kg, i.p.) or vehicle (10 mL/kg, p.o.) were given for 14 days. However, from the 8 to 14th day of treatment, group 2 was treated with vehicle (10 mL/kg, p.o.) and served as negative control, whereas groups 3–5 were treated with RUT (0.1, 0.2 and 0.4 mg/kg, p.o.) and group 6 received RIS (0.5 mg/kg, p.o.) additionally once daily with a 30 min interval between treatments (Scheme 1). Mice brains of animals treated with the three doses of rutin (0.1–0.4 mg/kg) were used for spectrophotometric biochemical (glutathione, malondialdehyde, acetylcholinesterase) and histomorphometric assays, while the most active dose of rutin (0.4 mg/kg) was used for the immunohistochemistry for GAD67 and Nox-2 assays.
Behavioral tests
Twenty four hours after the last treatment on the 14th day, behavioral evaluations were carried out according to previous protocols [5, 10]. These consist of hyperlocomotion test in the open-field test, memory performance in the Y-maze test, and social interaction test which represents social withdrawal. All behavioral tests listed in this order were assessed between 8:00 a.m. and 12:00 noon, by three trained observers who were unaware of treatment groups.
Open-field test
Prevention and reversal of RUT on KET-induced hyperlocomotion in mice was evaluated in an open field chamber (35 × 30 × 23 cm) with visible 16 squared lines. Hyperlocomotion based on number of line crossing activity was counted for a period of 5 min [27].
Y-maze test
The effect of RUT on KET-induced spatial memory impairment was evaluated based on spontaneous alternation behavior (SAB) in the Y-maze test (YMT), which has three identical arms (A, B and C), measuring 33 × 11 × 12 cm to which each arm are separated at 120°. Correct SAB (ABC, CAB or BCA) but not BAB was defined as entries into all three arms on sequential visitations for 5 min. Thereafter, the percentage of alternation was calculated as total alternation number/(total number of entries-2) × 100 [27].
Social interaction test (SIT)
Ketamine-induced social withdrawal was evaluated in the social interaction chamber, which included a Plexiglas box (60 × 40 cm) that is divided into three compartments (A, B and C) with a small opening (6 × 6 cm) in the dividers. A probe mouse placed in an iron restraining cage was fixed in one arm of the two side chambers (A) while the other chamber (C) was without a probe mouse in its restraining, to enable the assessment of the study of social and non-social activity. However, test mouse was placed in chamber (B, center chamber) and allowed to explore all chambers for a period of 5 min. Thereafter, an unfamiliar, same-sex probe mouse from the same experimental group was introduced in the restraining cage in chamber A, while chamber C was without mice. Test mouse was allowed to interact with all chambers. Social preference was taken as percentage of time spent in the social chamber minus the percentage time spent in the opposite chamber) [27].
Preparation of brain tissues for biochemical assays
Immediately after the behavioral tests, seven animals in the respective groups were decapitated and the brains were immediately removed. Afterward, each mouse brain was weighed, homogenized with 5 mL of 10% w/v phosphate buffer (0.1 M, pH 7.4) and each brain tissue homogenate was centrifuged at 10,000 rpm at 4 °C for 10 min. Thereafter, the homogenates were immediately separated into valves for the different biochemical assays and the pellets were discarded.
Evaluation of regional brain concentrations of glutathione (GSH) and malondialdehyde (MDA)
Regional brain concentration of glutathione was determined by the assay method described by Jollow et al. [28], as indicated by the formation of a stable yellow color when 5′,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB) is combined with sulfhydryl compounds. The concentration of reduced GSH in the striatum, prefrontal cortex and hippocampus were expressed as nanomoles per milligram protein (nmol/mg protein). In another experiment, the MDA generation in the form of thiobarbituric acid reacting substances in the striatum, prefrontal cortex and hippocampus were assayed according to the method previously described [29]. The regional brain concentrations of MDA were expressed as TBARS (nmol MDA/mg protein).
Determination of acetylcholinesterase activity in mice brain
Acetylcholinesterase (AChE) enzyme activity, a marker for cholinergic neurotransmission for cognitive effect of rutin was evaluated according to the method previously described [30]. Acetylcholinesterase enzyme activity of each treatment groups were estimated in the homogenates of the striatum, prefrontal cortex and hippocampus. The rate of acetylcholinesterase activity was calculated and expressed as µmol/min/mg tissue.
Estimation of regional brain protein contents
Protein content was assayed as earlier described [27] using bovine serum albumin (1 mg/mL) (standard reference).
Histopathological and histomorphometric evaluations of the effect of RUT on the striatum, prefrontal cortex and hippocampus of mice treated with ketamine
After the behavioral test, three animals were anaesthetized from each group, transcardially perfused cold normal saline and sodium phosphate buffered formalin. After perfusion, the brains were post-fixed in 4% paraformaldehyde in 100 mmol/L phosphate buffer for 48 h and then transferred to a cold sucrose solution (15%) in 0.1 M PBS containing 0.1% sodium azide at 4 °C. Section (20 µm thick) of the striatum, prefrontal cortex and hippocampus were done using a cryostat and collected in 100 mmol/L PBS containing 0.3% Triton X-100 (PBS-T). Previously described hematoxylin and eosin staining technique [31] was used. The viable neuronal cells of the striatum, prefrontal cortex and hippocampus were counted using Image J software (NIH, Bethesda, MD, USA) [31].
Determination of glutamic acid decarboxylase-67 (GAD67) and nicotiamide dinucleotide phosphate oxidase-2 (Nox-2) immnopositive cell expressions
The brain slices of selected regions of the brain (striatum, prefrontal cortex and hippocampus) expressions of the immunopositive proteins of GAD67 and Nox-2 as previously described by Ben-Azu et al. [10]. Briefly, the tissues slides for the different brain regions were incubated with the GAD67 and Nox-2 primary antibodies (1:300) for 20–30 min at 25 °C respectively. Additional incubation of tissue slides were carried with one-step horseradish peroxidase (HRP) polymer for about 20–30 min, and rinsed with PBS 4–6 consecutive times. Droplets of freshly prepared 3, 3′-diaminobenzidine (DAB) reagents were dropped on each slide and incubated for a period 6–10 min at room temperature which was immediately followed by washing with PBS 7–9 times. The slides were counter stained with hematoxylin for 30–60 s, rinsed with normal saline and dried. The photomicrograph of stained slides of each brain regions were viewed and obtained with Leica ICC50 E Digital Camera (Germany) connected to a computer interface (Magnafire) and an Olympus BX-51 Binocular research microscope. Immunoreactive cells were defined and analyzed with the aid of Image J software (NIH, Bethesda, MD, USA) [10].
Statistical analysis
Data were expressed as Mean ± S.E.M. (standard error of mean). After testing for normality distribution, behavioral data were analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc test for multiple comparisons where appropriate. Biochemical and neuronal densitometric data were analyzed in the striatum, prefrontal cortex and hippocampus using two-way ANOVA followed by Bonferroni post-hoc test. Data analyses were performed using GraphPad Prism software version 5 (GraphPad Software, Inc. La Jolla, CA 92,037 USA). A level of p ≤ 0.05 was considered as statistically significant for all analysis.
Results
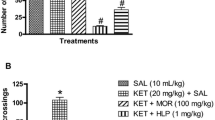
Rutin attenuates ketamine-induced hyperlocomotion
The effects of RUT on KET-induced hyperlocomotion in the preventive and reversal treatments are shown in Fig. 1a, b. One-way ANOVA and post hoc analysis with Bonferroni test showed that intraperitoneal injection of KET (20 mg/kg, i.p.) significantly (p < 0.05) increased the number of line crossings in the OFT in the preventive (Fig. 1a) and reversal (Fig. 1b) protocols when compared with vehicle-treated groups. However, RUT (0.2 and 0.4 mg/kg, p.o.) and RIS (0.5 mg/kg, p.o.) significantly (p < 0.05) attenuated KET-induced hyperlocomotion in the preventive [F (5, 36) = 19.76, p < 0.0001] and reversal [F (5, 36) = 14.44, p < 0.0001] treatments relative to KET-treated mice. But RUT (0.1 mg/kg, p.o.) did not prevent or reverse KET-induced hyperlocomotion when compared with KET-treated mice (Fig. 1a, b).
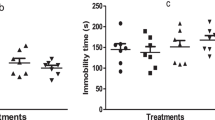
Rutin prevented and reversed ketamine-induced hyperlocomotion (a, b), spatial working memory impairments (c, d) and social interaction deficits (e, f). Bars represent the mean ± S.E.M of 7 animals/group. *p < 0.05 compared to vehicle (VEH) group and #p < 0.05 compared to KET group. One-way ANOVA followed by Bonferroni post-hoc test showed that there were significant differences between various treatment groups. VEH vehicle, KET ketamine, RUT rutin, RIS risperidone
Rutin reduces ketamine-induced spatial working memory impairment
The effects of RUT on KET-induced alteration in spatial working memory in the preventive and reversal treatments are shown in Fig. 1c, d. One way ANOVA revealed that KET (20 mg/kg, i.p.) significantly (p < 0.05) altered correct alternations in the YMT in both treatment protocols in comparison with vehicle-treated group. Both RUT (0.2 and 0.4 mg/kg, p.o.) and RIS (0.5 mg/kg, p.o.) significantly (p < 0.05) prevented (Fig. 1c) [F (5, 36) = 6.307, p < 0.0003] and reversed (Fig. 1d) [F (5, 36) = 7.018, p < 0.0001] KET-induced memory deficit when compared with KET-treated groups respectively. Meanwhile, RUT (0.1 mg/kg, p.o.) prevented, but failed to reverse the effect of KET on spatial working memory relative to KET treatment group (Fig. 1d).
Rutin abates ketamine-induced social interaction deficit
As shown in Fig. 1e, f, RIS (0.5 mg/kg, p.o.) and RUT (0.2 and 0.4 mg/kg, p.o.) but not 0.1 mg/kg significantly prevented KET-induced social withdrawal when compared with KET group (Fig. 1e) [F (5, 36) = 11.26, p < 0.0001]. However, only RIS (0.5 mg/kg, p.o.) and RUT (0.4 mg/kg, p.o.) reversed (p < 0.05) the effect of KET on social interaction in comparison with KET control group [F (5, 36) = 14.70, p < 0.0001] (Fig. 1f). RUT (0.1 and 0.2 mg/kg, p.o.) did not reverse KET-induced social deficit in mice.
Rutin inhibits ketamine-induced glutathione depletion in the striatum, prefrontal cortex and hippocampus of mice brains
Two-way ANOVA revealed that there were significant differences between treatment groups in the preventive treatment (Fig. 2a) {striatum [F (5, 36) = 4.990, p < 0.0014], prefrontal cortex [F (5, 36) = 4.712, p = 0.0021] and hippocampus [F (5, 36) = 8.755, p < 0.0001]}, and reversal treatment (Fig. 2b) {striatum [F (5, 36) = 16.24, p < 0.0001], prefrontal cortex [F (5, 36) = 6.988, p < 0.0001] and hippocampus [F (5, 36) = 6.807, p < 0.0001]} in GSH concentrations. Ketamine significantly decreased GSH concentrations in all brain regions in both treatment plans when compared with normal controls. Preventive treatment with both RUT (0.1, 0.2 and 0.4 mg/kg, p.o.) and RIS (0.5 mg/kg, p.o.) significantly (p < 0.05) increased GSH concentrations in the prefrontal cortex when compared with KET (20 mg/kg, i.p.) treatments. However, RUT (0.2 and 0.4 mg/kg, p.o.) increased striatal and hippocampal GSH levels relative to KET-treated groups (Fig. 2a). Furthermore, reversal treatment of RUT (0.1, 0.2 and 0.4 mg/kg, p.o.) significantly (p < 0.05) elevated GSH in the hippocampus when compared with KET (20 mg/kg, i.p.) treatment (Fig. 2b). RIS (0.5 mg/kg, p.o.) and RUT (0.2 and 0.4 mg/kg, p.o.), but not 0.1 mg/kg, also significantly (p < 0.05) increased GSH concentrations in the striatum and prefrontal cortex in comparison with KET-treated groups in the reversal study (Fig. 2b).
Rutin inhibits ketamine-induced glutathione alterations (a, b), malondialdehyde (c, d), nitrite (e, f) and acetylcholinesterase (g, h) up-regulations in the preventive and reversal treatments in the striatum, prefrontal cortex and hippocampus of mice brains. Bars represent the mean ± S.E.M of 7 animals/group. *p < 0.05 compared to vehicle group and #p < 0.05 compared to KET group (two-way ANOVA followed by Bonferroni post hoc test). VEH vehicle, KET ketamine, RUT rutin, RIS risperidone
Rutin suppressed ketamine-induced increased in malondialdehyde concentrations in the striatum, prefrontal cortex and hippocampus of mice brains
Two-way ANOVA and post-hoc analysis showed that intraperitoneal injection of KET (20 mg/kg, i.p.) produced a significant (p < 0.05) striatal [F (5, 36) = 5.936, p = 0.0004], prefrontal cortical [F (5, 36) = 6.367, p = 0.0002] and hippocampal [F (5, 36) = 3.799, p = 0.0073] increase in MDA concentrations of mice brains when compared with vehicle-treated animals (Fig. 2c). Treatment with RUT (0.1, 0.2 and 0.4 mg/kg, p.o.) and RIS (0.5 mg/kg, p.o.) significantly (p < 0.05) prevented KET-induced increase in MDA concentrations in the striatum and hippocampus when compared with KET-treated animals. Moreover, higher doses of RUT (0.2 and 0.4 mg/kg, p.o.) decreased MDA concentrations in the prefrontal cortex when compared with KET control (Fig. 2c). Also, in the reversal protocol, KET (20 mg/kg, i.p.) induced a significant (p < 0.05) increase in MDA levels {striatal [F (5, 36) = 6.569, p = 0.0002], prefrontal cortical [F (5, 36) = 6.938, p = 0.0001] and hippocampal [F (5, 36) = 5.008, p = 0.0014]} relative to vehicle groups (Fig. 2d). Although no significant effect was observed in the striatum with RUT treatment, RUT (0.2 and 0.4 mg/kg, p.o.) significantly (p < 0.05) reversed the effect of KET on MDA concentrations in the prefrontal cortex and hippocampus when compared with KET-treated mice. Meanwhile, RIS (0.5 mg/kg, p.o.) significantly (p < 0.05) decreased brain concentrations of MDA in the striatum, prefrontal cortex and hippocampus relative to KET-treated mice (Fig. 2d).
Effects of rutin on nitrergic levels in the striatum, prefrontal cortex and hippocampus in the preventive and reversal treatments in mice treated with ketamine
The effects of RUT on KET-induced alteration of nitrite concentrations in the striatum, prefrontal cortex and hippocampus of mice brains are shown in Fig. 2e, f. In the preventive treatment, two-way ANOVA showed that KET (20 mg/kg, i.p.) administration significant increased nitrite concentration in the striatum [F (5, 36) = 7.847, p < 0.0001] relative to vehicle control. No significant changes were observed in the prefrontal cortex and hippocampus compared with vehicle controls (Fig. 2e). RUT (0.2 and 0.4 mg/kg, p.o.) and RIS (0.5 mg/kg, p.o.) significantly (p < 0.05) attenuated KET-induced nitrite alteration in the striatum relative to KET groups (Fig. 2e). In the reversal treatment, KET (20 mg/kg, i.p.) caused a significant (p < 0.05) increase in brain levels of nitrite in the striatum [F (5, 36) = 6.149, p = 0.0003] and hippocampus [F (5, 36) = 0.1156, p = 0.9881], but not in the prefrontal cortex [F (5, 36) = 1.145, p = 0.3546] relative to vehicle control animals (Fig. 2f). However, RUT (0.4 mg/kg, p.o.) significantly decreased nitrite content in the prefrontal cortex when compared with KET group. Both RUT (0.2 and 0.4 mg/kg, p.o.) and RIS (0.5 mg/kg, p.o.) reduced KET-induced nitrite levels in the striatum and hippocampus when compared to KET controls. Rutin (0.1 mg/kg, p.o.) did not produce any significant change in the striatum and hippocampus (Fig. 2f).
Rutin decreases ketamine-induced up-regulation of acetylcholinesterase activity in the striatum, prefrontal cortex and hippocampus
In the preventive treatment protocol, two-way ANOVA together with post-hoc test showed that KET (20 mg/kg) significantly (p < 0.05) up-regulated AChE activity in the prefrontal cortex [F (5, 36) = 6.263, p = 0.0003] and hippocampus [F (5, 36) = 9.283, p < 0.0001], but not in the striatum [F (5, 36) = 2.400, p = 0.0561], when compared with vehicle groups (Fig. 3c). However, RUT (0.1, 0.2 and 0.4 mg/kg, p.o.) and RIS (0.5 mg/kg, p.o.) significantly (p < 0.05) attenuated AChE activity in the prefrontal cortex when compared with KET-treated mice. Moreover, RUT (0.2 and 0.4 mg/kg, p.o.) and RIS significantly (p < 0.05) decreased AChE activity in the hippocampus when compared with KET-treated mice (Fig. 2g). In the reversal treatment, KET (20 mg/kg, i.p.) significantly (p < 0.05) increased AChE activity in the striatum [F (5, 36) = 5.738, p = 0.0005], prefrontal cortex [F (5, 36) = 10.64, p < 0.0001] and hippocampus [F (5, 36) = 6.396, p = 0.0002] in comparison with KET-treated group. Both RUT (0.4 mg/kg, p.o.) and RIS (0.5 mg/kg, p.o.) significantly (p < 0.05) attenuated increased AChE activity due to KET injection in the striatum and hippocampus when compared with KET-treated group. Furthermore, RUT (0.2 and 0.4 mg/kg, p.o.) and RIS (0.5 mg/kg, p.o.) also reversed KET-induced increased AChE activity in the prefrontal cortex relative to KET group (Fig. 2h).
a–f Representative photomicrographs of the effect of rutin on ketamine-induced histo-architectural alterations in the striatum, prefrontal cortex and hippocampus of mice brains with ketamine in the preventive and reversal treatments. a = Vehicle 10 mL/kg, b = Ketamine 20 mg/kg, c = Rutin 0.1 mg/kg + Ketamine, d = Rutin 0.2 mg/kg + Ketamine, e = Rutin 0.4 mg/kg + Ketamine, and f = Risperidone 0.5 mg/kg + Ketamine. g, h Rutin increases the density of viable neuronal cells in the striatum, prefrontal cortex and hippocampus of mice treated with ketamine in the preventive (g) and reversal (h) treatments. Bars represent the mean ± S.E.M of 3 animals / group. *p < 0.05 compared to vehicle group and #p < 0.05 compared to KET group (Two-way ANOVA followed by Bonferroni post-hoc test). VEH vehicle, KET ketamine, RUT rutin, RIS risperidone
Rutin prevented and reversed ketamine-induced histo-architectural alterations in the striatum, prefrontal cortex and hippocampus of mice brains
The photomicrographs and densitometric counts of viable cells of the striatum, prefrontal cortex and hippocampus of mice brains treated with RUT on KET-induced cytoarchitectural and histomorphological alterations in the preventive and reversal treatments are shown in Fig. 3a–h. In both preventive and reversal treatments, hematoxylin and eosin staining revealed that KET (20 mg/kg, i.p.) produced a significant cytoarchitectural changes in the striatum (Fig. 3a, b) and prefrontal cortex (Fig. 3c, d), and CA1 subfield of the hippocampus of mice in reversal treatment (Fig. 3e) but not in the preventive treatment (Fig. 3f). This is characterized by increased population of highly condensed (pyknotic) and angulated neuronal cells. Also, as shown in Fig. 3g, h, KET (20 mg/kg, i.p.) significantly (p < 0.05) decreased the number of viable neuronal cells of the striatum [F (5, 12) = 8.347, p = 0.0013] and prefrontal cortex [F (5, 12) = 5.306, p = 0.0084] in the preventive study (Fig. 3g); and striatum [F (5, 12) = 10.38, p = 0.0005], prefrontal cortex [F (5, 12) = 4.497, p = 0.0153] and hippocampus [F (5, 12) = 6.942, p = 0.0029] for reversal treatment (Fig. 3h) in comparison with vehicle control groups. However, RUT (0.1, 0.2 and 0.4 mg/kg, p.o.) and RIS (0.5 mg/kg, p.o.) significantly reduced the cytoarchitectural alterations induced by KET, as evidenced by increased viable neuronal cells in the striatum and prefrontal cortex in the preventive treatment (Fig. 3g). Also, Rutin (0.2 and 0.4 mg/kg, p.o.) and RIS (0.5 mg/kg, p.o.) significantly (p < 0.05) decreased the loss of viable neuronal cells of the striatum and cortical regions when compared with KET treatment alone (Fig. 3h) in the reversal treatment. But, RUT (0.1 mg/kg) only increased viable neuronal cells in the striatum (Fig. 3h).
Rutin suppressed ketamine-induced up-regulation of Nox-2 immunoexpressions in the striatum, prefrontal cortex and hippocampus of mice brain
The photomicrographs and immunoexpressions of Nox-2 cells in the striatum, prefrontal cortex and hippocampus of mice brains treated with RUT on KET-induced immunohistochemical changes in the preventive and reversal treatments are shown in Fig. 4a–g. Chronic intraperitoneal injection of KET (20 mg/kg) produced significant (p < 0.05) immunohistochemical changes on Nox-2 expressions in the striatum (Fig. 4a, b), prefrontal cortex (Fig. 4c, d) and CA1 region of the hippocampus (Fig. 4e, f) in the preventive and reversal treatments. Post-hoc analysis indicates that there were significant differences between treatment groups in the preventive treatment (Fig. 4g) {striatum [F (3, 8) = 5.948, p = 0.0196], prefrontal cortex [F (3, 8) = 6.076, p = 0.0185] and hippocampus [F (3, 8) = 7.444, p = 0.0106]}, and reversal treatment (Fig. 4h) {striatum [F (3, 8) = 6.207, p = 0.0106], prefrontal cortex [F (3, 8) = 6.389, p = 0.0168] and hippocampus [F (3, 8) = 7.913, p = 0.0089]} in Nox-2 expressions. Immuno-densitometic expression revealed that KET increased the expressions of Nox-2 striatum, prefrontal cortex and hippocampus in the preventive (Fig. 4g) and reversal (Fig. 4h) treatments in comparison with vehicle-treated mice. Rutin (0.4 mg/kg, p.o.) and RIS (0.5 mg/kg, p.o.) significantly (p < 0.05) attenuated KET-induced expressions of Nox-2 immunopositive cells in the three brain regions when compared to KET-treated mice (Fig. 4g, h).
a–f Representative photomicrographs of the effect of rutin on ketamine-induced immunohistochemical changes and expressions of Nox-2 immunopositive cells in the striatum, prefrontal cortex and hippocampus of mice brains with ketamine in the preventive and reversal treatments. a = Vehicle 10 mL/kg, b = Ketamine 20 mg/kg, c = Rutin 0.4 mg/kg + Ketamine, and d = Risperidone 0.5 mg/kg + Ketamine. Vertical arrow indicates high immunopositive cell expression. Horizontal arrow indicates low immunopositive cell expression. g, h Rutin decreases the expression of Nox-2 immunopositive cells in the striatum, prefrontal cortex and hippocampus of mice treated with ketamine in the preventive (a) and reversal (b) treatments. Bars represent the mean ± S.E.M of 3 animals/group. *p < 0.05 compared to vehicle group and #p < 0.05 compared to KET group (Two-way ANOVA followed by Bonferroni post-hoc test). VEH vehicle, KET ketamine, RUT rutin, RIS risperidone
Rutin attenuates ketamine-induced down-regulation of GAD67 immunoexpressions in the striatum, prefrontal cortex and hippocampus of mice brain
The photomicrographs and immunoexpressions of GAD67 cells in the striatum, prefrontal cortex and hippocampus of mice brains treated with RUT on KET-induced immunohistochemical alteration in the preventive and reversal treatments are shown in Fig. 5a–h. Repeated administration of KET (20 mg/kg, i.p.) caused a significant (p < 0.05) immunohistochemical changes based decreased immunopositive expressions of GAD67 in the striatum [F (3, 8) = 6.396, p = 0.0161] (Fig. 5a and g), prefrontal cortex [F (3, 8) = 4.276, p = 0.0445] (Fig. 5c and g) and hippocampus [F (3, 8) = 8.906, p = 0.0063] (Fig. 5e and g), when compared with vehicle-treated groups. RUT (0.4 mg/kg, p.o.) significantly (p < 0.05) prevented KET-induced decreased GAD67 expressions in the preventive treatment in the striatum and prefrontal cortex but not significantly in the hippocampus. Although RIS (0.5 mg/kg, p.o.) did not show any effect in the striatum, it significantly (p < 0.05) increased GAD67 expression in the prefrontal cortex and hippocampus relative to KET-treated mice. In the reversal treatment, RIS (0.5 mg/kg, p.o.) significantly (p < 0.05) reversed the KET-induced down-regulation of GAD67 immunopositive cells in the striatum [F (3, 8) = 5.561, p = 0.0234] (Fig. 5b and h), prefrontal cortex [F (3, 8) = 16.48, p = 0.0009] (Fig. 5d and h) and CA1 region of the hippocampus [F (3, 8) = 6.091, p = 0.0184] (Fig. 5f and g) relative to KET-treated mice. On the other hand, RUT (0.4 mg/kg, p.o.) failed to reverse KET-induced decrease in GAD67 cell expressions.
a–f Representative photomicrographs of the effect of rutin on ketamine-induced immunohistochemical changes and expressions of GAD67 immunopositive cells in the striatum, prefrontal cortex and hippocampus of mice brains with ketamine in the preventive and reversal treatments. a = Vehicle 10 mL/kg, b = Ketamine 20 mg/kg, c = Rutin 0.4 mg/kg + Ketamine, and d = Risperidone 0.5 mg/kg + Ketamine. Vertical arrow indicates high immunopositive cell expression. Horizontal arrow indicates low immunopositive cell expression. g, h Rutin decreases the expression of GAD67 immunopositive cells in the striatum, prefrontal cortex and hippocampus of mice treated with ketamine in the preventive (a) and reversal (b) treatments. Bars represent the mean ± S.E.M of 3 animals/group. *p < 0.05 compared to vehicle group and #p < 0.05 compared to KET group (Two-way ANOVA followed by Bonferroni post-hoc test). VEH vehicle, KET ketamine, RUT rutin, RIS risperidone
Discussion
Findings from the present study showed that the exposure of mice to ketamine causes hyperlocomotion, social interaction deficit and memory impairment, which were prevented and reversed with rutin. Importantly, ketamine reduced the expression of GAD67 enzyme in the striatum, prefrontal cortex and hippocampus indicating decreased GABAergic neurotransmission which was only prevented by rutin in the striatum and prefrontal cortex. Moreover, the preventive and reversal treatments of mice with rutin also attenuated ketamine-induced increase in expression of the superoxide producing enzyme, Nox-2, as well as the oxidative/nitrergic stress and acetylcholinesterase activity in the striatum, prefrontal cortex and hippocampus in ketamine treated mice, respectively.
Ketamine-induced experimental psychosis is a widely accepted animal model for mimicking symptoms of schizophrenia [5, 6, 10, 27]. Hence, the discovery of compounds capable of ameliorating ketamine-induced hyperlocomotion, social and memory impairments could possibly be a novel drug for the management of schizophrenia [6, 10, 27]. In this study, ketamine-induced hyperlocomotion in the OFT, social isolation in the SIT and memory deficit in the YMT which corroborated previous studies [6, 10, 31]. However, ketamine-induced schizophrenic-like behaviors were ameliorated by preventive and reversal treatments with rutin which further confirms its antipsychotic-like activity [27].
It is well reported that repeated administration of ketamine up-regulate 5-HT2A expression, blocked phencyclidine allosteric NMDA channel complex in the frontal-parietal cortex [32], NMDA receptor channel complex in the ventral tegmental area as well as increased cortical extracellular brain levels of 5-HT, an action that has been linked to both negative and cognitive symptoms of schizophrenia [33]. More so, ketamine-induced up-regulation of 5-HT2A activity has been reported to reduce GABAA-mediated inhibitory current via decreased GAD67 enzyme activity [10, 13, 34]. Several post-mortem studies have consistently showed GAD67-dependent GABAergic alterations in schizophrenic brains [7, 8, 10]. Decreased GAD67 enzyme activity is believed to contributes to the reduced gamma band frequencies of the medial part of the prefrontal cortex, and cornu ammonis 1 (CA1) and 3 (CA3) of the hippocampus, as well as impaired dorsolateral prefrontal cortical- and hippocampal-dependent cognitive performance seen in schizophrenic patients [7, 10]. In this study, repeated administration of ketamine to mice down-regulates GAD67 immunopositive in the striatum, prefrontal cortex and hippocampus indicative of reduced GABAergic neurotransmission [10, 13]. However, ketamine-induced down-regulation of GAD67 immunoreactivity in the striatum and prefrontal cortex was prevented by rutin administration but not in the reversal study. It is well known that an increase in GAD67 immunoreactivity increases GABAergic-dependent pyramidal neuronal activity in the area of executive function with corresponding decrease in presynaptic dopamine release at the subcortical levels [14] as well as elevation of dorsolateral cortical (prefrontal cortex and hippocampus)-dependent cognitive and social performances [7]. It is worth mentioning that rutin has been previously reported to promote GABAergic activity in rats’ cerebral cortex [24]. Thus, it could be used as an adjunct in the treatment of neuropsychiatric conditions related to schizophrenia.
Recent studies have shown that repeated exposure of rodent to ketamine-induced oxidative stress via mechanisms associated with elevated expressions of Nox-2 [10, 35]. Interestingly, Nox-2 and reactive oxygen species serve as cellular patho-mechanisms that induce changes on NMDA/GABA receptors enshrined on GABAergic neurons [11, 13]. Indeed, prolonged Nox-2-mediated glutamate outflow has been reported to cause neuro-adaptative hypofunctionality of NMDA receptors [11], decreased GAD67 immunoreactivity [36] and consequently behavioral deficits relevant to schizophrenia pathology [35]. Interestingly, the findings from this study showed that ketamine profoundly increased the expressions of Nox-2 in the striatum, prefrontal cortex and hippocampus, which were abated by the preventive and reversal treatments of mice with rutin.
Increasing evidence shows that oxidative stress plays a role in the pathophysiology of schizophrenia [6, 10, 13, 37, 38]. It has been reported that total oxidant status (TOS), oxidative stress index (OSI) and 8-hydroxydeoxyguanosine (8-OHdG) levels were significantly higher in non-remission schizophrenic patients than in the controls [38]. The TOS and OSI levels were significantly higher in remission schizophrenic patients than in the controls [37]. Moreover, postmortem schizophrenic brain studies showed that altered cellular expression of the antioxidant master gene, nuclear factor erythroid 2-related factor 2 (Nrf2) [38]. Different studies have demonstrated that increase in GSH levels normalizes NMDA- and GAD67-dependent activities [36]. In this study, repeated administration of ketamine increased lipid peroxidation marker (MDA), nitrite levels with corresponding decrease in GSH concentrations as well as neuronal cell death in the striatum, prefrontal cortex and hippocampus, respectively. These findings are congruent with the previous observations which showed that repeated ketamine exposure to rodents causes oxidative/nitrergic stresses as well neuronal cell death [6, 10, 27, 31]. Furthermore, polymorphisms of the neuronal and inducible NO synthases have been implicated in schizophrenic brains [39] and these markers have been connected to neurochemical dysfunction [5]. However, the ability of rutin to significantly increase GSH concentrations, decreased MDA and nitrite levels as well as increase the population of viable neuronal cells in region-dependent manners further confirms its antioxidant property.
Additionally, chronic ketamine treatment is known to decrease the levels of acetylcholine (ACh), one of the main neurotransmitters implicated in the regulation of memory functions. Previous molecular studies have shown that ketamine-induced decreased concentration of ACh is connected with enhancement of acetylcholinesterase (AChE) postsynaptic activity [5, 6]. ACh through 7-alpha nicotinic acetylcholine receptor (α7nAChR) and calcium-dependent neuronal nitric oxide synthase (nNOS) activates NMDA receptors leading to increase nitric oxide influx and glutamate-mediated synaptic plasticity and increased cognitive function [5]. However, ketamine blocks α7nAChR to induce cognitive dysfunction via mechanism linked to downward regulation of NMDA-NO-induced glutamatergic neuro-adaptative hypofunctionality [5, 11]. Additionally, the increase in AChE by ketamine might also contribute to its ability to disrupt cholinergic-hippocampal-dependent higher order cognitive functions, which includes one of the pathological ensembles found in the brains of schizophrenia patients [5]. In agreement with previous studies [5, 6, 31], our results also showed that ketamine induces memory impairment via increased AChE activity in the striatum, prefrontal cortex and hippocampus, which also correlates with the cholinergic dysfunction associated with schizophrenia [6]. Thus, the ability of rutin to decrease AChE activity in the striatum, prefrontal cortex and hippocampus of mice treated with ketamine, further suggests the possibility of increase ACh levels and beneficial effect against psychiatric disorders associated with cognitive impairment. Various studies have showed that rutin improves memory impairments in neurological disorders associated with oxidative stress and neuroinflammation because of its strong antioxidant and anti-inflammatory potentials [17, 21, 22, 26]. Rutin attenuated streptozotocin-induced hippocampal damage by down-regulating inflammatory mediators and thus improving cognitive function [17]. Also, rutin prevents diabetic-induced neuropathy by attenuation of oxidative stress via Nrf2 signaling pathway in rats [40]. It important to mention here that substantial body of evidence have shown that rutin inhibits AChE activity [41, 42] by binding to the active pocket of some residue proteins of human AChE [43,44,45]. Rutin has also been shown to inhibit the formation and stabilization of β-amyloid deposit via inhibition of β-secretase (BACE-1), the penultimate β-amyloid synthesizing enzyme, thereby suppressing the initiation of β-induced cognitive pathology [43].
It is worthy of note that pharmacokinetic studies of rutin conducted in rat revealed that rutin is quickly absorbed after oral administration into the bloodstream, crosses the blood brain barrier (BBB) however, metabolically hydrolyzed in the liver and intestinal tract into by cecal microflora to sulfate and glucuronide intermediates [46]. While the metabolic transformation and degradation by bacterial enzymes of the intestinal tract have been shown to limit the oral bioavailability of rutin [47], the high permeability of rutin across the BBB has been ascribed to its wide range of central nervous system activities [17, 21,22,23,24,25,26]. Although the choice of doses of rutin used in this present investigation was based on results mined from earlier studies [21, 25], the reason why rutin produced better effects in the reversal treatment than in the preventive protocol in some activities requires further investigation. However, the observed effects from this investigation could be in part due to its antioxidant and anti-inflammatory actions as well as GABAergic modulating activity, mechanisms that have been attributed to the reversal effects of many conventional antipsychotic drugs [6, 10, 12, 27].
Conclusion
Our study reinforced the strong connection between chronic ketamine injection and the schizophrenia-related behavior, which were prevented and reversed by rutin. We therefore showed that rutin attenuated ketamine-induced hyperactivity, social withdrawal and cognitive deficit via inhibition of oxidative/nitrergic stress, acetylcholinesterase activity, suppression of Nox-2 expression and up-regulation of GAD67-dependent GABAergic neurotransmissions in a brain region specific manner in mice.
Abbreviations
- GAD67:
-
Glutamic-acid decarboxylase-67
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NMDA:
-
N-methyl-d-Aspartate
- KET:
-
Ketamine
- RUT:
-
Rutin
- SAB:
-
Spontaneous alternation behavior
- YMT:
-
Y-maze test
- SIT:
-
Social interaction test
- DTNB:
-
5′,5′-Dithio-bis-(2-nitrobenzoic acid)
- CA:
-
Cornu ammonis
- NO:
-
Nitric oxide
- GSH:
-
Glutathione
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide-dismutase
- AChE:
-
Acetyl-cholinesterase
- PFC:
-
Prefrontal cortex
- ST:
-
Striatum
- HC:
-
Hippocampus
References
Orrico-Sánchez A, López-Lacort M, Muñoz-Quiles C, Sanfélix-Gimeno G, Díez-Domingo J (2020) Epidemiology of schizophrenia and its management over 8-years period using real-world data in Spain. BMC Psychiatry 20:149
Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, McGrath JJ, Whiteford HA (2018) Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr Bull 44:1195–1203
Larson MK, Walker EF, Compton MT (2010) Early signs, diagnosis and therapeutics of the prodromal phase of schizophrenia and related psychotic disorders. Expert Rev Neurother 10:1347–1359
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD (1994) Subanaesthetic effects of the non-competitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Chatterjee M, Rajkumar V, Surajit G, Gautam P (2012) Neurochemical and molecular characterization of ketamine-induced experimental psychosis model in mice. Neuropharmacology 63:1161–1171
Ben-Azu B, Aderibigbe AO, Eneni AO, Ajayi AM, Umukoro S, Iwalewa EO (2018) Orin attenuates neurochemical changes and increased oxidative/nitrergic stress in brains of mice exposed to ketamine: prevention and reversal of schizophrenia-like symptoms. Neurochem Res 43:1745–1755
Fujihara K, Hideki M, Toshikazu K, Ryosuke K, Masahiko M, Chiyoko T, Nobuaki T, Yuchio Y (2015) Glutamate decarboxylase 67 deficiency in a subset of GABAergic neurons induces schizophrenia-related phenotypes. Neuropsychopharmacology 40:2475–2486
Schmidt MJ, Mirnics K (2015) Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology 40:190–206
Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van den Oever MC (2014) Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci 8:230
Ben-Azu B, Aderibigbe AO, Ajayi AM, Eneni AO, Umukoro S, Iwalewa EO (2018) Involvement of GABAergic, BDNF and Nox-2 mechanisms in the prevention and reversal of ketamine-induced schizophrenia-like behavior by morin in mice. Brain Res Bull 139:292–306
Sorce S, Krause KH (2009) NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal 11:2481–2504
Ben-Azu B, Aderibigbe AO, Ajayi AM, Eneni AO, Omogbiya IA, Owoeye O, Umukoro S, Iwalewa EO (2019) Morin decreases cortical pyramidal neuron degeneration via inhibition of neuroinflammation in mouse model of schizophrenia. Int Immunopharmacol 70:338–435
Behrens MM, Ali SS, Laura LD (2008) Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci 28:13957–13966
Włodarczyk A, Szarmach J, Cubała WJ, Wiglusz MS (2017) Benzodiazepines in combination with antipsychotic drugs for schizophrenia: GABA-ergic targeted therapy. Psychiatr Danub 29:345–348
Nunes EA, Leila C, de Oliveira L, de Luca RD, João Q, Zugno A, Peregrino A, Alexandre J (2012) Effects of pregabalin on behavioral alterations induced by ketamine in rats. Rev Bras Psiquiatr 34:329–333
Kreft S, Knapp M, Kreft I (1997) Extraction of rutin from buckwheat (Fagopyrum esculentum Moench) seeds and determination by capillary electrophoresis. J Agric Food Chem 47:4649–4652
Javed H, Khan MM, Ahmad A, Vaibhav K, Ahmad ME, Khan A, Ashafaq M, Islam F, Siddiqui MS, Safhi MM, Islam F (2012) Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience 17:340–352
Ahmed OM, Moneim AA, Yazid IA (2010) Antihyperglycemic, antihyperlipidemic and antioxidant effects and the probable mechanisms of action of ruta graveolens infusion and rutin in Nicotinamide streptozotocin-induced diabetic rat. Diabetol Croat 39:15–35
Webster RP, Gawde MD, Bhattacharya RK (1996) Protective effect of rutin, a flavonol glycoside, on the carcinogen-induced DNA damage and repair enzymes in rats. Cancer Lett 109:185–191
Panasiak W, Wleklik M, Oraczewska A, Luczak M (1989) Influence of flavonoids on combined experimental infections with EMC virus and Staphylococcus aureus in mice. Acta Microbiol Pol 38:185–188
Fernandez SP, Wasowski C, Loscalzo LM, Granger RE, Johnston GA, Paladini AC, Marder M (2006) Central nervous system depressant action of flavonoid glycosides. Eur J Pharmacol 539:168–176
Nieoczym D, Socała K, Raszewski G, Wlaz P (2014) Effect of quercetin and rutin in some acute seizure models in mice. Prog Neuropsychopharmacol Biol Psychiatry 54:50–58
Paladini AC, Marder M, Viola H, Wolfman C, Wasowski C, Medina JH (1999) Flavonoids and the central nervous system: from forgotten factors to potent anxiolytic compounds. J Pharm Pharmacol 51:519–526
Wang FM, Shing Y, Huen SY, Xue TH (2005) Neuroactive flavonoids interacting with GABAA receptor complex. Curr Drug Targets CNS Neurol Disord 4:575–585
Pandy V, Vijeepallam K (2017) Antipsychotic-like activity of scopoletin and rutin against the positive symptoms of schizophrenia in mouse models. Exp Anim 66:417–423
Bishnoi M, Chopra K, Kulkarni SK (2007) Protective effect of rutin, a polyphenolic flavonoid against haloperidol-induced orofacial dyskinesia and associated behavioural, biochemical and neurochemical changes. Fundam Clin Pharmacol 21:521–529
Monte AS, de Souza GC, Mclntyre RS, Joanna KS, dos Santos JV, Rafaela CC, Bruna MM, de Lucena RDF (2013) Prevention and reversal of ketamine-induced schizophrenia related behaviour by minocycline in mice: possible involvement of antioxidant and nitrergic pathway. J Psychopharmacol 27:1032–1043
Jollow DJ, Michell JR, Zampaglione H, Gillete J (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione an evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Ohkawa H, Ohishi N, Yagi E (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Ellman GL, Courtney KD, Andres V, Feather-Stone RM Jr (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ben-Azu B, Aderibigbe AO, Omogbiya IA, Ajayi AM, Owoeye O, Olonode T, Iwalewa EO (2018) Probable mechanisms involved in the antipsychotic-like activity of morin in mice. Biomed Pharmacother 105:1079–1090
Becker A, Grecksch G (2004) Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Test of predictive validity. Prog Neuropsychopharmacol Biol Psychiatry 28:1267–1277
Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK (2010) Animal models of cognitive dysfunction and negative symptoms of schizophrenia: Focus on NMDA receptor antagonism. Pharmacol Ther 128:419–432
Johnston A, Chris J, McBain AF (2014) 5-Hydroxytryptamine1A receptor-activation hyperpolarizespyramidal cells and suppresses hippocampal gammaoscillations via Kir3 channel activation. J Physiol 592:4187–4199
Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, Cuomo V, Trabace L et al (2009) Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry 66:384–392
Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM (2006) A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci 26:1604–1615
Alpak MG, Unal A, Ari M, Savas HA (2015) Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry Res 229(1–2):200–205
Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT (2011) Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 14:123–130
Shinkai T, Ohmori O, Hori H, Nakamura J (2002) Allelic association of the neuronal nitric oxide synthase (NOS1) gene with schizophrenia. Mol Psychiatry 7:560–563
Tian R, Yang W, Xue Q, Gao L, Huo J, Ren D, Chen X (2016) Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur J Pharmacol 771:84–92
Szwajgier D, Borowiec K, Zapp J (2020) Activity-guided isolation of cholinesterase inhibitors quercetin, rutin and kaempferol from Prunus persica fruit. Z Naturforsch 75:87–96
Ademosun AO, Oboh G, Bello F, Ayeni PO (2016) Antioxidative properties and effect of quercetin and its glycosylated form (rutin) on acetylcholinesterase and butyrylcholinesterase activities. J Evid Based Complement Altern Med 21:11–17
Omar SH, Scott CJ, Hamlin AS, Obied HK (2018) Biophenols: enzymes (β-secretase, cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.). Fitoterapia 128:118–129
Vijayakumar S, Manogar P, Prabhu S, Singh RA (2018) Novel ligandbased docking; molecular dynamic simulations; and absorption, distribution, metabolism, and excretion approach to analyzing potential acetylcholinesterase inhibitors for Alzheimer’s disease. J Pharm Anal 8:413–420
Yan X, Chen T, Zhang L, Du H (2018) Study of the interactions of forsythiaside and rutin with acetylcholinesterase (AChE). Int J Biol Macromol 119:1344–1352
Ou-yang Z, Cao X, Wei Y, Wei-Wan-Qi Z, Ming Z, Jin-ao D (2013) Pharmacokinetic study of rutin and quercetin in rats after oral administration of total flavones of mulberry leaf extract. Rev Bras Farmacogn 23:776–782
Manach C, Morand C, Demigné C, Texier O, Régérat F, Rémésy C (1997) Bioavailability of rutin and quercetin in rats. FEBS Lett 409:12–16
Acknowledgements
The Authors are grateful to Dr. Theophilus Jarikre of the Department of Veterinary Pathology, University of Ibadan for his technical assistance during the immunohistochemistry
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
All experiments were approved and performed under the guidelines of University of Lagos’s Animals Ethic Committee (CMUL/HREC/01/19/481) and the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication number: 85–23, revised 1985).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oshodi, T.O., Ben-Azu, B., Ishola, I.O. et al. Molecular mechanisms involved in the prevention and reversal of ketamine-induced schizophrenia-like behavior by rutin: the role of glutamic acid decarboxylase isoform-67, cholinergic, Nox-2-oxidative stress pathways in mice. Mol Biol Rep 48, 2335–2350 (2021). https://doi.org/10.1007/s11033-021-06264-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06264-6