Abstract
Schizophrenia is a neurological disorder that alters the behavior and affects the quality of life of a patient. It is characterized by hallucinations, disorganized behavior, cognitive dysfunction, hyperlocomotion, and loss of the reward system. Schizophrenia constitutes three symptoms’ domains, viz. positive, negative and cognitive. Typical and atypical antipsychotics do not fully resolve all the symptoms’ domains thus paving the way to the genesis of the glutamatergic hypothesis, i.e. N-methyl-d-aspartate (NMDA) receptor hypofunction in the pathophysiology of schizophrenia. Positive modulation of NMDA receptors by enhancing co-agonist, glycine effect is proposed to produce a therapeutic effect in schizophrenia. Hence, sarcosine (N-methyl glycine), natural amino acid, and a glycine transporter inhibitor (GlyT-1) which also acts on NMDA receptors were used in the present study. The present study unravels the role of sarcosine in the attenuation of ketamine-induced three symptom domains in a rat model through modulation of oxidative stress, mitochondrial dysfunction, and neuroinflammatory pathways. The animal model of schizophrenia was established by injecting ketamine intraperitoneal (ip) at a 30 mg/kg dose for 10 consecutive days, after which sarcosine (300, 600 mg/kg, ip) as a treatment was given for 7 days followed by behavioral, biochemical, molecular, and histopathological analysis. It was revealed that sarcosine reversed ketamine-induced behavioral impairments. Moreover, sarcosine ameliorated oxidative and nitrosative stress, mitochondrial dysfunction, and neuroinflammation and showed protective effects in histopathological examination by hematoxylin and eosin staining. Hence, conclusively, sarcosine was regarded to attenuate the behavioural symptoms of schizophrenia by alleviating oxidative stress, neuroinflammation, and mitochondrial dysfunction established by the ketamine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is a neurological disorder in which the thinking and behavior of an individual are severely affected (Bueno-Antequera and Munguía-Izquierdo 2020). People with schizophrenia often lose connection with reality followed by delusional behavior. Schizophrenics usually manifest paranoid delusions and hallucinations as positive symptoms; blunted effect and anhedonia as negative symptoms along with different degrees of cognitive impairment (Batinic 2019).
Various reviews and other biochemical and pharmacological studies form the basis for the dopamine (DA) hypothesis which states that schizophrenia is dependent on relatively higher DA-mediated neuronal activity. The dopamine hypothesis arising due to dopamine hyperfunction results in hallucinations and delusions (positive symptoms). Over the year dopamine pathway was studied widely and gave rise to different antipsychotics including risperidone which acts by blocking the dopamine receptor-2 (D2). In addition, risperidone has strong action to downregulate serotonergic and dopaminergic pathways in the brain region, thereby possessing the potential to diminish schizophrenic signs and symptoms (Karl et al. 2006). However, D2 blockers are not effective in managing negative and cognitive symptoms, in addition, positive symptoms are also not fully resolved by these typical and atypical antipsychotics (Steeds et al. 2015). More recently, various lines of findings have converged to highlight the hypothesis of persistent dysregulation in the transmission of glutamatergic transmission via N-methyl-d-aspartate (NMDA) receptors. Hypofunction of NMDA receptors is thought to recapitulate some of the behavioral symptoms of schizophrenia by irregular glutamate transmission through NMDA receptors which are evidenced in various studies (Tanqueiro et al. 2021). Phencyclidine and ketamine (ket) as NMDA receptor antagonists are being used to induce animal models that more closely mimic the pathophysiology of schizophrenia as compared to other models (Wang et al. 2020). It was found that chronic antipsychotic therapy is proficient to treat only positive symptoms amongst all three symptoms domains but treatment resistance to schizophrenia has been observed with the use of atypical antipsychotics (Fattal et al. 2006). Hence, stimulation of glutamatergic transmission using glutamate agonists could be one of the possible targets for the treatment of schizophrenia. Glutamate is an agonist while glycine act as a co-agonist to NMDA receptor (Strzelecki et al. 2016).
Furthermore, NMDA receptor hypofunction and dysregulated glutamatergic neurotransmission have been depicted to be positively correlated to oxidative imbalance or oxidative stress which can be the causative factors in schizophrenia (Genius et al. 2013). In addition, neuroinflammatory markers like cytokines can also be the triggers of symptoms of schizophrenia, which is again linked to NMDA receptor antagonism (Anderson and Maes 2013).
Recent clinical evidence shows the involvement of glycine transporter inhibitor (GlyTI) in brain diseases, owing to their presence in brain regions controlling mood, memory, and behavior, especially in schizophrenia (Fleischhacker et al. 2021). GlyTI has been investigated pre-clinically in an acute model of schizophrenia and Alzheimer’s disease which depicted its role in working memory deficit but there are no shreds of evidence that suggest the role of GlyTI in ameliorating the cognitive dysfunction along with positive and negative symptoms of schizophrenia (Harada et al. 2012). Interestingly, most of the studies of glycine transporter inhibitor/s have not investigated its role in chronic ketamine-induced animal models of schizophrenia. In this regard, our study could be considered a novel one. Furthermore, chronic ketamine administration in our study resulted in all three symptom domains of schizophrenia; positive, negative, and cognitive. Positive symptoms include hallucinations and delusions which are demonstrated by hyperlocomotion while negative symptoms include anhedonia evident by the sucrose preference test. Moreover, for the cognitive symptoms, authors have performed Barnes Maze Test and a novel object recognition test. The authors have tried to elucidate the mechanism of action of glycine transporter inhibitor (Sarcosine) by various molecular and histological analyses.
Sarcosine is N-methyl glycine in chemical nature and a glycine transporter inhibitor that also has the potential to decline the levels of reactive oxygen species (Pinto et al. 2012). This feature of sarcosine could regulate mitochondrial energy generation since oxidative stress is correlated to a neuronal mitochondrial abnormality in schizophrenia (Prabakaran et al. 2004). The present study explores the role of glycine transporter inhibitor (sarcosine) in positive, negative, and cognitive symptoms of schizophrenia by different behavioral and biochemical estimations in ketamine-induced schizophrenia.
Material and methods
Animals
Male Wistar rats (adult) aged 4 weeks and weighed around 180–250 g were used in this study. Animals were obtained and housed in the Central animal house (CAH), Panjab University, Chandigarh, India. Free access to food and water was given to the animals placed in respective cages under a 12-h dark and light cycle at 23 ± 1 °C in a 60% relative humidity-controlled environment. The experimentation procedure was approved by Institutional Animal Ethics Committee, Panjab University, Chandigarh, India (Approval No.: PU/45/99/CPCSEA/IAEC/2017/12).
Drugs/chemicals
Marketed formulation of ketamine hydrochloride (KETMIN®50) to induce schizophrenia-like symptoms. Sarcosine and risperidone were obtained from Tokyo chemical industry (TCI) India, Pvt. Ltd. Chemicals used for biochemical estimation were of analytical grade. Ketamine, sarcosine, and risperidone were dissolved in 0.9% saline and injected intraperitoneally (ip) at a dose of 0.2 mg/kg. Drugs were freshly prepared and administered ip at a volume of 5 mL/kg.
Experimental design
Animals were randomly divided into six groups having the same number of animals (n = 8) in each group (Table 1). Animals were subjected to ketamine injection (ip) for 10 days at a 30 mg/kg dose (Nikiforuk and Popik 2012). Drug treatment was done for 7 days starting from the 10th day of protocol (Fig. 1). All the treatments were given between periods of 9:00 a.m. to 5:00 p.m. The behavioral tests were performed from the 17th day to the 23rd day as per Fig. 1. Sarcosine and risperidone were given at preselected doses based on a previous study (Kumar et al. 2016).
Behavioral parameters
Locomotion
The locomotion of the animal was evaluated by an instrument named an actophotometer obtained from IMCORP, Ambala, India. Each animal was placed in an actophotometer for 5 min to habituate the chamber after that ambulatory and rearing parameters were recorded digitally in the instrument (Yadav et al. 2018).
Open field test (OFT)
OFT was conducted using a larger circular arena of 110 cm in diameter (Bruijnzeel et al. 2016). Each animal was placed at the center of the instrument for 5 min of habituation. After that total number of line crossing, rearing, and center entries were video-recorded for 5 min for further analysis. While counting the scores, the observer was blinded to the groups.
Sucrose preference test (SPT)
SPT was conducted using sucrose solution (1%) and tap water. It is used to evaluate negative symptoms, especially anhedonia (Sun et al. 2017). Animals were placed in each animal per cage with access to food. Two identical bottles of equal volumes were filled with sucrose and tap water, respectively, placed on the two sides of a cage for 72 h. Meanwhile, both bottles were switched three times at the interval of 24 h. Sucrose preference was determined by measuring the amount of final volume of each bottle at the end of 24 h for 3 consecutive days (Kandratavicius et al. 2015). Sucrose preference was calculated by the percentage of the ratio of sucrose intake (mL) to the sum of sucrose and water intake (mL).
Novel object recognition test (NORT)
NORT evaluates recognition memory. The test was conducted on an open field arena with two different objects of similar heights and volume. Animals were habituated by allowing them to explore the empty arena for 10 min and after 24 h they were subjected to explore the arena with two similar objects. On the very next day, one object was replaced by a novel object, and animals were placed to explore to test the recognition memory (Akhtar et al. 2020). The entire procedure was video-recorded for further analysis. The experimenter was blinded to the groups while observing exploration time. Exploration time was counted as sniffing, touching, etc. The discrimination index was calculated.
Barnes maze test
The Barnes maze test is used to measure spatial learning and memory. It consisted of a black, circular platform with a diameter of 122 cm and an elevation of 70 cm from the ground. The maze has 20 evenly spaced holes (diameter 10 cm), 5 cm from the maze perimeter. An escape box designed as a goal location was placed under one of the holes. The test consisted of 7 days. On the first day of the training session, each animal was habituated in an escape cage for 2 min and then placed over the center of the maze for 5 min to find the escape box. Similarly, for the rest of the days, animals were trained by placing them at the center of the maze for 5 min. Latency is the time taken by the animal to find the escape box and errors when the animal placed its head or paw on fake holes were noted Gawel et al. 2019.
Biochemical estimations
Preparation of blood samples
The rats were anesthetized after the completion of behavioral assessments and blood was collected via retro-orbital plexus. For the collection of plasma from the blood, EDTA was used as an anticoagulant, and blood was centrifuged at 10,000 rpm for 10 min. Plasma was separated and further stored at − 80 °C for further analysis.
Preparation of brain homogenate
The rats were killed, and their brains were isolated followed by the removal of the cerebral cortex and hippocampus for further biochemical assays. Cortex and hippocampi tissues were divided into two halves for further biochemical and mitochondrial estimations and ELISA. Tissues were then homogenized in 10% (w/v) homogenization buffer (consisting of 10 mM Tris–HCl, 150 mM MgCl2, 1mM EDTA, 1% Triton X 100, pH 7.4). The post-nuclear fraction was obtained by centrifugation of the homogenates at 4000g for 20 min at 4 °C. The post-nuclear fraction as brain homogenate obtained was used for estimation of superoxide dismutase (SOD) lipid peroxidation (LPO), catalase, and reduced glutathione (GSH) in both cortex and hippocampus.
Lipid peroxidation
Lipid peroxidation was quantitatively determined in the form of thiobarbituric acid reactive substances by a previously described method (Bose et al. 1989). Tris–HCl (0.5 mL) was added to 0.5 mL of brain homogenate supernatant and incubated for 2 h at 37 °C followed by the addition of 1 mL of 10% trichloroacetic acid and centrifugation at 300g for 10 min. The supernatant was collected and 1 mL of supernatant was mixed with an equal volume of 0.67% of thiobarbituric acid and kept in boiling water for 10 min. 1 mL of double distilled water was added after cooling. Absorbance was measured at 532 nm (Perkin Elmer UV/Vis Spectrophotometer, Lambda 20). The concentration of malondialdehyde (MDA) was calculated by a molar extinction coefficient of 1.56 × 105 M−1 cm-1 and expressed as nanomoles of MDA equivalents per milligram protein.
Catalase
Catalase activity was measured according to the previously designed method (Escarabajal et al. 2000). The final volume of 3 mL was made by mixing 1.95 mL phosphate buffer of 0.05 M, pH 7.0, 1 mL of 0.019 M of hydrogen peroxide, and 0.05 mL of 10% of brain homogenate supernatant. Absorbance changes were recorded at 240 nm using a Spectrophotometer (Perkin Elmer UV/Vis, Lambda 20) for 1 min. Quantification of catalase activity was measured by mM extinction coefficient of H2O2 (0.07 mM) and expressed as µM of H2O2 decomposed per minute per milligram protein.
Reduced glutathione (GSH)
1 mL of 10% of brain homogenate supernatant was precipitated with an equal volume of 4% of sulfosalicylic acid (4%) and kept at 4 °C for 1 h. Centrifuged the solution at 1200 rpm for 15 min. The method was previously described for GSH by Jollow et al. (1974). Final volume was made by adding 0.1 mL supernatant, 2.7 mL phosphate buffer (0.1 M, pH 7.4), and 0.2 mL of Ellman’s reagent (0.1 mM, pH 8.0). The absorbance of the developed yellow color was measured at 412 nm using a Spectrophotometer (Perkin Elmer UV/Vis, Lambda 20). The concentration of GSH was calculated by a molar extinction coefficient of 1.36 × 104 M−1 cm−1 and expressed as µM per milligram protein.
Superoxide dismutase (SOD)
The assay consisted of a mixture of 0.1 mM EDTA, 50 mM sodium carbonate, and 96 mM of nitro blue tetrazolium (NBT). 2 mL of the mixture was transferred in the cuvette, and 0.05 mL of hydroxylamine and 0.05 mL of the brain homogenate supernatant were added. Auto-oxidation of hydroxylamine was measured absorbance (Perkin Elmer UV/Vis Spectrophotometer, Lambda 20) at 560 nm for 2:30 min intervals (Kono 1978).
Plasma nitrite
Plasma and Griess reagent was mixed at equal volume (0.1% naphthyl ethylene diamine dihydrochloride and 1% sulphanilamide in 5% phosphoric acid) according to the method followed by Green et al. (1982). The mixture was kept in dark for 10 min for incubation at room temperature and absorbance was measured at 540 nm. Sodium nitrite was used as standard and levels of plasma nitrite were calculated by standard curve.
Mitochondrial estimation
Isolation of mitochondria
The cerebral cortex and hippocampus were separated from the rat brain. Mitochondrial isolation was done using an ice-cold isolation buffer. Buffer was maintained at 7.2 pH and prepared by mannitol (215 mM), sucrose (75 mM), BSA (0.1%), HEPES (20 mM), and 1 mM of EGTA with EGTA. Prepared homogenates were centrifuged at 1000g for 5 min at 4 °C and pellets were re-suspended with buffer containing EGTA. Re-spun the solution at 13,000g for 5 min at 4 °C. Supernatants were collected and topped off using isolation buffer with EGTA, again spun at 13,000g for 10 min at 4 °C. Repeated the centrifugation process with pellet re-suspended at 13,000g for 5 min at 4 °C and incubated with 1 mL digitonin for 10 min. The mixture was centrifuged for one last time at 13,000g for 10 min at 4 °C (King et al. 2006). Mitochondria were re-suspended in pellet having isolation buffer without EGTA.
Complex I activity
Complex I known as nicotinamide adenine dinucleotide (NADH) dehydrogenase was assessed in the cerebral cortex and hippocampus. The method involved catalytic oxidation of NADH to NAD+ with subsequent reduction of cytochrome c. The reaction mixture was prepared by mixing 0.2 M glycyl glycine buffer with 6 mM NADH in 2 mM glycyl glycine buffer, and 10.5 mM cytochrome c. Mitochondrial sample was solubilized with the reaction mixture, and absorbance change was noted at 550 nm for 3 min at a 1-min interval. The results were calculated as moles of NADH oxidized/min/mg protein.
Complex II activity
Succinate dehydrogenase activity or complex II activity was measured in the cortex and hippocampal region of the rat brain according to the method previously described by Cassina and Radi (1996). The reaction occurs at the oxidation of succinate by an artificial electron acceptor potassium ferricyanide. The reaction mixture was prepared by the addition of phosphate buffer (0.2 M) at pH 7.8 with 1% BSA, succinic acid (0.6 M), and potassium ferricyanide (0.03 M). A mitochondrial sample was added to the reaction mixture to initiate the reaction and absorbance change was measured at 420 nm for 3 min at 1 min interval. The results were expressed as n moles of substrate/min/mg protein.
Complex III activity
Methylthiazolyldiphenyltetrazolium bromide (MTT) is used to assess the complex III activity. MTT is a pale yellow substrate that produces purple color product when the mitochondrial sample is added. Solubilization with dimethyl sulfoxide (DMSO) measures the number of viable cells per well-concerning production of the purple product. Mitochondrial samples were incubated with 10 µL of MTT in each well in a humidified atmosphere (5% CO2 95% air) at 37 °C for 3 h. the medium was aspirated off and lysed with DMSO (50%). Absorbance change was measured by ELISA reader at 580 nm Han et al. 2015.
Complex IV activity
Complex IV (cytochrome oxidase) activity was assessed in the cortex and hippocampal mitochondrial samples. The assay mixture was prepared by reduced cytochrome c (3 mM) in phosphate buffer (75 mM) (Galpern and Cudkowicz 2007). A mitochondrial sample was added to the assay mixture to initiate the reaction. Absorbance change was recorded at 550 nm for 3 min at a 1-min interval. The results were expressed as l moles of cytochrome-c reductase/min/mg protein.
ELISA
Estimation of IL-6 levels
Interleukin-6 (IL-6) is a pleiotropic cytokine that acts in the acute phase reaction and neuroinflammation. The method was followed according to the manual provided by the ELISA kit procured from R&D systems.
Procedure
The reagents, samples, and standard dilutions were used as directed by the manufacturer’s instructions. Assay diluent (50 µL) was added to the center of each well. Each well was filled with 100 μL of standard/control/sample. The plate was incubated for 2 h at room temperature and covered with an adhesive strip provided with the kit. Wash buffer 400 μL was used to aspirate and wash each well four times. After that, the plate was inverted and blotted against the clean paper. Biotinylated detection antibody was added at 100 μL volume and incubated for 1 h at room temperature. The plate was washed 4 times and 100 μL of rat IL-6 conjugate was added to each well. The reaction mixture was incubated for 2 h at room temperature. Washing was repeated and 100 uL of substrate solution was added to each well followed by incubation for 30 min at room temperature. Stop solution (uL) was added to each well and immediately the readings were taken using an ELISA reader at 450 nm.
Hematoxylin and eosin (H&E) staining for histopathological analysis
Animal brains were kept in formaldehyde (10% v/v) solution after killing. Brain tissues were processed in a series of alcohol (50, 70, 80, 95, and 100%) for dehydration and treated with xylene solution. Now the tissues were kept in hot paraffin solution for 2 h and blocks were prepared in paraffin by embedding brain tissues. The brain sections of 5–10 μm thickness were cut and mounted on slides for staining using a microtome. Slides were dewaxed and stained with hematoxylin and eosin. Finally, slides were subjected to observation under alight microscope (Snyder et al. 2013).
Statistical analysis
Graph pad Prism software was used for data analysis. Values were measured as mean ± SEM. For behavioral and biochemical analysis, one-way ANOVA followed by Tukey’s test for multiple comparisons was applied however, Barnes maze test (behavioral parameter), oxidative stress parameters were analyzed by two-way ANOVA followed Bonferroni post hoc test for multiple comparisons. The significance level was considered at p < 0.05.
Results
Effect of sarcosine, risperidone, and combination of both on ketamine-induced hyperlocomotion
Testing animals for locomotor activity has been widely used in modeling the positive symptoms of schizophrenia, and ketamine in the present study induced hyperlocomotion in rats. However, sarcosine (300, 600 mg/kg, ip) and risperidone (0.2 mg/kg, ip) injection significantly decreased the locomotion in ketamine pre-treated groups as compared to the ketamine alone group. Combination of sarcosine (300 mg/kg, ip) and risperidone (0.2 mg/kg, ip) also significantly decreased the locomotion in ketamine pre-treated rats as compared to ketamine group alone and their individual effects [F(5, 23) = 23.39, p < 0.05] (Fig. 2).
Effect of sarcosine, risperidone, and combination of both on ketamine induced hyperlocomotion: one-way ANOVA followed by Tukey’s multiple comparison test was used in obtained data values and expressed as mean ± SEM. *p < 0.05 as compared to vehicle control, #p < 0.05 as compared to ket (30), $p < 0.05 as compared to [ket (30) + sar (300)], &p < 0.05 as compared to [ket (30) + risp (0.2)]. Ket (30): ketamine (30 mg/kg); sar (300): sarcosine (300 mg/kg); sar (600): sarcosine (600 mg/kg); risp (0.2 mg/kg): risperidone (0.2 mg/kg)
Effect of sarcosine, risperidone, and combination of both on open field test in ketamine-induced rats
-
(A)
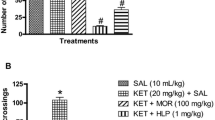
Ambulatory score Ambulatory score was evaluated by a total number of line crossings. Sarcosine (300, 600 mg/kg, ip) and risperidone (0.2 mg/kg, ip) in ketamine-induced rats significantly decreased the ambulatory score when compared to the ketamine group. Combination of sarcosine (300 mg/kg, ip) and risperidone (0.2 mg/kg, ip) in ketamine injected rats have significantly decreased the line crossing response as compared to ketamine treated group alone [F(5, 25) = 6.877, p < 0.0004] (Fig. 3A).
-
(B)
Rearing Sarcosine (300, 600 mg/kg, ip) and risperidone (0.2 mg/kg, ip) in ketamine-induced rats significantly reduced rearing. Combination drugs also significantly decreased ketamine-induced increased rearing [F(5, 25) = 11.84, p < 0.0001] (Fig. 3B).
-
(C)
Frequency of center entry Increased frequency of center crossing represents avoidance behavior in rats while being at the periphery shows anxiety. Sarcosine (300, 600 mg/kg, ip) and risperidone (0.2 mg/kg, ip) in ketamine injected rats significantly increased the exploratory behaviour measured by center entries when compared with ketamine group alone [F(5, 25) = 6.954, p < 0.05] (Fig. 3C).
Effect of sarcosine, risperidone, and combination of both on open field test in ketamine-induced rats: one-way ANOVA followed by Tukey’s multiple comparison test was used in obtained data values and expressed as mean ± SEM. *p < 0.05 as compared to vehicle control, #p < 0.05 as compared to ket (30). Ket (30): ketamine (30 mg/kg); sar (300): sarcosine (300 mg/kg); sar (600): sarcosine (600 mg/kg); risp (0.2 mg/kg): risperidone (0.2 mg/kg)
Effect of sarcosine, risperidone, and combination of both on sucrose preference test (SPT) in ketamine-induced rats
The sucrose preference test was measured by % consumption of sucrose preference. Percentage sucrose preference was significantly reduced in ketamine treated groups as compared to vehicle control representing negative symptoms of schizophrenia. Sarcosine (300, 600 mg/kg, ip), risperidone (0.2 mg/kg, ip) and their combination (sarcosine 300 mg/kg + risperidone 0.2 mg/kg) in ketamine pre-treated rats have significantly increased the percent sucrose preference when compared with ketamine alone group [F(5, 25) = 115.0, p < 0.05] (Fig. 4).
Effect of sarcosine, risperidone, and combination of both on sucrose preference test in ketamine induced rats: one-way ANOVA followed by Tukey’s multiple comparison test was used in obtained data values and expressed as mean ± SEM. *p < 0.05 as compared to vehicle control. #p < 0.05 as compared to ket (30). Ket (30): ketamine (30 mg/kg); sar (300): sarcosine (300 mg/kg); sar (600): sarcosine (600 mg/kg); risp (0.2 mg/kg): risperidone (0.2 mg/kg)
Effect of sarcosine, risperidone, and combination of both on novel object recognition (NORT) test in ketamine-induced rats
Novel object recognition test is evaluated by discrimination index (DI). Sarcosine (300, 600 mg/kg, ip), risperidone (0.2 mg/kg, ip) and their combination (sarcosine 300 mg/kg + risperidone 0.2 mg/kg) in ketamine pre-treated rats have significantly increased DI as compared to ketamine group [F(5, 25) = 14.91, p < 0.05] (Fig. 5).
Effect of sarcosine, risperidone, and combination of both on novel object recognition test in ketamine-induced rats: one-way ANOVA followed by Tukey’s multiple comparison test was used in obtained data values and expressed as mean ± SEM. *p < 0.05 as compared to vehicle control. #p < 0.05 as compared to ket (30). Ket (30): ketamine (30 mg/kg); sar (300): sarcosine (300 mg/kg); sar (600): sarcosine (600 mg/kg); risp (0.2 mg/kg): risperidone (0.2 mg/kg)
Effect of sarcosine, risperidone, and combination of both on Barnes maze test in ketamine-induced rats
The Barnes maze is used to evaluate spatial learning and memory. It was assessed by two parameters, latency (seconds) and the number of errors. Ketamine (30 mg/kg) administration significantly increased latency and number of errors in the Barnes maze test when compared with vehicle control on days 4, 5, 6, and 7. Sarcosine (300, 600 mg/kg, ip) treatment significantly decreased latency to enter the escape box only on day 7 as compared to ketamine. Risperidone (0.2 mg/kg) and its combination with sarcosine (300 mg/kg) significantly decreased latency to enter escape boxes compared to the ketamine group on days 5, 6, and 7. Sarcosine (300, 600 mg/kg, ip) and its combination with risperidone significantly decreased number of errors in ketamine treated rats when compared with ketamine group on day 4, 5, 6 and 7 [F(6, 175) = 31.74, p < 0.05] [F(6, 175) = 40.02, p < 0.05] (Fig. 6A, B).
Effect of sarcosine, risperidone, and combination of both on Barnes maze test in ketamine-induced rats. A Latency, B number of errors: two-way ANOVA followed by Bonferroni’s post hoc test was used in obtained data values and expressed as mean ± SEM. Ket (30) *p < 0.05 as compared to vehicle control and #p < 0.05 as compared to ket (30). Ket (30): ketamine (30 mg/kg); sar (300): sarcosine (300 mg/kg); sar (600): sarcosine (600 mg/kg); risp (0.2 mg/kg): risperidone (0.2 mg/kg)
Effect of sarcosine, risperidone, and combination of both on parameters of oxidative stress in ketamine-induced rats
Sarcosine (300, 600 mg/kg, ip), risperidone (0.2 mg/kg, ip) and their combination significantly increased SOD, GSH, and catalase levels in isolated brain regions (cortex and hippocampus) as compared to ketamine (30 mg/kg) (Fig. 7A–C). A significant increase in MDA levels were observed in cortex and hippocampus of rats treated with ketamine for 10 days. However, sarcosine (300, 600 mg/kg, ip), risperidone (0.2 mg/kg, ip) and their combination (sarcosine 300 mg/kg + risperidone 0.2 mg/kg) treatment significantly decreased MDA levels as compared to ketamine group, thus, suggesting attenuation of oxidative stress in rat cortex and hippocampus (Fig. 7D). SOD [F(5, 12) = 16.86, p < 0.05] [F(5, 12) = 7.989, p < 0.05], GSH [F(5, 12) = 31.53, p < 0.05] [F(5, 12) = 6.531, p < 0.05], catalase [F(5, 12) = 4.623, p < 0.05] [F(5, 12) = 5.286, p < 0.05] and MDA [F(5, 12) = 8.212, p < 0.05] [F(5, 12) = 10.62, p < 0.05] (Fig. 7A–D).
Effect of sarcosine, risperidone and combination of both on parameters of oxidative stress in ketamine induced rats. A SOD, B GSH, C Catalase and D LPO: two-way ANOVA followed by Bonferroni’s post hoc test was used in obtained data values and expressed as mean ± SEM. *p < 0.05 as compared to vehicle control, #p < 0.05 as compared to ket (30), $p < 0.05 as compared to sar (300). Ket (30): ketamine (30 mg/kg); sar (300): sarcosine (300 mg/kg); sar (600): sarcosine (600 mg/kg); risp (0.2 mg/kg): risperidone (0.2 mg/kg)
Effect of sarcosine, risperidone, and combination of both on plasma nitrite level in ketamine-induced rats
Sarcosine (300, 600 mg/kg, ip), risperidone (0.2 mg/kg, ip) and combination (sarcosine 300 mg/kg + risperidone 0.2 mg/kg) treatment significantly decreased plasma nitrite levels as compared to ketamine group [F(5, 12) = 7.136, p<0.05] (Fig. 8).
Effect of sarcosine, risperidone, and combination of both on plasma nitrite level in ketamine-induced rats: one-way ANOVA followed by Tukey’s multiple comparison test was used in obtained data values and expressed as mean ± SEM. *p < 0.05 as compared to vehicle control, #p < 0.05 as compared to ket (30). Ket (30): ketamine (30 mg/kg); sar (300): sarcosine (300 mg/kg); sar (600): sarcosine (600 mg/kg); risp (0.2 mg/kg): risperidone (0.2 mg/kg)
Effect of sarcosine, risperidone, and combination of both on mitochondrial complexes (I, II, III, and IV) in cortex and hippocampus of ketamine-induced rats
Sarcosine (300, 600 mg/kg, ip), risperidone (0.2 mg/kg, ip) and combination (sarcosine 300 mg/kg + risperidone 0.2 mg/kg) treatment significantly increased the activity of these complexes in both cortex and hippocampus when compared with ketamine group (Fig. 9A–D). Complex-I [F(5, 12) = 22.53, p < 0.05] [F(5, 12) = 11.94, p < 0.05], complex-II [F(5, 12) = 11.20, p < 0.05] [F(5, 12) = 51.86, p < 0.05], complex-III [F(5, 12) = 20.47, p < 0.05] [F(5, 12) = 21.52, p < 0.05] and complex-IV [F(5, 12) = 57.34, p < 0.05] [F(5, 12) = 58.57, p < 0.05].
Effect of sarcosine, risperidone and combination of both on mitochondrial complexes I, II, III and IV in cortex and hippocampus of ketamine-induced rats. A Complex-I, B Complex-II, C Complex-III, D Complex-IV: one-way ANOVA followed by Tukey’s multiple comparison test was used in obtained data values and expressed as mean ± SEM. *p < 0.05 as compared to vehicle control, #p < 0.05 as compared to ketamine, $p < 0.05 as compared to [ket + sar (300)] and &p < 0.05 as compared to [ket + sar (300) + risp (0.2)]. Ket (30): ketamine (30 mg/kg); sar (300): sarcosine (300 mg/kg); sar (600): sarcosine (600 mg/kg); risp (0.2 mg/kg): risperidone (0.2 mg/kg)
Effect of sarcosine, risperidone, and combination of both on interleukin-6 (IL-6) in isolated brain regions (cortex and hippocampus) of ketamine-induced rats
Sarcosine (300, 600 mg/kg, ip), risperidone (0.2 mg/kg, ip) and combination (sarcosine 300 mg/kg + risperidone 0.2 mg/kg) treatment significantly decreased the levels of IL-6 in cortex (Fig. 10A) and hippocampus (Fig. 10B) when compared with ketamine treated animals. [F5, 12 = 8.963, p < 0.05] [F5, 12 = 10.20, p < 0.05].
Effect of sarcosine, risperidone, and combination of both on interleukin-6 (IL-6) in cortex and hippocampus of ketamine-induced rats: one-way ANOVA followed by Tukey’s multiple comparison test was used in obtained data values and expressed as mean ± SEM. *p < 0.05 as compared to vehicle control, #p < 0.05 as compared to ketamine. Ket (30): ketamine (30 mg/kg); sar (300): sarcosine (300 mg/kg); sar (600): sarcosine (600 mg/kg); risp (0.2 mg/kg): risperidone (0.2 mg/kg)
Effect of sarcosine on histopathological changes in cortex and hippocampus region of ketamine-induced rats
The tissue architecture of the cortical and hippocampal region of vehicle control was found to be normal. Histopathological analysis of ketamine-induced rats demonstrated necrosis (evident by hyperchromatic pyknotic nuclei) and perinuclear vacuolated tissue in rat cortical and hippocampal (CA3) region while no morphological changes were found in the hippocampus, especially CA1 and CA2 region. Treatment with sarcosine caused significantly diminished ketamine-induced histopathological hyperchromatic nuclei and perinuclear vacuolization in the cortex and hippocampus (CA3) region (Fig. 11A–D).
Effects of sarcosine on histopathological changes in cortex and hippocampus region of ketamine-induced rats: necrosis (evident by hyperchromatic pyknotic nuclei) and perinuclear vacuolated tissue in rat cortical and hippocampal (CA3) region represented by yellow and red arrow, respectively. Normal cells are represented by black arrows. No morphological changes were found in the CA1 and CA2 regions of the hippocampus (A–D)
Discussion
NMDA receptor antagonist drugs like ketamine often cause symptoms and behavioral alterations closely related to observed in schizophrenia and are well-established tools for inducing schizophrenia-like behavior in animal model. Moreover, several clinical and animal studies have demonstrated that alteration in glutamate transmission is involved in the pathophysiology of schizophrenia. This is further supported by several environmental and genetic factors converging on the N-methyl-d-aspartate (NMDA) receptor-mediated glutamate transmission and resulting in NMDA receptor hypofunction in the limbic system (Zhang et al. 2009). However, direct activation of NMDA receptors using agonists can enhance excitotoxicity globally. Given that there may be less of a chance for neurotoxicity and seizures, the glycine binding site is a more desirable target for improving NMDAR function than the glutamate binding site. Because they might raise the glycine concentration available to activate these receptors, sarcosine and other GlyT1 inhibitors offer a potential method for improving NMDAR functionality (Reilly et al. 2006). Therefore, a better approach is focused on the glycine modulatory site of the NMDA receptor (Fig. 12).
Risperidone targets dopamine receptors (D2) and is ideal for positive and negative symptoms of schizophrenia. Additionally, a study suggested that risperidone worsens spatial memory in patients with schizophrenia (Zhou et al. 2018). While many other studies decline the fact that atypical antipsychotic risperidone worsens cognitive impairments. A recent clinical trial suggested the role of risperidone in improving cognitive functions in patients with schizophrenia. The possible mechanism of action for improving memory function might be due to strong antagonism of the D2 receptor and alteration in the serotonergic system (Moghaddam and Javitt 2012).
Therefore, the author tests the alternative therapy to check the other possible molecular mechanism of sarcosine and its combination with risperidone. We found that all the symptoms domains of schizophrenia, i.e. positive negative and cognitive are reversed by sarcosine as well as risperidone and their combination. In relation to the results, we found that sarcosine might be acting on other molecular targets including glycine transporter inhibition. Recent study found attenuated psychotic effects of NMDA receptor antagonists after using glycine and d-serine glycine site agonists (Tollefson et al. 1995). Hence, investigation and identification of positive modulators of NMDA receptors using the ketamine model of schizophrenia can serve as a prospect and possibility for the treatment of schizophrenia.
The present study evaluated the effect of sarcosine after the administration of ketamine in rats. Ketamine administration for 10 days produced a pattern of neurochemical and behavioral changes that produced a model of schizophrenia in this study. Furthermore, chronic administration of ketamine produced both positive (hyperlocomotion) and negative (anhedonia) symptoms. Moreover, it decreased discrimination capacity in novel object recognition tests and increased latency and number of errors to find the escape cage in the Barnes maze test, thus, depicting cognitive deficits, these findings are in-line with previous findings on ketamine (Kandratavicius et al. 2015). There are several reports that GlyT-1 inhibitors are the positive modulator of NMDA receptors and improve cognitive deficits in animal behavior (Heresco-Levy 2006).
Ketamine-induced hyperlocomotion represented positive symptoms of psychosis. Overactive dopaminergic system in rodents leads to hyperlocomotion, (horizontal locomotor activity and rearing) or stereotypy at higher doses. Sarcosine significantly reduced hyperlocomotion and combination treatment with risperidone produced a potentiated effect in reducing hyperlocomotion. In addition, sarcosine significantly reversed ketamine-induced anhedonia, exploratory behavior and produced a significant increase in discrimination index, indicating improvement in cognition. Similarly, improvement in cognitive function was observed when tested in the Barnes maze. We found that sarcosine decreased latency and number of errors in the Barnes maze test and significantly increased discrimination ability in the novel object recognition test. Thus, we can say that sarcosine was found to be effective in attenuating positive, negative, and cognitive symptoms. Sarcosine treatment in this study is in synchronous with the findings of previous studies. Preliminary clinical studies suggest that sarcosine improves the positive and negative symptoms of schizophrenia. Moreover, in previous clinical and preclinical evaluations, sarcosine alone or add-on treatment has been reported to attenuate all three symptoms’ domains in schizophrenic patients (Pei et al. 2019; Ben-Azu et al. 2016).
To activate the NMDA receptors, sarcosine targets the glycine transporter-1 and make the concentration of glycine more available for binding with the receptors. However, other mechanisms of sarcosine should also be considered like oxidative stress, mitochondrial complexes and inflammatory markers. The brain is susceptible to oxidative stress and degeneration due to its low antioxidant enzyme activity. The studies suggested, NMDA receptor hypofunction by ketamine leads to oxidative stress and mitochondrial dysfunction (Phensy et al. 2017; Struys et al. 2010). The present study shows, ketamine administration increased lipid peroxidation, plasma nitrite and decreased the levels of antioxidant defence systems like SOD, GSH, and catalase. Similarly, numerous preclinical studies have demonstrated increased oxidative damage and neuroinflammation in ketamine-induced oxidative stress Valvassori et al. 2021. In addition, the ketamine-induced model has also mediated mitochondrial dysfunction through the oxidative stress pathway. Both oxidative stress and mitochondrial dysfunction were reversed by sarcosine, concurrent with previous studies (Pinto et al. 2012). Therefore, the inter-link between the oxidative stress normalizing character and glycine can be drawn from our study. GlyTI also regulates the NF-κB pathway, which is somehow accountable for cytokine release. Therefore, our results with 1L-6 give further indication that sarcosine reduces cytokine-mediated neuroinflammation through this pathway.
Sarcosine's potential for use in the treatment of schizophrenia can be attributed to a number of factors. Sarcosine is an endogenous compound that, when administered intraperitoneally, enters brain and inhibits the glycine transporter-1. Sarcosine as glycine transporter inhibitor was previously tested on cognitive symptom domains in patients with schizophrenia. However, other studies were showing different findings even with the opposite outcomes (Jentzmik et al. 2011) (Moller et al. 2015). It became the basis for us to find the significant effect of sarcosine in three different symptom domains. In this study, ketamine administration also increased the levels of IL-6 in the cortex and hippocampus, suggesting a role of neuroinflammation in ketamine-induced schizophrenia-like behavior. Treatment with sarcosine alone and in combination with risperidone decreased the levels of IL-6. Reversal of neuroinflammation with sarcosine is in line with an already reported study (Pinto et al. 2012).
Oxidative stress, mitochondrial dysfunction and neuroinflammatory markers could have strong link with neurotransmitters involved in schizophrenia. Hence, providing an inference of the role sarcosine on neurotransmitters involved in schizophrenia (Yadav et al. 2018). Treatment with sarcosine caused diminished ketamine-induced histopathological lesions such as hyperchromatic nuclei and perinuclear vacuolization in the cortex and hippocampus [46]. These results further strengthen and support the findings that sarcosine has normalized schizophrenia-like behavior. However, the limitation of the study can be counted that neurotransmitters, proteins, and gene expression evaluation of glycine transporter and NMDA were not done. This could be the futuristic approach to unravel the treatment mechanism of schizophrenia. The reason for superior treatment action of the combination therapy might be due to synergistic action of the combination treatment on multiple receptors involved in the generation of schizophrenic symptoms. Risperidone possesses more extrapyramidal side effects and also increases prolactin levels including weight gain, metabolic problems, cardiac effects, sedation and seizures. Furthermore, sarcosine is present endogenously in brain and possess lesser amount of adverse effects as compared to risperidone (anxiety, aggression, agitation, etc.).
Our current work is preliminary study which is a preclinical one and even though most of the results showed improvement with risperidone treatment alone which was found to comparable with the effects of sarcosine. Still the improving action of sarcosine cannot be ruled out. Further animal studies using different animal models or different combination of sarcosine can be investigated further. It can possibly give rise to superior results to risperidone if studied clinically in future.
Conclusion
The reversal of cognitive impairment, hyperlocomotion, increased exploratory behavior, and decreased sucrose preference in ketamine treated animals indicated that sarcosine may have a beneficial effect in the treatment of three symptoms’ domains involved in schizophrenia: positive, negative, and cognitive probably by affecting glutamate transmission with increasing glycine concentration at the synapse. The present study demonstrated that sarcosine is capable of reversing the behavioral alterations as well as biochemical and neuroinflammatory changes induced by ketamine injection repeatedly.
Data availability
The authors confirm that the data supporting the project's findings are included in the publication and its supplemental materials and that the corresponding author can provide the raw data upon reasonable request.
Abbreviations
- CA:
-
Cornu ammonis
- DA:
-
Dopamine
- DI:
-
Discrimination index
- DMSO:
-
Dimethyl sulfoxide
- GlyT-1:
-
Glycine transporter inhibitor
- GlyTIs:
-
Glycine transporter inhibitors
- GSH:
-
Reduced glutathione
- ip:
-
Intraperitoneally
- IL-6:
-
Interleukin-6
- Ket:
-
Ketamine
- MDA:
-
Malondialdehyde
- MTT:
-
Methylthiazolyldiphenyl-tetrazolium bromide
- NADH:
-
Nicotinamide adenine dinucleotide
- NMDA:
-
N-methyl-d-aspartate
- NORT:
-
Novel object recognition test
- OFT:
-
Open field test
- RI:
-
Recognition index
- Risp:
-
Risperidone
- Sar:
-
Sarcosine
- SOD:
-
Superoxide dismutase
- SPT:
-
Sucrose preference
References
Akhtar A, Dhaliwal J, Saroj P, Uniyal A, Bishnoi M, Sah SP (2020) Chromium picolinate attenuates cognitive deficit in ICV-STZ rat paradigm of sporadic Alzheimer’s-like dementia via targeting neuroinflammatory and IRS-1/PI3K/AKT/GSK-3β pathway. Inflammopharmacology 28:385–400
Anderson G, Maes M (2013) Schizophrenia: linking prenatal infection to cytokines, the tryptophan catabolite (TRYCAT) pathway, NMDA receptor hypofunction, neurodevelopment and neuroprogression. Prog Neuro-Psychopharmacol Biol Psychiatry 42:5–19
Batinic B (2019) Cognitive models of positive and negative symptoms of schizophrenia and implications for treatment. Psychiatr Danub 31:181–184
Ben-Azu B, Aderibigbe AO, Ajayi AM, Iwalewa EO (2016) Neuroprotective effects of the ethanol stem bark extracts of Terminalia ivorensis in ketamine-induced schizophrenia-like behaviors and oxidative damage in mice. Pharm Biol 54(12):2871–2879
Bose R, Sutherland GR, Pinsky C (1989) Biological and methodological implications of prostaglandin involvement in mouse brain lipid peroxidation measurements. Neurochem Res 14:217–220
Bruijnzeel AW, Qi X, Guzhva LV, Wall S, Deng JV, Gold MS, Febo M, Setlow B (2016) Behavioral characterization of the effects of cannabis smoke and anandamide in rats. PLoS ONE 11:e0153327
Bueno-Antequera J, Munguía-Izquierdo D (2020) Exercise and schizophrenia. Adv Exp Med Biol 1228:317–332
Cassina A, Radi R (1996) Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328:309–316
Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G (2012) Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Novel Antischizophr Treat 267–295
Escarabajal D, Miquel M, Aragon CM (2000) A psychopharmacological study of the relationship between brain catalase activity and ethanol-induced locomotor activity in mice. J Stud Alcohol 61:493–498
Fattal O, Budur K, Vaughan AJ, Franco K (2006) Review of the literature on major mental disorders in adult patients with mitochondrial diseases. Psychosomatics 47:1–7
Fleischhacker WW, Podhorna J, Gröschl M, Hake S, Zhao Y, Huang S, Keefe RS, Desch M, Brenner R, Walling DP, Mantero-Atienza E (2021) Efficacy and safety of the novel glycine transporter inhibitor BI 425809 once daily in patients with schizophrenia: a double-blind, randomised, placebo-controlled phase 2 study. Lancet Psychiatry 8:191–201
Galpern WR, Cudkowicz ME (2007) Coenzyme Q treatment of neurodegenerative diseases of aging. Mitochondrion 7:S146–S153
Gawel K, Gibula E, Marszalek-Grabska M, Filarowska J, Kotlinska JH (2019) Assessment of spatial learning and memory in the Barnes maze task in rodents—methodological consideration. Naunyn-Schmiedeb. Arch. Pharmacol 392(1):1–8
Genius J, Geiger J, Dölzer AL, Benninghoff J, Giegling I, Hartmann AM, Möller HJ, Rujescu D (2013) Glutamatergic dysbalance and oxidative stress in in vivo and in vitro models of psychosis based on chronic NMDA receptor antagonism. PLoS ONE 8:5939
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Analbiochem 126:131–138
Han ZM, Huang HM, Wang FF (2015) Brain-derived neurotrophic factor gene-modified bone marrow mesenchymal stemcells. Exp Ther Med 9(2):519–22
Harada K, Nakato K, Yarimizu J, Yamazaki M, Morita M, Takahashi S, Aota M, Saita K, Doihara H, Sato Y, Yamaji T (2012) A novel glycine transporter-1 (GlyT1) inhibitor, ASP2535 (4-[3-isopropyl-5-(6-phenyl-3-pyridyl)-4H-1, 2, 4-triazol-4-yl]-2, 1, 3-benzoxadiazole), improves cognition in animal models of cognitive impairment in schizophrenia and Alzheimer’s disease. Eur J Pharmacol 685(1–3):59–69
Heresco-Levy U (2006) Adding sarcosine, but not d-serine, to risperidone improves symptoms in people with acute phase schizophrenia. Evid-Based Ment Health 9:48
Jentzmik F, Stephan C, Lein M, Miller K, Kamlage B, Bethan B, Kristiansen G, Jung K (2011) Sarcosine in prostate cancer tissue is not a differential metabolite for prostate cancer aggressiveness and biochemical progression. J Urol 185(2):706–711
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Kandratavicius L, Balista PA, Wolf DC, Abrao J, Evora PR, Rodrigues AJ, Chaves C, Maia-de-Oliveira JP, Leite JP, Dursun SM (2015) Effects of nitric oxide-related compounds in the acute ketamine animal model of schizophrenia. BMC Neurosci 16:1–9
Karl T, Duffy L, O’Brien E, Matsumoto I, Dedova I (2006) Behavioural effects of chronic haloperidol and risperidone treatment in rats. Behav Brain Res 171(2):286–294
King A, Selak MA, Gottlieb E (2006) Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene 25:4675–4682
Kono Y (1978) Generation of superoxide radicals during auto-oxidation of hydroxyl-amine hydrochloride an assay for SOD. Arch BiochemBiophys 186:189–195
Kumar V, Ahmad M, Najmi A, Akhtar M (2016) Effect of sarcosine (a glycine transport 1 inhibitor) and risperidone (an atypical antipsychotic drug) on MK-801 induced learning and memory deficits in rats. Drug Res 66:11–17
Moghaddam B, Javitt D (2012) From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 37:4–15
Moller M, Swanepoel T, Harvey BH (2015) Neurodevelopmental animal models reveal the convergent role of neurotransmitter systems, inflammation, and oxidative stress as biomarkers of schizophrenia: implications for novel drug development. ACS Chem Neurosci 6(7):987–1016
Nikiforuk A, Popik P (2012) Effects of quetiapine and sertindole on subchronic ketamine-induced deficits in attentional set-shifting in rats. Psychopharmacology 220:65–74
Pei JC, Hung WL, Lin BX, Shih MH, Lu LY, Luo DZ, Tai HC, Studer V, Min MY, Lai WS (2019) Therapeutic potential and underlying mechanism of sarcosine (N-methylglycine) in N-methyl-d-aspartate (NMDA) receptor hypofunction models of schizophrenia. J Psychopharmacol 33:1288–1302
Phensy A, Driskill C, Lindquist K, Guo, Jeevakumar V, Fowler B, Du H, Kroener S (2017) Antioxidant treatment in male mice prevents mitochondrial and synaptic changes in an NMDA receptor dysfunction model of schizophrenia. ENeuro 4(4)
Pinto MCX, Mourão FAG, Binda NS, Leite HR, Gomez MV, Massensini AR, Gomez RS (2012) Pharmacological induction of ischemic tolerance in hippocampal slices by sarcosine preconditioning. Neurochem Int 61:713–720
Prabakaran S, Swatton J, Ryan M, Huffaker S, Huang JJ, Griffin J, Wayland M, Freeman T, Dudbridge F, Lilley K (2004) Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry 9:684–697
Rademann P, Weidinger A, Drechsler S, Meszaros A, Zipperle J, Jafarmadar M, Dumitrescu S, Hacobian A, Ungelenk L, Röstel F, Kaszaki J (2017) Mitochondria-targeted antioxidants SkQ1 and MitoTEMPO failed to exert a long-term beneficial effect in murine polymicrobial sepsis. Oxid Med Cell Longev
Reilly JL, Harris MS, Keshavan MS, Sweeney JA (2006) Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch Gen Psychiatry 63(11):1189–1197
Rosenfeld CS, Ferguson SA (2014) Barnes maze testing strategies with small and large rodent models. JoVE (J Vis Exp) e51194
Snyder MA, Adelman AE, Gao WJ (2013) Gestational methylazoxymethanol exposure leads to NMDAR dysfunction in hippocampus during early development and lasting deficits in learning. Neuropsychopharmacology 38:328–340
Steeds H, Carhart-Harris RL, Stone JM (2015) Drug models of schizophrenia. Ther Adv Psychopharmacol 5:43–58
Struys EA, Heijboer AC, van Moorselaar J, Jakobs, Blankenstein MA (2010) Serum sarcosine is not a marker for prostate cancer. Ann Clin Biochem 47(3):282–282
Strzelecki D, Kałużyńska O, Wysokiński A (2016) BDNF serum levels in schizophrenic patients during treatment augmentation with sarcosine (results of the PULSAR study). Psychiatry Res 242:54–60
Sun ZY, Gu HS, Chen X, Zhang L, Li XM, Zhang JW, Li L (2017) A novel flavanone derivative ameliorates cuprizone-induced behavioral changes and white matter pathology in the brain of mice. Psychiatry Res 257:249–259
Tanqueiro SR, Mouro FM, Ferreira CB, Freitas CF, Fonseca-Gomes J, Simões do Couto F, Sebastião AM, Dawson N, Diógenes MJ (2021) Sustained NMDA receptor hypofunction impairs brain-derived neurotropic factor signalling in the PFC, but not in the hippocampus, and disturbs PFC-dependent cognition in mice. J Psychopharmacol 35(6):730–743
Tollefson GD, Beasley CM, Tran PV, Sanger T (1995) Olanzapine: an exciting atypical antipsychotic—the clinical experience. Eur Neuropsychopharmacol 3:256
Valvassori SS, Cararo JH, Menegas S, Possamai-Della T, Aguiar-Geraldo JM, Araujo SL, Mastella GA, Quevedo J, Zugno AI (2021) Haloperidol elicits oxidative damage in the brain of rats submitted to the ketamine-induced model of schizophrenia. Brain Res Bull 170:246–53
Wang C, Inselman A, Liu S, Liu F (2020) Potential mechanisms for phencyclidine/ketamine-induced brain structural alterations and behavioral consequences. Neurotoxicology 76:213–219
Yadav M, Parle M, Jindal DK, Dhingra S (2018) Protective effects of stigmasterol against ketamine-induced psychotic symptoms: possible behavioral, biochemical and histopathological changes in mice. Pharmacol Rep 70:591–599
Zhang HX, Hyrc K, Thio LL (2009) The glycine transport inhibitor sarcosine is an NMDA receptor co-agonist that differs from glycine. J Physiol 587(13):3207–3220
Zhou Y, Li G, Li D, Cui H, Ning Y (2018) Dose reduction of risperidone and olanzapine can improve cognitive function and negative symptoms in stable schizophrenic patients: a single-blinded, 52-week, randomized controlled study. J Psychopharmacol 32(5):524–532
Acknowledgements
The authors are grateful to the All India Council for Technical Education, New Delhi, for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there was no conflict of interest.
Additional information
Communicated by Sreedharan Sajikumar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, A., Akhtar, A., Kuhad, A. et al. Sarcosine (glycine transporter inhibitor) attenuates behavioural and biochemical changes induced by ketamine, in the rat model of schizophrenia. Exp Brain Res 241, 451–467 (2023). https://doi.org/10.1007/s00221-022-06530-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-022-06530-4