Abstract
Azotobacter chroococcum and A. salinestris do not possess significant and distinct morphological and physiological differences and are often mistaken with each other in microbiological research. In this study, 12 isolates of Azotobacter isolated by standard protocol from soils were identified morphologically and physiologically as A. chroococcum. The isolates were more closely investigated for the molecular differentiation and diversity of A. chroococcum and A. salinestris. For this purpose, the ARDRA technique including HpaII, RsaI, and AluI restriction enzymes, and REP, ERIC, and BOX markers were used. The nifD and nifH genes were also utilized to evaluate the molecular identification of these two species. The 16S rDNA evaluation showed that only four out of the 12 isolates were identified as A. chroococcum and the rest were A. salinestris. The results revealed that HpaII was able to differentiate A. chroococcum from A. salinestris whereas RsaI and AluI were not able to separate them. Moreover, BOX and REP markers were able to differentiate between A. chroococcum and A. salinestris. However, ERIC marker and nifD and nifH genes were unable to separate these species. According to the results, HpaII restriction enzyme is suggested to save time and cost. BOX and REP markers are recommended for differentiation and clear discrimination not only between A. chroococcum and A. salinestris but also among their strains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Azotobacter is a Gram-negative, aerobe, heterotrophic, non-symbiotically nitrogen fixer, and polymorphism from the rod, cocci to ovoid, which is capable of cyst formation [1]. Azotobacter belongs to the Pseudomonadaceae family and seven species of this genus have been identified so far, including Azotobacter chroococcum, A. vinelandii, A. Beijerinckii, A. nigricans, A. paspali, A. salinestris and A. armeniacus [1]. Laboratory identification of Azotobacter species is difficult because of their morphological similarity, especially between A. chroococcum and A. salinestris. A. chroococcum is the most famous species of Azotobacter, which has considerable variations among its strains in terms of cell shape, colony size, and pigmentation [2]. Page [3] reported that some strains of A. chroococcum isolated from slightly saline soils of western Canada were dependent on sodium (Na+) for growth. Page and Shivprasad [4] proposed a new species named A. salinestris for the Na+-dependent strains of A. chroococcum. These two bacterial species have many physiological and morphological similarities and, in many cases, are mistaken for each other. Therefore, it is important to develop a simple and quick molecular identification method to differentiate between the two species. The sequence of 16S rDNA as a molecular tool has been widely used to identify bacteria to the level of genus or species. For more accurate identification and differentiation between these two species, other molecular techniques are required. Amplified ribosomal DNA restriction analysis (ARDRA) is commonly used for the microbial identification and classification at the level of genus and species [5, 6]. The 16S-ARDRA is based on amplified ribosomal DNA restriction analysis of 16S rDNA, which is a simple method routinely used in laboratories owing to not requiring specialized equipment and being cheaper than 16S rDNA sequencing. In the ARDRA technique, strains are identified by comparing the gel electrophoresis profiles of restriction fragments of the strains with the profiles of reference strains [7]. There is a small volume of the literature on the molecular differences between A. chroococcum and A. salinestris. The power of differentiation of ARDRA depends on the type of restriction enzymes. There are various PCR fingerprinting techniques like ERIC, REP, and BOX-PCR for the amplification of repetitive DNA sequences present in the genomes of most Gram-negative and several Gram-positive bacteria. Rep-PCR is based on primers targeting the 35- to 40-bp repetitive extragenic palindromic sequence [8]. ERIC-PCR is based on primers targeting the highly conserved enterobacterial repetitive intergenic consensus (124- to 127-bp). ERIC-PCR is highly sensitive and useful in detecting microorganisms from any environment [9, 10]. BOX-PCR is based on primers targeting the highly conserved repetitive DNA sequences of the BOXA subunit (59 bp) of the BOX element [11]. BOX-PCR is superior to other techniques in creating distinctive fingerprint patterns; however, the other two methods were used as primary methods for genotyping. Most importantly, BOX-PCR does not share any sequence homology with either REP or ERIC-PCR [12, 13]. The advantages of repetitive element-based PCR (Rep-PCR) include the ability to distinguish between closely related strains, as well as being a simple, reliable, cheap, and quick method [14].
Therefore, in this study, some types of enzymes were examined. Moreover, different Rep-PCR techniques including REP, ERIC, and BOX were compared for the molecular differentiation of native A. chroococcum and A. salinestris, and their strain diversities were also evaluated. In addition, nifD and nifH genes were used to evaluate the molecular identification of the two species.
Materials and methods
Soil sampling and bacterial isolation
Rhizosphere soils were sampled both from cultivated (under crop plants) and uncultivated lands (under pastures) in arid and semi-arid regions across Tehran, Alborz, Qazvin and Qom Provinces of Iran. For soil sampling, whole plants with roots and surrounding soil were removed from the ground. The samples were transferred to the laboratory and stored at 4 °C for further investigations. Fifty gram of each soil sample were mixed with 0.5 g of pyruvic acid sodium salt and saturated with distilled sterile water. The soil paste was transferred to a sterile 9 cm Petri dish and the surface of the paste was smoothed and leveled by a sterile spatula. After 5–10 days incubation at 30 °C, the soil paste—plates presenting growth of A. chroococcum were revealed by the appearance of brown to black colonies [2]. The Winogradsky medium was used for growth of bacteria [1]. The geographical coordinates and some characteristics of the soils from which Azotobacter was separated are presented in Table 1. Gram stain, pigmentation, cell morphology, and cyst formation were employed for purification and individual identification of the isolates [1]. Two control strains of A. salinestris (strain AS-FE: CCSM-B00469) and A. chroococcum (strain AC-SW15: CCSM-B00477) were taken from the culture collection of soil microorganism (CCSM, WDCM 891) of the Soil and Water Research Institute, Iran.
16S rDNA amplification and ARDRA analysis
Genomic 16S rDNA was amplified using 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-TACGGTTACCTTGTTACGACTT-3′) primers [15]. PCR was performed with a Techne, Genius FGEN02TP thermocycler in a total volume of 50 μl solution containing 5 μl of PCR buffer (10 ×), 1 μl of 10 mM dNTP, 1 μl of 50 mM MgCl2, 1 μl of each primer (10 pmol), 0.6 μl of Taq DNA polymerase (Smartaq; 5 U/µl) and 3 μl of DNA template. The amplification condition was 5 min at 94 °C for initial denaturation; 30 cycles of 45 s at 94 °C, 45 s at 52 °C, and 2 min at 72 °C, and the final extension at 72 °C for 7 min [16]. Amplified 16S rDNA products were digested with AluI, HpaII, and RsaI restriction enzymes for 2 h at 37 °C according to the manufacturer’s instructions.
Amplification of nifD and nifH genes
The nitrogen-fixing nifD and nifH genes were amplified using the primers FdB260 (TCRTTIGCIATRTGRTGNCC)/FdB261 (TGGGGICCIRTIAARGAYATG) and PolF (TGCGAYCCSAARGCBGACTC)/PolR (ATSGCCATCATYTCRCCGGA), respectively [17, 18]. The reaction components for the amplification of nifD and nifH genes were 5 μl of PCR buffer (10 ×), 0.5 μl of 20 mM dNTP, 1.5 μl of 50 mM MgCl2, 2 μl of each primer (10 pmol), 0.6 μl of Taq DNA polymerase (Smartaq; 5 U/µl), and 1.5 μl of DNA template. The amplification conditions were 5 min initial denaturation at 94 °C; 35 cycles of 1 min denaturation at 94 °C; 1 min annealing step at 53 °C (nifD) or 57 °C (nifH); 1 min extension at 72 °C, and a final extension for 10 min at 72 °C.
Rep-PCR fingerprinting and profile analyses
Genomic fingerprints were generated using a single primer BOXA1R (5′-CTACGGCAAGGCGACGCTGACG-3′) and the primer pairs of ERIC1R (5′-ATGTAAGCTCCTGGGGATTCAC-3′)/ERIC2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′); and REP1R (5′-IIIICGICGICATCIGGC-3′)/REP2I (5′-ICGICTTATCIGGCCTAC-3′) [19, 20]. The optimized Rep-PCR reactions were performed in a total volume of 30 μl solution containing 2 μl of DNA template, 3 μl of PCR buffer (10 ×), 0.24 μl of 20 mM dNTP, 1.2 μl of 50 mM MgCl2, 1.8 μl of each primer (10 pmol), 0.48 μl of Taq DNA polymerase (Smartaq; 5 U/µl), and 1 μl of each primer (10 pmol). The amplification program comprised 1 cycle of 6 min at 95 °C; 35 cycles of 1 min at 94 °C; then 1 min at 40 °C (REP) or 50 °C (BOX) or 53 °C (ERIC); then 4 min at 72 °C, and finally, 1 cycle of 10 min at 72 °C.
The 16S rDNA, nifD and nifH products were analyzed by gel electrophoresis on 1% agarose gel stained with 5 μl of SimplySafe™ (EURX) for 1.5 h at a constant voltage (100 V). The digested products were electrophoresed on 2% (w/v) agarose gels stained with 5 μl of SimplySafe™ (EURX) for 2 h at 90 V. For Rep-PCR, the gels were run on 1.5% agarose gel for 2 h at 100 V. A 100-bp DNA ladder (CinnaGene Inc., Iran) was used as a DNA size marker.
The 16S rDNA, nifD and nifH products were purified and sequenced using the mentioned primers by the Bioneer Company, South Korea. The sequences were compared with those of the most closely related bacterial species using the BLAST program on the NCBI and EzTaxon servers [21, 22]. Finally, the sequence data were deposited in the GenBank database.
A maximum parsimony tree was constructed using the 16S rDNA region of the 12 isolates of Azotobacter, AS-FE and AC-SW15 control strains, four reference sequences from NCBI (Accession Numbers: NR_114165, NR_041038, MF805703, MH763851), and one out-group from NCBI (Accession numbers: DQ133506) using the MEGA 6.06 software with bootstrap values calculated from 1000 replicates [23].
The PCR products from REP, ERIC, and BOX analyses were scored according to either presence (1) or absence (0) to construct the binary data matrices. In order to ensure the repeatability of the bands, the experiments were performed in three replications. The Jaccard similarity coefficient (J) was used to estimate the genetic similarities among the isolates, and the dendrograms were generated according to the unweighted pair-group mean arithmetic method (UPGMA) using NTSYSpc (numerical taxonomy and multivariate analysis system) software, Version 2.0 [24].
Results
Bacterial isolation
Twelve isolates of Azotobacter were isolated from rhizosphere soil samples of pastures and crops grown in arid and semi-arid regions of Iran. Young cells of the isolates showed polymorphism, but all the isolates were Gram-negative, produced light to dark brown water-insoluble pigments, and formed cysts. Moreover, all the isolates grew on the nitrogen-free medium but did not produce water-soluble pigments. Some morphological and physiological characteristics of the isolates are given in Table 2. The results revealed that the morphological and physiological characteristics of all the isolates were similar to those of A. chroococcum.
Molecular identification
The 16S rDNA, nifD and nifH gene sequences were compared to those of the most closely related bacterial species using the NCBI BLAST program (http://www.ncbi.nlm.nih.gov/BLAST). The lengths of 16S rDNA, nifD and nifH gene sequences for each strain are shown in Table 3. The above-mentioned sequences were submitted to the GeneBank/NCBI database under the accession numbers provided in Table 3.
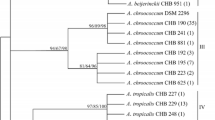
The analyses of the 16S rDNA regions revealed that the AC-63, AC-22, AC-58, and AC-LAB isolates were A. chroococcum, whereas the other eight isolates were identified as A. salinestris (Table 3). The maximum parsimony tree based on the 16S rDNA region sequence showed two groups. One group consisted of eight isolates of A. salinestris (AS-12, AS-71, AS-62, AS-26, AS-70, AS-66, AS-11, and AS-69), AS-FE control strain, and two reference sequences (AS-NBRC102611, AS-ATCC49674). The other group included AC-SW15 control strain, four isolates of A. chroococcum (AC-63, AC-22, AC-58, and AC-LAB), and two reference sequences (AC-AzXU1, AC-DC4) (Fig. 1). The nifD and nifH genes were unable to separate the species based on GeneBank/NCBI database.
A maximum parsimony tree based on the 16S rDNA region sequences (1390 bp) of the 12 isolates of Azotobacter, AS-FE and AC-SW15 control strains, four reference sequences from Genbank (Accession Numbers: NR_114165, NR_041038, MF805703, MH763851).The tree rooted with Pseudomonas putida PP-GM6 (DQ133506). Only bootstrap values > 60% (1000 replications) are shown at the branches
ARDRA profile of isolates
The results of ARDRA showed that HpaII was able to differentiate A. chroococcum from A. salinestris whereas RsaI and AluI were unable to separate these species. The ARDRA profiles of the Azotobacter isolates are shown in Fig. 2.
ARDRA profiles of the 12 Azotobacter isolates obtained by digestion of the 16S rDNA region by a HpaII, b RsaI, and c AluI. M: 100 bp DNA ladder. Lane 1 and 10 (controls): Azotobacter salinestris AS-FE and Azotobacter chroococcum AC-SW15, respectively. Lane 2–9: Azotobacter salinestris isolates; AS-12, AS-62, AS-66, AS-26, AS-11, AS-71, AS-70, and AS-69, respectively. Lane 11–14: Azotobacter chroococcum isolates; AC-22, AC-LAB, AC-63, and AC-58, respectively
Rep-PCR fingerprinting and profile analyses
The profiles of genomic DNA fingerprinting of BOX, ERIC, and REP of the Azotobacter isolates and their dendrogram are shown in Figs. 3, 4, and 5, respectively. The number of amplified bands was between 6 and 16 and the band sizes ranged from 340 to 4000 bp with BOX primer. The isolates were divided into two groups based on BOX patterns. The dendrogram showed that one group consisted of AS-FE and eight isolates of A. salinestris (AS-12, AS-71, AS-62, AS-26, AS-70, AS-66, AS-11, and AS-69) and the other group included AC-SW15 and four isolates of A. chroococcum (AC-63, AC-22, AC-58, and AC-LAB). The BOX marker could split the two A. chroococcum and A. salinestris species. In addition, the number of polymorphic bands was 25, which showed 100% polymorphism (Fig. 3).
BOX profiles of the 12 Azotobacter isolates. M: 100 bp DNA ladder. Lane 1 and 10 (controls): Azotobacter salinestris AS-FE and Azotobacter chroococcum AC-SW15, respectively. Lane 2–9: Azotobacter salinestris isolates; AS-12, AS-62, AS-66, AS-26, AS-11, AS-71, AS-70, and AS-69, respectively. Lane 11–14: Azotobacter chroococcum isolates; AC-22, AC-LAB, AC-63, and AC-58, respectively. Dendrogram was constructed based on BOX profiles by UPGMA method
ERIC profiles of the 12 Azotobacter isolates. M: 100 bp DNA ladder. Lane 1 and 10 (controls): Azotobacter salinestris AS-FE and Azotobacter chroococcum AC-SW15, respectively. Lane 2–9: Azotobacter salinestris isolates; AS-12, AS-62, AS-66, AS-26, AS-11, AS-71, AS-70, and AS-69, respectively. Lane 11–14: Azotobacter chroococcum isolates; AC-22, AC-LAB, AC-63, and AC-58, respectively. Dendrogram was constructed based on ERIC profiles by UPGMA method
REP profiles of the 12 Azotobacter isolates. M: 100 bp DNA ladder. Lane 1 and 10 (controls): Azotobacter salinestris AS-FE and Azotobacter chroococcum AC-SW15, respectively. Lane 2–9: Azotobacter salinestris isolates; AS-12, AS-62, AS-66, AS-26, AS-11, AS-71, AS-70, and AS-69, respectively. Lane 11–14: Azotobacter chroococcum isolates; AC-22, AC-LAB, AC-63, and AC-58, respectively. Dendrogram was constructed based on REP profiles by UPGMA method
The ERIC patterns represented that the number of amplified bands was between 2 and 11 and the band sizes ranged from 240 to 2100 bp. Moreover, the number of polymorphic bands was 18, which showed 94.7% polymorphism (Fig. 4).
The number of amplified bands of REP was between 3 and 15 and the band sizes ranged from 320 and 3500 bp. The analysis of REP profiles revealed that the isolates were divided into two groups. The REP marker could split the two species A. chroococcum and A. salinestris. Further, the number of polymorphic bands was 22, which showed 100% polymorphism (Fig. 5).
The consensus tree derived from ERIC, BOX, and REP profiles revealed the ability to differentiate between A. chroococcum and A. salinestris species and their strains (Fig. 6).
Discussion
Azotobacter is used for inoculation of plants as a biofertilizer due to its rapid growth, ability to fix N2 and production of plant growth substances. It forms cysts that help to cope with stress and grows better in saline conditions such as Iranian soils. Therefore, an accurate study of Azotobacter in agriculture and natural resources is particularly important for the production of biological fertilizers. In this study, 20 Azotobacter isolates were first isolated from 77 slightly saline soil samples in arid and semiarid regions. Based on the physiological and morphological characteristics, 12 isolates were selected for more investigation. During the sampling, according to the various growth stages of plants, it was not possible to identify all of them, particularly pasture plants. It is necessary to mention that Azotobacter is a free-living bacterium and does not need a plant for growth, but due to root exudates, its population is larger in the rhizosphere. However, soil characteristics have a greater impact on the growth, activity, and population of Azotobacter than the plant type. Therefore, Azotobacter isolates were obtained from soils and not from the roots using the soil paste method. This method was recommended as a reliable technique for the isolation and preliminary identification of A. chroococcum from soil samples [2]. These isolates were investigated in terms of young cell morphology, colony appearance, cyst formation, and water-soluble and insoluble pigment production (Table 2). The isolates were identified based on morphological and physiological characteristics as A. chroococcum. The analyses of the 16S rDNA regions revealed that only four out of the 12 isolates were identified as A. chroococcum and the rest were A. salinestris. The 16S rDNA has been used in many types of research for the molecular identification of bacteria such as Azotobacter. Chen et al. [25] employed 16S rDNA for identification of Azotobacter species including A. chroococcum, A. vinelandii, A.beijerinckii, and A. tropicalis from the rice rhizosphere of Taiwan.
In this study, the isolates were further investigated to differentiate between the two species using other molecular methods. It is necessary to mention that some Na+-dependent isolates of A. chroococcum isolated from slightly saline soils were proposed as a new species named A. salinestris [4].
Based on the previous investigations, AluI, HpaII, and RsaI restriction enzymes have been mainly used for identification of Azotobacter genus from other genera [26, 27]. But in our research, these restriction enzymes were used for differentiation at the species level. Aquilanti et al. [26] reported that the 16S rDNA region and ARDRA method with five restriction enzymes including RsaI, HhaI, HpaII, FnuDII, and AluI were useful markers for identification of Azotobacter genus from other free nitrogen-fixing bacteria. Jiménez et al. [27] expressed similar results based on morphological characteristics, 16S rDNA, and ARDRA with AluI, HpaII, and RsaI to identify Azotobacter species from soil samples. The number of restriction enzymes required for species differentiation varies depending on the species and the presence of the restriction sites. Some studies have revealed that at least four restriction enzymes are essential to resolve the 16S rRNA gene of different species [28, 29]. In contrast, species-level identification has been reported to be achievable even with three or fewer restriction enzymes [7, 30].
In our research, the ARDRA evaluation showed that among three enzymes, the best restriction enzyme for differentiating between A. salinestris and A. chroococcum was HpaII. This led to the clear discrimination between the two species. AluI and RsaI showed the same pattern characteristic of the genus Azotobacter, due to the presence of same restriction sites on the entire rRNA gene copies present in a single genome. Our results were in agreement with those of Rubio et al. [31], which reported that RsaI produced identical bands among A. chroococcum, A. salinestris, and A. armeniacus. The results are in line with results reported by Mazinani and Asgharzadeh [32], who noted that genetic diversity of A. chroococcum, A. vinelandii and A. beijernckii by RsaI was very low and it was because of the same digestion regions in their conserved sites. On the other hand, the use of HpaII and HhaI on strains led to the separation among the Azotobacter species. Other studies have employed the ARDRA technique for the diversity evaluation of Azotobacter [33, 34].
The findings of Rep-PCR fingerprinting revealed that BOX and REP markers could separate the two species, but the ERIC marker was incapable of fully separating them. The percentages of polymorphic bands obtained with BOX and REP markers were 100%; however, the ERIC marker did not show completely polymorphic bands. Few studies have distinguished between A. chroococcum and A. salinestris using REP, ERIC, and BOX. Lenart-Boroń et al. [35] reported that the high level of genetic diversity observed using Random Analysis of Polymorphic DNA and BOX markers. These markers could show the genetic diversity of the A. salinestris, A. chroococcum, and A. vinelandii. In addition, similar to our experiment, no monomorphic band was observed using BOX. Rubio et al. [31] reported that Rep-PCR is a useful tool for the taxonomic classification of Azotobacter isolates and they found high genetic diversity among A. chroococcum, A. salinestris, and A. armeniacus.
Rep-PCR was reported as a distinguishable marker for differentiation between A. chroococcum and Azospirillum brasilense strains [36]. Similar to our experiment, Chen et al. [37] proved that Rep-PCR was a remarkable tool for genotyping of bacterial species. They concluded that a combination of Box, Eric and Miniprimer-PCR results was a fast and reliable method for segregation among P. fluorescens isolates. In another research, the Box PCR technique was used to discriminate between P. aeruginosa isolates [38]. A high degree of clonal diversity among P. aeruginosa strains was observed using REP and ERIC markers [39, 40].
The NCBI BLAST of nifD and nifH gene sequences showed that these genes could not distinguish between the two species.One reason that nif genes were unable to differentiate between the two species can be the shorter length and similarity of the sequenced region as well as the lack of sufficient data in the database. However, these sequences were submitted to the GeneBank/NCBI database under the accession numbers provided in Table 3.
Conclusion
Azotobacter chroococcum and A. salinestris as beneficial soil bacteria are almost completely similar in terms of morphological and physiological characteristics; thus, the exact diagnosis of these two species requires molecular investigations. The sequence of 16S rDNA was used for the preliminary separation of these two species. In this research, additional molecular techniques including ARDRA and different Rep-PCR were used to study the diversity between these two species more closely. Our findings suggested that HpaII was a suitable restriction enzyme for differentiation and clear discrimination between A. chroococcum and A. salinestris. Although the results of 16S rDNA and HpaII were similar in separating these two species, the use of this restriction enzyme is suggested because of no need for sequencing and time and cost savings. BOX and REP markers were able to differentiate not only between the two species but also among their strains. Therefore, these two markers are recommended for the diversity studies of these two species.
References
Kennedy C, Rudnick P, MacDonald ML, Melton T (2015) Azotobacter. Bergey’s Man Syst Archaea Bact 17:1–33
Becking JH (1981) The family Azotobacteraceae. In: Ballows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (eds) The prokaryotes: a handbook on habitats, isolation, and identification of bacteria. Springer, Heidelberg, pp 795–817
Page WJ (1986) The sodium-dependent growth of Azotobacter chroococcum. Appl Environ Microb 51:510–514
Page WJ, Shivprasad S (1991) Azotobacter salinestris sp. nov., a sodium-dependent, micro-paedophilic, and aero adaptive nitrogen- fixing bacterium. Int J Syst Bacteriol 41:369–376
Grimont F, Grimont PAD (1986) Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol 137B:165–175
Massol-Deya AA, Odelson DA, Hickey RF, Tiedje JM (1995) Bacterial community fingerprinting of amplified 16S and 16-23S ribosomal DNA gene sequences and restriction endonuclease analysis (ARDRA). Mol Microbial Ecol Man 3.3.2:1–8
Kšicová K, Dušková M, Karpíšková R (2013) Differentiation of Lactobacillus species by ARDRA. Czech J Food Sci 31:180–188
Stern MJ, Ames GFL, Smith NH, Robinson EC, Higgins CF (1984) Repetitive extragenic palindromic sequences: a major component of the bacterial genome. Cell 37(3):1015–1026
Frye SR, Healy M (2006) Molecular strain typing using repetitive sequence-based PCR. In: Tang Y-W, Stratton CW (eds) Advanced techniques in diagnostic microbiology, 1st edn. Springer, New York, pp 444–471
Hulton CSJ, Higgins CF, Sharp PM (1991) ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol Microbiol 5(4):825–834
Martin B, Humbert O, Camara M, Guenzi E, Walker J, Mitchell T, Andrew P, Prudhomme M, Alloing G, Hakenbeck R, Morrison DA (1992) A highly conserved repeated DNA element located in the chromosome of Streptococcus pneumoniae. Nucleic Acids Res 20(13):3479–3483
Das S, Dash HR, Mangwani N, Chakraborty J, Kumari S (2014) Understanding molecular identification and polyphasic taxonomic approaches for genetic relatedness and phylogenetic relationships of microorganisms. J Microbiol Method 103:80–100
Olive DM, Bean P (1999) Principles and applications of methods for DNA-based typing of microbial organisms. J Clin Microbiol 37:1661–1669
Bilung LM, Pui CF, Su’ut L, Apun K (2018) Evaluation of BOX-PCR and ERIC-PCR as molecular typing tools for pathogenic Leptospira. Dis Markers 2018:1–9
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematic. Wiley, New York, pp 115–175
Jiang H, Dong H, Zhang G, Yu B, Chapman LR, Fields MW (2006) Microbial diversity in water and sediment of Lake Chaka, an Athalassohaline lake in northwestern China. Appl Environ Microb 72:3832–3845
Poly F, Monrozier LJ, Bally R (2001) Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res Microbiol 152:95–103
Stoltzfus JR, So RMPP, Malarvithi PP, Ladha JK, De Bruijn FJ (1997) Isolation of endophytic bacteria from rice and assessment of their potential for supplying rice with biologically fixed nitrogen. Plant Soil 194:25–36
Versalovic J, Koeuth T, Lupski R (1991) Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 19:6823–6831
Versalovic J, Schneider M, De Bruijn FJ, Lupski JR (1994) Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol Cell Biol 5(1):25–40
Altschul SF, MaddenTL Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729
Rohlf FJ (1993) NTSYS-PC: numerical taxonomy and multivariate analysis system, version 1.80. Steauket, New York
Chen SL, Tsai MK, Huang YM, Huang CH (2018) Diversity and characterization of Azotobacter isolates obtained from rice rhizosphere soils in Taiwan. Ann Microbiol 68:17–26
Aquilanti L, Mannazzu I, Papa R, Cavalca L, Clementi F (2004) Amplified ribosomal DNA restriction analysis for the characterization of Azotobacteraceae: a contribution to the study of these free-living nitrogen-fixing bacteria. J Microbiol Methods 57:197–206
Jiménez DJ, Montaña JS, Martínez MM (2011) Characterization of free nitrogen fixing bacteria of the genus Azotobacter in organic vegetable-grown Colombian soils. Braz J Microbiol 42:846–858
Dec M, Puchalski A, Urban-Chmiel R, Wernicki A (2016) 16S-ARDRA and MALDI-TOF mass spectrometry as tools for identification of Lactobacillus bacteria isolated from poultry. BMC Microbiol 16(105):1–16
Moyer CL, Tiedje JM, Dobbs FC, Karl DM (1996) A computer-simulated restriction fragment length polymorphism analysis of bacterial small-subunit rRNA genes: efficacy of selected tetrameric restriction enzymes for studies of microbial diversity in nature. Appl Environ Microbiol 62(7):2501–2507
Nel S, Davis SB, Endo A, Dicks LMT (2019) Differentiation between Bacillus amyloliquefaciens and Bacillus subtilis isolated from a South African sugarcane processing factory using ARDRA and rpoB gene sequencing. Arch Microbiol. https://doi.org/10.1007/s00203-019-01698-w
Rubio EJ, Montecchia MS, Tosi M, Cassán FD, Perticari A, Correa OS (2013) Genotypic characterization of Azotobacteria isolated from Argentinean soils and plant-growth-promoting traits of selected strains with prospects for biofertilizer production. Sci World J. https://doi.org/10.1155/2013/519603
Mazinani Z, Asgharzadeh A (2014) Genetic diversity of Azotobacter strains isolated from soils by amplified ribosomal DNA restriction analysis. Cytol Genet 48(5):293–301
Bhatia R, Ruppel S, Narula N (2008) Diversity studies of Azotobacter spp. from cotton-wheat cropping systems of India. J Basic Microbiol 48:455–463
Cinnadurai C, Gopalaswamy G, Balachandar D (2013) Diversity of cultivable Azotobacter in the semi-arid alfisol receiving long-term organic and inorganic nutrient amendments. Ann Microbiol 63:1397–1404
Lenart-Boroń AM, Wolny-Koładka KA, Boroń PM, Mitka JR (2014) The molecular marker-based comparison of Azotobacter spp. populations isolated from industrial soils of Cracow-Nowa Huta steelworks (southern Poland) and the adjacent agricultural soils. J Environ Sci Health A 49:1054–1063
Tejera N, Lluch C, Martinez-Toledo MV, Gonzalez-Lopez J (2005) Isolation and characterization of Azotobacter and Azospirillum strains from the sugarcane rhizosphere. Plant Soil 270:223–232
Chen Q, Qi P, Xu R, Tambong JT, Djama ZR, Li W (2011) Comparison of three typing methods for evaluating the diversity of Pseudomonas fluorescens in the rhizosphere. J Plant Sci 6(2):52–65
Wolska K, Kot B, Jakubczak A, Rymuza K (2011) BOX-PCR is an adequate tool for typing of clinical Pseudomonas aeruginosa isolates. Folia Histochem Cytobiol 49:734–738
Han MM, Mu LZ, Liu XP, Zhao J, Liu XF, Liu H (2014) ERIC-PCR genotyping of Pseudomonas aeruginosa isolates from haemorrhagic pneumonia cases in mink. Vet Rec Open 1(1):e000043
Serrano I, De Vos D, Santos JP, Bilocq F, Leitão A, Tavares L, Pirnay JP, Oliveira M (2016) Antimicrobial resistance and genomic rep-PCR fingerprints of Pseudomonas aeruginosa strains from animals on the background of the global population structure. BMC Vet Res 13:58
Acknowledgements
This work was conducted at the Soil and Water Research Institute and was financially supported by the National Science and Technology Foundation of Iran affiliated to the Vice Presidents of Science and Technology of Iran.
Funding
The National Science and Technology Foundation affiliated to the Vice President of Science and Technology of Iran funded this study (Project No. 94000889).
Author information
Authors and Affiliations
Contributions
All authors of this research paper have directly participated in the planning, execution, or analysis of this study. All authors in this manuscript have read and approved the current version of the manuscript. The contents of this manuscript have not been copyrighted or published previously.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khosravi, H., Dolatabad, H.K. Identification and molecular characterization of Azotobacter chroococcum and Azotobacter salinestris using ARDRA, REP, ERIC, and BOX. Mol Biol Rep 47, 307–316 (2020). https://doi.org/10.1007/s11033-019-05133-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05133-7