Abstract
Oxidative stress occurs due to an imbalance between antioxidant defenses and pro-oxidant agents in brain. This condition has been associated to the pathogenesis of several brain diseases; therefore, increasing the use of compounds that exert antioxidant activity. Thus, the objective of this study was to evaluate, in vitro, the effect of isoflavones in: (1) lipid peroxidation, catalase activity and thiol groups in the presence of pro-oxidants: sodium nitroprusside or Fe2+/EDTA complex in rat brain homogenates; (2) the activity of the enzyme monoamine oxidase (MAO). As a result, the isoflavones reduced lipid peroxidation in a manner dependent on the concentration and protected against the reduction of catalase activity as well as the induced thiol oxidation in brain tissue. In addition, isoflavones inhibited MAO activity (MAO-A and MAO-B). Taken together, our results showed that isoflavones avoided oxidative stress and decreased the MAO activity, suggesting a promissory use in the treatment of neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress is a biological condition that occurs due to an imbalance between antioxidant defenses and reactive oxygen species (ROS) and/or reactive nitrogen species (RNS) generated from normal oxidative metabolism or from pro-oxidant environmental exposures [1, 2]. This condition has been described to be involved in neurodegenerative disorders such as Parkinson’s (PD), Alzheimer’s (AD) and Huntington’s (HD) disease [3, 4], where ROS can contribute to their worsening by causing alterations in the cell membrane (lipid peroxidation and protein oxidation) and DNA mutations [5, 6].
It is known that the brain is vulnerable to oxidative damage because of a relative lack of antioxidants and abundance of oxidizable substrates like polyunsaturated fatty acids and catecholamines such as dopamine [7, 8]. The toxic potential of dopamine is mainly due its oxidation by monoamine oxidase (MAO), which generates hydrogen peroxide (H2O2) [9].
Two isoforms of MAO (MAO-A and MAO-B) have been identified in humans which are found in the outer mitochondrial membrane and are responsible for the metabolism of monoamine neurotransmitters in the brain and peripheral tissues.
Studies have demonstrated that MAO is associated with psychiatric and neurological disorders, including depression, PD and AD [10]. Furthermore, it was demonstrated the inhibition of MAO-A prevents cell apoptosis [11], suggesting its important role in neurodegenerative diseases.
In this context, alternative ways have been considered as adjuvant treatment of numerous diseases, mainly those associated with oxidative stress [12, 13] and a special attention has been given to natural products as sources of antioxidants [14].
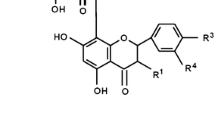
Isoflavones are phenolic compounds present in high concentrations in leguminous plants, such as soybeans. When ingested isoflavones are hydrolyzed in the intestine by intestinal glycosidases, releasing the main bioactive forms: aglycones, daidzein, genistein and glycitain [15] that will promote beneficial action in human body, including the decrease in menopausal symptoms [16] and treatment of hormonal diseases [17]. It is generally believed that many of the beneficial effects of isoflavones are at least partially associated with their antioxidant activity [18, 19] which may be related to the number of hydroxyl groups present in their chemical structure [18]. In addition, isoflavones are capable of inhibiting lipid peroxidation in vitro by free radical scavenging [15]. Pharmacologically, isoflavones are phytoestrogens because of their ability to bind to estrogen receptors in vivo [20]. There are reports in the literature suggesting isoflavones as promising agents in the treatment of neurodegenerative diseases due to their ability to cross the blood brain barrier, have a half-life (15–22 h) and low oral toxicity in vivo [21].
Thus, the objective of this study was to investigate, in vitro, the antioxidant effect and inhibitory activity on the activity of MAO, using isoflavones extracted from soy, because it is the formulation that is marketed in pharmacies and the way in which the population has access and use.
Materials and methods
Animals
Male Wistar rats (± 2 months old), weighing between 200 and 250 g, from breeding colony of UFSM (Brazil) were kept in cages with free access to food and water in a room with controlled temperature (22 ± 1 °C) and in 12 h light/dark cycle with lights on at 7:00 am. The brain tissues used were brain samples that left over from control animals from other experiments that were previously approved by the Ethics Committee on Animal Use (CEUA) of the UFSM that had been stored at − 80 °C until the use.
Reagents
Tris–HCl, thiobarbituric acid, malonaldehyde bis(dimethyl acetal) (MDA), 5,5-dithiobis(2-nitrobenzoic acid), l-Glutathione reduced, Folin and Ciocalteu’s phenol reagent, kynuramine dihydrobromide, clorgyline, and pargyline were obtained from Sigma (St. Louis, MO, USA). Hydrogen peroxide and trichloroacetic acid (TCA), sodium nitroprusside, ferrous sulfate, and EDTA were obtained from Merck (Brazil).
Isoflavones
Isoflavones were acquired from Xi’an Green Life Natural Products®. The certificated of analysis contain the following information: 41.73% of the powder was composed by isoflavones. Among them: daidzein (35.8%), daidzin (3.11%), glycitin (1.21), genistin (1.18%), glycitein (0.32%), genistein (0.11%). The concentrations of isoflavones varied according with their potencies in each test since in some tests they presented effect only in higher concentrations than another.
Preparation of isoflavones solution
The isoflavones were weighted and dissolved in distilled water. For each experiment, a new solution was prepared.
Determination of thiobarbituric acid reactive substances (TBARS)
To evaluate the effects of isoflavones on TBARS production induced by different pro-oxidants in vitro, the rat brain tissue was homogenized in 10 mM Tris–HCl, pH 7.4 (1:10) and centrifuged at 3000 rpm for 10 min. After, an aliquot of the supernatant (S1) was incubated for 1 h at 37 °C with pro-oxidants and in the presence or absence of different concentrations of isoflavones (equivalent to 25, 50, 100, 200 and 300 µg/mL of isoflavones). After, TBARS reaction was performed by adding thiobarbituric acid (0.6%), acetic acid/HCl buffer (pH 3.4), sodium dodecyl sulfate (8.1%) and incubated at 95 °C for 1 h. As pro-oxidant agents, sodium nitroprusside (SNP 5 µM) and Fe2+/EDTA (100 µM/100 µM) were used. Malondialdehyde (MDA) was used as standard and samples were read at 532 nm [22].
Catalase activity
The antioxidant activity of isoflavones was also verified through to its ability in to maintaining the enzyme catalase activity. To this brain homogenate (S1) was incubated at 37 °C for 1 h with the pro-oxidant agents: SNP (5 µM) and Fe2+/EDTA (100 µM) in the presence or absence of isoflavones (equivalent to 25, 50, 100, 200 and 300 µg/mL of isoflavones). Immediately after, the reaction was centrifuged and an aliquot was used to measuring the activity of catalase. An aliquot of supernatant was added to potassium phosphate buffer 50 mM, pH 7.4 at 25 °C and then hydrogen peroxide (H2O2, 0.5 M). The reading was made at 240 nm during 2 min and the data were expressed as µmol of H2O2/min/g tissue [23].
Oxidation of thiols groups
The following concentrations of isoflavones were used for this experiment: 25; 50; 100; 200 and 300 µg/mL and, as pro-oxidants Fe2+/EDTA (100 µM) or SNP (5 µM). An aliquot of S1 was incubated under the same experimental conditions described above for catalase activity and, after 1 h, levels of protein and non-protein thiol were determined. The reagent 5,5′-dithiobis (2-nitrobenzoic acid)—DTNB was added to the samples and the chromogen formed was measured spectrophotometrically at 412 nm. For levels of non-protein thiol, 10% trichloroacetic acid (TCA) was added to the pre-incubated aliquot, centrifuged at 500 g for 5 min and the supernatant was used. The results were expressed in µmol of protein thiol/g of tissue and non-protein thiol/g of tissue [24].
Activity of the enzyme monoamine oxidase (MAO)
Monoamine oxidase (MAO) activity was determined by measuring the kynuramine oxidation to 4-hydroxiquinoline [25,26,27]. For assessing the effect of isoflavones on the activity of MAO, the following concentrations were used: 12.5; 25; 50; 100; 200; 300; 600 µg/mL. The whole brain was homogenized in buffer containing: 16.8 mM, Na2PO4, 10.6 mM, KH2PO4, 3.6 mM KCl pH 7.4. Brain homogenates (0.25 mg of protein) were pre-incubated during 10 min at 37 °C with MAO-A (chlorgiline, 250 nM) or MAO-B (pargyline, 250 nM) inhibitors in the presence or absence of different concentrations of isoflavones. After this, kynuramine was added as MAO substrate in sub maximal concentration (60 µM). The reaction was incubated during 30 min at 37 °C. After this time, the reaction was stopped with 10% trichloroacetic acid (TCA). The samples were centrifuged at 500×g for 5 min and the supernatant was used to estimate the MAO activity. It was added 1 mL of 1N NaOH with an equal volume of supernatant. The product of reaction was measured spectrofluorimetrically at 315 nm for excitation and 380 nm for emission [28]. The results are represented as nmol 4-HQ/mg of protein/min.
Statistical analysis
The results were expressed as the means ± SEM. Differences between groups were evaluated for significance using one-way analysis of variance (ANOVA) followed by Tukey’s test or unpaired t-test. Significance was considered when p < 0.05.
Results
Effect of isoflavones on lipid peroxidation induced by SNP or Fe2+/EDTA
The protective effect of isoflavones against pro-oxidants-induced lipid peroxidation was measured using the TBARS test. Both NPS and Fe2+/EDTA complex increased TBARS in brain homogenates of rat (123.7 ± 3.35 nmol MDA/g of tissue [NPS], 305.7 ± 8.17 nmol MDA/g of tissue [Fe2+/EDTA], p < 0.0001, Fig. 1a, b). Isoflavones reduced this increase in a concentration-dependent manner with an IC50 of 71.39 ± 2.01 µg/mL to SNP and 124.5 ± 8.13 µg/mL to Fe2+/EDTA.
Effect of isoflavones on lipid peroxidation induced by SNP or Fe2+/EDTA complex in rat brain homogenates. The data show the mean ± SEM of 3 experiments performed in duplicate and analyzed by one-way ANOVA followed by Tukey’s test. *, ****(p < 0.05 and p < 0.0001) Significant differences from basal. ##, ####p < 0.01 and p < 0.0001) Significant differences with induced by SNP or Fe2+/EDTA
Effect of isoflavones on catalase activity
To investigate whether the protective effect of isoflavones against oxidative stress was associated with the preservation of antioxidant enzymes, catalase activity was measured in brain tissue incubated with pro-oxidants. NPS 5 µM (Fig. 2a) and Fe2+/EDTA 100 µM (Fig. 2b) complex decreased catalase activity (20.31 ± 3.30 µmol of H2O2/min/g tissue and 10.73 ± 2.69 µmol of H2O2/min/g tissue, respectively) when compared to control (41.14 ± 2.14 µmol of H2O2/min/g tissue). However, isoflavones avoided the decrease of catalase activity at the concentration of 100 and 200 µg/ml (32.58 ± 1.92 µmol of H2O2/min/g tissue and 38.08 ± 3.02 µmol of H2O2/min/g tissue) compared to that induced with SNP (Fig. 2a), and at concentrations of 200 and 300 µg/mL (38.97 ± 3.90 µmol of H2O2/min/g tissue and 42.42 ± 1.13 µmol of H2O2/min/g tissue) when compared with the induced with Fe2+/EDTA (Fig. 2b).
Effect of isoflavones on catalase levels in brain homogenates incubated with NPS 5 µM (a) or Fe2+/EDTA 100 µM (b). Data show the mean ± S.E.M. of 3 experiments performed in duplicate and analyzed by one-way ANOVA followed by Tukey’s test. *, **, ***, ****(p < 0.05, p < 0.01, p < 0.001 and p < 0.0001) Significant differences from basal. #, ##, ####(p < 0.05, p < 0.01 and p < 0.0001) Significant differences compared with SNP or Fe2+/EDTA
Effect of isoflavones on oxidation of thiols groups
Results show that both pro-oxidant agents caused oxidation of protein thiol groups (18.78 ± 0.45 µmol of protein thiol/g of tissue [NPS] and 15.29 ± 0.31 µmol of protein thiol/g of tissue [Fe2+/EDTA]) and non-protein thiol (3.18 ± 0.41 non-protein thiol/g of tissue [SNP] and 2.15 ± 0.21 non-protein thiol/g of tissue [Fe2+/EDTA]) when compared to control (20.53 ± 037 µmol of protein thiol/g of tissue and 4.23 ± 0.21 non-protein thiol/g of tissue, respectively). Isoflavones were able to protect against thiol oxidation at concentrations of 50–200 µg/mL for protein thiol (24.03 ± 0.99 µmol of protein thiol/g of tissue at 200 µg/mL of isoflavones) and non-protein thiol (5.24 ± 0.30 non-protein thiol/g of tissue at 200 µg/mL of isoflavones) compared with induced by SNP (p < 0.05–p < 0.0001; p < 0.05 and p < 0.001, respectively) (Fig. 3a, c). Similarly, concentrations of 100–300 µg/mL for protein thiol (23.35 ± 0.18 µmol of protein thiol/g of tissue at 300 µg/mL of isoflavones) and the concentrations of 200–300 µg/mL for non-protein thiol (4.19 ± 0.30 non-protein thiol/g of tissue at 300 µg/mL of isoflavones) presented significant difference compared to induced by Fe2+/EDTA (p < 0.001 and p < 0.0001; p < 0.05 respectively) (Fig. 3b, d).
Effect of isoflavones on thiol content (protein and non-protein) in brain homogenates of rats incubated with pro-oxidant agents. Oxidation of protein thiol induced by SNP (a) and induced by Fe2+/EDTA (b). Oxidation of non-protein thiol induced by SNP (c) and induced by Fe2+/EDTA complex (d). Data show the mean ± SEM of 3 experiments performed in duplicate and analyzed by one-way ANOVA followed by Tukey’s test. *, **, ***, ****(p < 0.05, p < 0.01, p < 0.001 and p < 0.0001) Represents significant differences from basal. #, ###, ####(p < 0.05, p < 0.001 and p < 0.0001) Significant differences compared to that induced with pro-oxidants
Effect of isoflavones on MAO activity
Isoflavones showed a significant inhibitory effect (p < 0.0001) on the activity of MAO-A at all tested concentrations (12.5–600 µg/mL [0.050 ± 0.000 nmol 4-HQ/mg of protein/min at 600 µg/mL of isoflavones]; IC50 = 196.4 ± 9.59 µg/mL) compared with control (0.419 ± 0.001 nmol 4-HQ/mg of protein/min; Fig. 4a). With regard to MAO-B activity, all concentrations tested of isoflavones, except the lowest concentration (12.5 µg/mL) inhibited it ([0.056 ± 0.001 nmol 4-HQ/mg of protein/min at 600 µg/mL of isoflavones]; IC50 = 161 ± 6.70 µg/mL) when compared to the control (0.72 ± 0.01 nmol 4-HQ/mg of protein/min; p < 0.0001) (Fig. 4b).
Discussion
Estrogen is an agent related to the neuroprotective effect in insults in central nervous system [29, 30]. Thus, this study aimed to evaluate in vitro, the antioxidant potential of isoflavones and their inhibitory effect on MAO enzyme in rat brain homogenate, once the increased metabolism of monoamines is responsible for the production of reactive species and pro-apoptotic events.
Isoflavones are phytoestrogens found in various grains, particularly soybeans. Basically, the isoflavones have the capacity to act beneficially in the body in 4 different ways: (1) estrogens and antiestrogens; (2) inhibitors of enzymes linked to the development of cancer; (3) antioxidant; (4) anti-inflammatory [31].
In this context, our first aim was to evaluate the antioxidant potential of isoflavones in vitro by using pro-oxidant agents in brain tissue. SNP has been suggested to cause cytotoxicity via the release of cyanide and/or nitric oxide (NO) [32, 33]. NO is a RNS and has several roles in mammals, but unregulated RNS production can cause adverse effects (e.g., cell damage or cell death) through reaction with biological target molecules such as DNA, lipids, and proteins [34]. Also, NO has the ability to inhibit the activity of certain enzymes such as catalase, and it occurs through of the NO binding on enzyme active site [35, 36]. Besides, the iron and the complexes Fe3+/EDTA can react with H2O2 via Fenton reaction [37] to form the hydroxyl radical which is highly reactive and one of the responsible to initiate lipid peroxidation, causing damage on cell membranes by disrupting fluidity and permeability [38, 39]).
Then, the effect of isoflavones was tested in oxidative (lipid peroxidation) and antioxidant markers (catalase activity and thiol levels) in the presence of pro-oxidant agents (SNP or Fe2+/EDTA), which are widely used to cause lipid peroxidation. Here, the results show that isoflavones were able to reduce brain lipid peroxidation induced by SNP or Fe2+/EDTA in brain homogenates. Lipid peroxidation is a complex process occurring in cells which reflects the interaction between ROS and polyunsaturated fatty acids. The products of lipid peroxidation are reactive aldehydes and malondialdehyde, many of which are highly toxic to cells [40], being present in neurodegenerative disorders [41]. Corroborating with our results, a study using genistein at 100 µM significantly reduced the iron-induced TBARS in neurons culture [42].
We decided to test the effect of isoflavones on catalase activity because it is one of the most important endogenous antioxidants, which acts by catalyzing the reduction of H2O2 into molecular oxygen and water [43] and protects the tissues from highly reactive hydroxyl radicals that could be generated from H2O2. The present results show that the presence of isoflavones in the reaction in a concentration-dependent manner avoided the decrease in catalase activity induced by pro-oxidants. Corroborating with this, a study conducted by Zhang et al. [44] verified that tectorigenin, an isoflavone, reduced H2O2-induced death of Chinese hamster lung fibroblasts (V79-4) and increased the activity and protein expression of catalase in a time-dependent manner, thus highlighting the antioxidant effect of isoflavones.
In addition to the effect of isoflavones on the catalase enzyme, we demonstrated that isoflavones protect against thiol groups oxidation induced by SNP or Fe2+/EDTA. Glutathione (GSH) is a powerful antioxidant and is the major soluble, non-enzymatic antioxidant in cells. It is the major intracellular thiol compound (non-protein thiol—NPSH) synthesized intracellularly from cysteine, glycine and glutamate. GSH is capable of scavenging hydroxyl radical and is important in maintaining –SH groups in other molecules including proteins. Also, –SH groups react with H2O2 and the OH˙ radical and may prevent tissue damage [45, 46]. It is hypothesized that isoflavones could avoid the oxidation of thiol groups induced by pro-oxidant agents either by promoting the regeneration, probably through redox system [47]. In addition, this hypothesis may also justify the maintenance of the catalase activity and the protection against lipid peroxidation, as we observed in this study.
Dysregulation of redox states is being increasingly recognized in many illnesses, such as PD, where the increase in the enzymatic metabolism of dopamine by MAO-B could lead to the formation of H2O2 and OH˙ [48]. Considering that the increase in the activity of the MAO enzyme can lead to mitochondrial damage and neurodegenerative disturbances [49], and that the enzyme inhibition is used as part of the treatment of neurodegenerative diseases [50, 51], we resolved to verify if the isoflavones would be able to inhibit the activity of the enzymes MAO.
MAO is an enzyme that catalyzes the oxidative deamination of monoamines. In humans, the MAO activity increases with age [52] and is also depleted in certain neurodegenerative diseases [53, 54]. Therefore, an inhibition of MAO-B activity has been suggested to delay the neurodegenerative process and, consequently, improve the quality of life, especially of the seniors [53]. Moreover, it was demonstrated the inhibition of MAO-A prevents cell apoptosis [11]. Clinically, MAO-A inhibitors are used as antidepressants agents, while MAO-B inhibitors are used as therapeutics for AD and PD [9].
However, there are few information about the inhibitory effects of isoflavones on MAO enzymes. Zarmouh et al. [55] reported that genistein inhibits non-selectively MAO-A and MAO-B with IC50 values of 9.7 and 6.8 µM, respectively. Recently, a study published by Zarmouh et al. [56] showed that Biochanin-A, an isoflavone, is a reversible and competitive inhibitor MAO with high selectivity index and high affinity to inhibit MAO-B. The predicted interactions of Biochanin-A with the active site amino acids involve reversible H-bonds and hydrophobic interactions. Here, in our study, isoflavones showed a significant inhibitory effect on the activity of MAO-A and MAO-B, with an IC50 = 196.4 ± 9.59 µg/mL and IC50 = 161 ± 6.70 µg/mL, respectively.
Isoflavones are considered phytoestrogens due to its structural similarity with estradiols [17]. In addition, the classical actions of estrogens and phytoestrogens are mediated via the transcriptional activation of genes responsive to estrogen, involving intracellular receptors [57], exhibiting a greater affinity to ERβ receptors than to ERα [58]. Numerous publications point out hormones, such as estrogen, as responsible for the activation or inhibition of some enzymes that act on the synthesis of neurotransmitter [59]. A study by Gundlah et al. [60] analyzed the effect of ovarian steroid hormones on MAO as well as its molecular expression and demonstrated that brain areas with a predominance of ERβ receptors showed regulation for MAO-A and lower interaction for MAO-B and ERα receptor with greater regulation for MAO-B and lower for MAO-A. ERβ is highly expressed in nerve tissue and can be expected to have a greater effect on the expression of MAO-A than ERα [57]. With this information we can suggest that the mechanisms of inhibition of MAO activity by isoflavones are complex, either through the modulation of the estrogen receptor or by acting directly on the active site of the enzyme.
Conclusion
Studies with natural compounds are important, in particular because the population believes in the therapeutic action due to their natural origin, and generally have low toxicity. In this study, isoflavones were able to reduce brain lipid peroxidation and protect against the reduction of the activity of catalase, and oxidation of thiols induced by well-known pro-oxidants agents in brain tissue. Moreover, isoflavones inhibited the activity of the MAO (MAO-A and MAO-B), which is related to the reactive species production during catecholamines metabolism. Considering the effects of isoflavones, they could be considered as an alternative to the prevention of degenerative diseases. However, more studies must be performed to investigate its mechanism of action with the aim of exploring the whole therapeutic potential of isoflavones.
References
Berg D, Youdim MB, Riederer P (2004) Redox imbalance. Cell Tissue Res 318:201–213
Kohen R, Nyska A (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30:620–650
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Rego AC, Oliveira CR (2003) Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res 28:1563–1574
Duffy S, So A, Murphy TH (1998) Activation of endogenous antioxidant defenses in neuronal cells prevents free radical mediated damage. J Neurochem 71:69–78
Silva JP, Coutinho OP (2010) Free radicals in the regulation of damage and cell death—basic mechanisms and prevention. Drug Discov Ther 4:144–167
Cohen G (1988) Oxygen radicals and Parkinson’s disease. In: Halliwell B (ed) Oxygen Radicals and Tissue Injury. FASEB, Bethesda, pp 130–135
Halliwell B, Gutteridge JMC (1985) Oxygen radicals and the nervous system. Trends Neurosci 8:22–26
Youdim MB, Bakhle YS (2006) Monoamine oxidase: isoforms and inhibitors in Parkinson’s disease and depressive illness. Br J Pharmacol 147:287–296
Sun Y, Chen J, Chen X, Huang L, Li X (2013) Inhibition of cholinesterase and monoamine oxidase-B activity by tacrine–homoisoflavonoid hybrids. Bioorg Med Chem 21:7406–7417
Ou XM, Chen K, Shih JC (2006) Monoamine oxidase A and repressor R1 are involved in apoptotic signaling pathway. Proc Natl Acad Sci USA 103:10923–10928
Pereira RP, Fachinetto R, Souza AP, Puntel RL, Santos GNS, Heinzmann BM, Boschetti TK, Athayde ML, Burger ME, Morel AF, Morsch VM, Rocha JB (2008) Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citrates. Neurochem Res 34:973–983
Peroza LR, Busanello A, Leal CQ, Ropke J, Boligon AA, Meinerz D, Libardoni M, Athayde ML, Fachinetto R (2013) Bauhinia forficata prevents vacuous chewing movements induced by haloperidol in rats and has antioxidant potential in vitro. Neurochem Res 38:789–796
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477
Cook NC, Samman S (1996) Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem 7:66–76
Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J (2011) Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial. Arch Intern Med 171:1363–1369
Morton MS, Arisaka O, Miyake N, Morgan LD, Evans BA (2002) Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J Nutr 132:3168–3171
Naim M, Gestetner B, Bondi A, Birk Y (1976) Antioxidative and antihemolytic activities of soybean isoflavones. J Agric Food Chem 24:1174–1177
Wei H, Wei L, Frenkel K, Bowen R, Barnes S (1993) Inhibition of tumor promoter-induced hydrogen peroxide formation in vitro and in vivo by genistein. Nutr Cancer 20:1–12
Dixon RA (2004) Phytoestrogens. Annu Rev Plant Biol 55:225–261
Takimoto CH, Glover K, Huang X, Hayes SA, Gallot L, Quinn M, Jovanovic BD, Shapiro A, Hernandez L, Goetz A, Llorens V, Lieberman R, Crowell JA, Poisson BA, Bergan RC (2003) Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol Biomark Prev 12:1213–1221
Ohkawa H, Ohishi H, Yagi K (1979) Assay for lipid peroxide in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Aebi H (1984) Catalase in vitro methods enzymol. Academic Press 105:121–126
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Reis EM, Schreiner Neto FW, Cattani VB, Peroza LR, Busanello A, Leal CQ, Boligon AA, Lehmen TF, Libardoni M, Athayde ML, Fachinetto R (2014) Antidepressant-like effect of Ilex paraguariensis in rats. Biomed Res. https://doi.org/10.1155/2014/958209
Soto-Otero R, Méndez-Alvarez E, Hermida-Ameijeiras A, Sánchez-Sellero I, Cruz-Landeira A, Lamas ML (2001) Inhibition of brain monoamine oxidase activity by the generation of hydroxyl radicals: potential implications in relation to oxidative stress. Life Sci 69:879–889
Villarinho JG, Oliveira SM, Silva CR, Cabreira TN, Ferreira J (2012) Involvement of monoamine oxidase B on models of postoperative and neuropathic pain in mice. Eur J Pharmacol 690:107–114
Morinan A, Garratt HM (1985) An improved fluorimetric assay for brain monoamine oxidase. J Pharmachol Methods 13:213–223
Zhao L, Wu TW, Brinton RD (2004) Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res 1010:22–34
Sribnick EA, Ray EA, Banik SK (2004) Estrogen as a multi-active neuroprotective agent in traumatic injuries. Neurochem Res 29:2007–2014
Esteves EA, Monteiro JB (2001) Efeitos benéficos das isoflavonas de soja em doenças crônicas. Rev Nutr 14:43–52
Bates JN, Baker MT, Guerra R, Harrison DG (1991) Nitric oxide generation from nitroprusside by vascular tissue. Biochem Pharmacol 42:157–165
Rauhala P, Khaldi A, Mohanakumar KP, Chiueh CC (1998) Apparent role of hydroxyl radicals in oxidative brain injury induced by sodium nitroprusside. Free Radic Biol Med 24:1065–1073
Yen GC, Lai HH (2003) Inhibition of reactive nitrogen species effects in vitro and in vivo by isoflavones and soy-based food extracts. J Agric Food Chem 51:7892–7900
Brown GC (1995) Reversible binding and inhibition of catalase by nitric oxide. Eur J Biochem 232:188–191
Cooper CE (1999) Nitric oxide and iron proteins. Biochim Biophys Acta 1411:290–309
Graf E, Mahoney JR, Bryant RG, Eaton JW (1984) Iron catalyzed hydroxyl radical formation: stringent requirement for free iron coordination site. J Biol Chem 259:3620–3624
Dean RT, Fu S, Stocker R, Davies MJ (1997) Biochemistry and pathology of radical-mediated protein oxidation. Biochem J 324:1–18
Halliwell B, Gutteridge JMC (1989) Lipid peroxidation: a radical chain reaction. Free Radic Biol Med. 2nd Edition Clarendon Press, Oxford
Yu BP, Yang R (1996) Critical evaluation of the free radical theory of aging. A proposal for oxidative stress hypothesis. Ann N Y Acad Sci 786:1–11
Bradley-Whitman MA, Lovell MA (2015) Biomarkers of lipid peroxidation in Alzheimer disease (AD): an update. Arch Toxicol 89:1035–1044
Ho KP, Li L, Zhao L, Qian ZM (2003) Genistein protects primary cortical neurons from iron-induced lipid peroxidation. Mol Cell Biochem 247:219–222
Matés JM, Sánchez-Jiménez F (1999) Antioxidant enzymes and their implications on pathophysiologic processes. Front Biosci 4:339–345
Zhang R, Piao MJ, Oh MC, Park JE, Shilnikova K, Moon YK, Kim DH, Hung U, Kim IG, Hyun JW (2016) Protective effect of an isoflavone, tectorigenin, against oxidative stress-induced cell death via catalase activation. J Cancer Prev 21:257–269
Fang Y, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18:872–879
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Int J Biochem Cell Biol 39:44–84
Boadi WY, Thaire L, Kerem D, Yannai S (1991) Effects of dietary factors on antioxidant enzymes in rats exposed to hyperbaric oxygen. Vet Hum Toxicol 33:105–109
Mariani E, Polidori MC, Cherubini A et al (2005) Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B 827:65–75
Bolasco A, Carradori S, Fioravanti R (2010) Focusing on new monoamine oxidase inhibitors. Expert Opin Ther Pat 20:903–909
Hou WC, Lin RD, Chen C, Lee MH (2005) Monoamine oxidase B (MAO-B) inhibition by active principles from Uncaria rhynchophylla. J Ethnopharmacol 100:216–220
Seif-El-Nasr M, Amina SA, Rania MA (2008) Effect of MAO-B inhibition against ischemia-induced oxidative stress in the rat brain. Drug Res 58:160–167
Fowler JS, Logan J, Volkow ND, Wang GJ, MacGregor R, Ding Y (2002) Monoamine oxidase: radiotracer development and human studies. Methods 27:263–277
Girgin SF, Sozmen EY, Ersoz B, Mentes G (2004) Link between monoamine oxidase and nitric oxide. Neurotoxicology 25:91–99
Nagatsu T (2004) Progress in monoamine oxidase (MAO) research in relation to genetic engineering. Neurotoxicology 25:11–20
Zarmouh NO, Messeha SS, Elshami FM, Soliman KF (2016) Evaluation of the isoflavone genistein as reversible human monoamine oxidase-a and-b inhibitor. Evid Based Complementary Altern Med 2016:1–12
Zarmouh NO, Eyunni S, Soliman KF (2017) The benzopyrone biochanin-A as a reversible, competitive and selective monoamine oxidase B inhibitor. BMC Complement Altern Med 17:34
Kuiper G, Lemmen J, Carlsson BO, Corton JC, Safe S, Van Der Saag P, Gustafsson J (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocr Rev 139:4252–4263
McDonnell DP, Norris JD (2002) Connections and regulation of the human estrogen receptor. Science 296:1642–1644
Bach AWJ, Lan NC, Johnson SL, Abell CW, Bembenek ME, Kwan S-W, Seeburg PH, Shih JC (1988) cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci USA 85:4934–4938
Gundlah C, Lu NZ, Bethea CL (2002) Ovarian steroid regulation of monoamine oxidase-A and B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology 160:271–282
Acknowledgements
We acknowledge fellowships from CNPq (R.F.) and CAPES (L.F.S., A.B., C.M.F, L.R.P).
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001, and CAPES/PROEX (23038.005848/2018-31; support number: 0737/2018). Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul – FAPERGS/Brazil (2080–2551/13-5-PqG-001/2013) and Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq/Brazil (475210/2013-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they do not hold any conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva Schmitz, I., Schaffer, L.F., Busanello, A. et al. Isoflavones prevent oxidative stress and inhibit the activity of the enzyme monoamine oxidase in vitro. Mol Biol Rep 46, 2285–2292 (2019). https://doi.org/10.1007/s11033-019-04684-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04684-z