Abstract
miRNAs have emerged as crucial regulators in the regulation of development as well as human diseases, especially tumorigenesis. The aims of this study are to evaluate miR-30b-5p expression pattern and mechanism in gastric carcinogenesis due to which remains to be determined. Expression of miR-30b-5p was analyzed in 51 gastric cancer cases and 4 cell lines by qRT-PCR. The effect of DNA methylation on miR-30b-5p expression was assessed by MSP and BGS. In order to know whether DNMT1 increased miR-30b-5p promoter methylation, DNMT1 was depleted in cell lines AGS and BGC-823. The role of miR-30b-5p on cell migration was evaluated by wound healing assays. Decreased expression of miR-30b-5p was found in gastric cancer samples. In tumor, the expression level of miR-30b-5p was profound correlated with lymph node metastasis (P = 0.019). The level of miR-30b-5p may be restored by DNA demethylation and DNMT1 induced miR-30b-5p promoter methylation. In vitro functional assays implied that enforced miR-30b-5p expression affected cell migration, consistent with tissues analysis. Our findings uncovered that miR-30b-5p is significantly diminished in gastric cancer tissues, providing the first insight into the epigenetic mechanism of miR-30b-5p down-regulation, induced by DNMT1, and the role of miR-30b-5p in gastric cancer carcinogenesis. Overexpression of miR-30b-5p inhibited cell migration. Thus, miR-30b-5p may represent a potential therapeutic target for gastric cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Gastric cancer is the fourth most common cancer and one of the leading causes of cancer related deaths in the world, though its incidence has decreased in the last decade [1]. The poor prognostic methods used to detect gastric cancer and the major clinical limitation in detecting cancer at an early stage lead to less than 5 percent people survive for more than five years [2]. In recent times, gastric cancer is generally considered as the results of deregulation in complex biological processes including many genes which regulate activities such as cell growth, death or apoptosis, DNA repair, etc. The alterations in gene regulation activities result from various underlying genetic instabilities and epigenetic changes [3, 4]. However, current tumor markers are not ideal in relatively low specificity and sensitivity. Thus, there is an urgent need to identify more specific and sensitive novel markers for clinical management of gastric cancer.
MicroRNAs (miRNAs) are non-coding small RNAs of 19–25 nucleotides that have a crucial function in post-transcriptional regulation by binding to the 3′UTR of mRNA, inhibiting translation or mRNA degradation. It has been reported that as many as 30–60 % of protein-encoding genes may be regulated by miRNAs [3, 4]. Depending on their mRNA target, miRNAs can function as a tumor suppressor or promoter of oncogenesis. Many studies have focused on aberrantly expressed miRNAs and their effects on tumorigenesis. Human microRNA-30b (miR-30b), a member of miR-30 family, has been reported to be associated with various human malignancies. In TRAIL-resistant glioma cells, miR-30b expression is particularly increased and impairs TRAIL-dependent apoptosis by targeting caspase-3 [5]; High miR-30b expression in combination with other miRNAs significantly predicts a shorter recurrence-free survival in hepatocellular carcinoma [6]; Up-regulation of miR-30b plays a role in blocking of terminal B cell differentiation in primary central nervous system lymphoma (PCNSL) [7]; However, Van Laere et al. found that miR-30b was significantly down-regulated in breast cancer patients in comparison with non- inflammatory breast cancer [8]; Also in Ehrlich ascites tumor cells, miR-30b expression was low, contributing to the cells with drug-resistant phenotype [9]; miR-30b was significantly dysregulated between parathyroid carcinoma and parathyroid adenoma [10]. Thus the expression pattern of miR-30b is different due to various tumors. However, most of previous study reported miR-30b expression and function in different tumors; they didn’t distinguish miR-30b-5p from miR-30b-3p, two mature miRNAs spliced from 5′ or 3′ end of pre-miR-30b. miR-30b-5p may be a novel biomarker for pancreatic ductal adenocarcinoma [11]. Nevertheless, the expression profile and clinical significance of miR-30b-5p in gastric cancer are not clear, not to mention the mechanism that miR-30b-5p deregulation.
In the present study, we set out to evaluate the expression of miR-30b-5p in gastric cancer tissues and analyze its correlation with clinicopathological factors of gastric cancer patients, and the epigenetic mechanism of miR-30b-5p down-regulation in gastric cancer cell lines, which will provide a potential molecular therapeutic target for human gastric cancer.
Materials and methods
Cell culture and tissue specimens
Human gastric adenocarcinoma cell lines AGS, MGC-803, SGC-7901, BGC-823 were purchased from the Cell Bank of Chinese Academy of Sciences. Cells were routinely cultured with RPMI-1640 medium supplemented with 10 % fetal bovine serum at 37 °C in a humidified atmosphere with 5 % CO2. Gastric cancer and adjacent non-cancerous tissue specimens were obtained from the 3rd Affiliated Hospital of Harbin Medical University. The samples and our study were approved by the Committees for Ethical Review of Research at the 3nd Affiliated Hospital of Harbin Medical University in China and the patients signed informed consent forms. The clinicopathological features are shown in Supplementary Table 1.
Real-time reverse transcriptase quantitative PCR (qRT-PCR)
Total RNA was extracted from cells and tissues samples with Trizol reagent (Invitrogen, USA). The quality and quantity of the RNA samples were assessed by standard electrophoresis and spectrophotometric methods. The first-strand cDNA was synthesized using reverse transcription kit (TAKARA, Japan) according to the manufacturer’s instruction. Hsa-miR-30b-5p gene expression was examined using a SYBR-Green PCR kit (TAKARA, Japan).The primers were purchased from RIBO Corporation (Guangzhou, China) and U6 small nuclear RNA was used as a normalizer. PCR was performed at 95 °C for 30 s, followed by 40 cycles of 95 °C for 30 s and 60 °C for 30 s. The relative expression ratio of hsa-miR-30b-5p in gastric cancer tissues and cells was quantified by the 2−△△CT method.
5′-Aza treatment, Methylation-Specific PCR (MSP), Bisulfite Genome Sequencing (BGS)
A total of 1.5 × 105 AGS or BGC-823 cells were plated in 6-well plates. The cells were cultured in medium containing 0, 10 or 50 µM of the DNA methyltransferase inhibitor 5′-Aza (Sigma, USA) for 72 h. Then RNA was extracted and first-strand cDNA was synthesized for qRT-PCR.
Genomic DNA was extracted from the cells using the phenol–chloroform method followed by bisulfite modification. Methylation-specific PCR (MSP) and bisulfite genome sequencing (BGS) were performed as previously described [12]. Bisulfite-treated genomic DNA from AGS-cMT1 and AGS-SiMT1 cells was amplified using methylated miR-30b-5p primers: MF: 5′-TCGGGTATCGGATATGTTTAGTAAC-3′; MR: 5′-TTAAAAAATAATTTAAACCTCCGCC-3′ and unmethylated primers: UF: 5′-TGGGTATTGGATATGTTTAGTAATGT-3′; UR: 5′-TTAAAAAATAATTTAAACCTCCACC-3′. Primers for BGS were: 5′-GATTGAGTTGGTTTGGTTGAGTATT-3′ (forward) and 5′-TCCCATTTTAAAAACTCTCCTACTC-3′ (reverse). PCR products for the bisulfite sequencing were gel-purified, sub-cloned into a pMD19-T vector system (TAKARA, Japan). At least ten colonies were sequenced to assess the degree of methylation in each CpG site.
A vector-based DNMT1 siRNA construct transfection to gastric cancer cell lines AGS and BGC-823
SiRNAs targeting DNMT1 were designed and prepared as previous described [13]. The siRNA sequences against DNMT1 were designed as sense and anti-sense oligonucleotides corresponding to nucleotide position 2,620–2,638 of human DNMT1 (GenBank Accession No. NM001379.1). There was no homology with other human genes by scanning the GenBank of NCBI using this siRNA. The human gastric cancer cell lines were seeded one day before transfection in order that they were 70–80 % confluent the next day. 4 μg of DNMT1 siRNA construct was transfected by transfection reagent from Roche. Control cells were treated with pSUPER-GFP plasmid. Cells were grown and selectively cultured in 0.4 mg/ml Geneticin (Life Technologies, USA) for 2 months after the initial transfection. AGS and BGC-823 cells transfected with DNMT1 siRNA construct were labeled as AGS-SiMT1, BGC-SiMT1; those transfected with DNMT1 scramble sequence were labeled as AGS-cMT1 or BGC-cMT1.
Western blot analysis
Western blot analysis was performed to detect DNMT1 protein expression in gastric cancer cell lines. The protein concentration of each extract was standardized using the BCA assay (Pierce, USA). The DNMT1 primary antibody (1:1000, Santa, USA) and the mouse monoclonal anti-β-actin antibody (1:8000, Sigma, USA) were used to detect DNMT1 protein levels. The intensities of specific protein bands were quantified with Gel Pro 3.2 (UVP, CLL, USA), corrected for the intensity of the respective β-actin band.
Wound healing detection
Cell migration was assessed by measuring the movement of cells into a scraped area created by a 200-µl pipette tip, and the spread of the wound closure was observed after the cells transfected with miR-30b-5p mimics (100 nM) for 24 h. miR-30b-5p mimics (UGUAAACAUCCUACACUCAGCU) and negative control were designed and synthesized by GenePharma (Shanghai, China). The cells were photographed under a microscope.
Statistical analysis
Correlations between the miRNA expression levels and pathological features were analyzed with the χ2 test using SPSS 13.0 software for Windows. Differences were analyzed by Fisher’s exact test. The independent Student’s t-test was used to compare the results, which were expressed as the mean ± SD between any two preselected groups.
Results
Expression of miR-30b-5p is significantly down-regulated in gastric cancer and associated with cancer metastasis
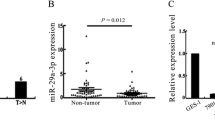
The miR-30b-5p levels were detected by qRT-PCR in gastric tumor, matched non-tumor tissue specimen and 4 gastric cancer cell lines. We confirmed that reduced expression of miR-30b-5p was a frequent event in gastric cancer tissue and the frequency of miR-30b-5p down-regulation was 62.75 % (32/51). The expression levels of miR-30b-5p in gastric cancer were lower than that in non-tumor tissues (P = 0.007) (Fig. 1a). The decreased expression of miR-30b-5p was found in AGS and BGC-823 cells, compared to normal tissues (five pooled normal tissues) (P = 0.023; P = 0.017) (Fig. 1b).
Relative miR-30b-5p expression is determined in gastric cancer tissues and cell lines by qRT-PCR. Actin was used as a internal control. a The top and bottom horizontal lines of the box indicate the 25th and 75th percentiles, respectively. The bold lines within the box indicate the median values. Every dot indicates one sample. N : non-tumor tissues. b Detection of miR-30b-5p expression in gastric cancer cell lines and 5 pooled normal tissues (N). * P < 0.05
In order to correlate the expression pattern of hsa-miR-30b-5p with clinicopathological features of patients in gastric cancer, patients were categorized into two groups according to the expression level difference (≥1.5-fold) of miR-30b-5p. However, there was no statistically significant association between miR-30b-5p and clinicopathological features, including age, gender, differentiation, invasion degree and lymph node metastasis (Supplementary Table 1).
While the 51 pairs specimens were categorized based on clinical feature, differentiation and invasive degree had no relationship with miR-30b-5p expression (P = 0.736; P = 0.536) (Fig. 2a, b), but we found the expression level of miR-30b-5p was profound correlated with lymph node metastasis (P = 0.019) (Fig. 2c). These data indicated that decreased miR-30b-5p expression may play an important role in the metastasis process of gastric cancer.
Silenced miR-30b-5p was restored by methylation inhibitor 5′-Aza
Corcoran et al. [14] predicted the promoter of miR-30b contains CpG islands and has a statistically significant Pol II signal. To examine whether miR-30b-5p down-regulation due to epigenetic inactivation, gastric cancer cell lines AGS and BGC-823 were treated with DNA methylation inhibitor 5-aza-2′-deoxycytidine (5′-Aza). miR-30b-5p expression was restored with different dose of 5′-Aza treatment in AGS (Fig. 3a, P = 0.011, P = 0.005) and BGC-823 cell lines (Fig. 3b, P = 0.045, P = 0.011) depending on dosage. These findings imply the expression of miR-30b-5p is affected by methylation in gastric cancer cells.
miR-30b-5p was induced after DNMT1 knockdown in gastric cancer cell lines
5′-Aza, a potent inhibitor of DNMT1, is known to induce demethylation and reactivation of silenced genes. 5′-Aza incorporates into DNA forming covalent adducts with cellular DNMT1, thereby depleting the cells from enzyme activity and causing de-methylation of genomic DNA as a secondary consequence [15, 16]. Thus we knocked down DNMT1 protein expression in AGS and BGC-823 cell lines (Fig. 4a, c) to further explore whether miR-30b-5p expression was affected by DNMT1. The results showed that miR-30b-5p expression increased in AGS-SiMT1 and BGC-SiMT1 compared to scramble SiRNA transfected cell lines AGS-cMT1 and BGC-cMT1 (Fig. 4b, P = 0.026 and 4d, P = 0.025).
miR-30b-5p expression increased in DNMT1 knocked-down gastric cancer cell lines. a and c, DNMT1 expression of protein level was inhibited in AGS and BGC cell lines by western blot detection. b and d, miR-30b-5p expression increased in AGS-SiMT1 and BGC-SiMT1 compared to scramble SiRNA transfected cell lines AGS-cMT1 and BGC-cMT1
Depletion of DNMT1 induced miR-30b-5p expression via demethylation of the promoter
In order to evaluate whether promoter methylation status contribute to induced miR-30b-5p by DNMT1 RNAi, MSP and BGS were employed to detect methylation status in miR-30b-5p promoter region (Fig. 5). MSP showed that the restoration of miR-30b-5p was associated with its promoter demethylation in AGS-SiMT1 and BGC-SiMT1 cell lines (Fig. 5b, P < 0.05 and Fig. 4). Subsequently, a 218 bp (−3164/− 2947) fragment that contained part of the miR-30b-5p promoter was chosen for further methyaltion analysis by bisulfite sequencing (Fig. 5c). Ten individual colonies were sequenced to identify methylated cytosine residues. We found that all 4 CpG sites were methylated in AGS-cMT1. In contrast, different CpG sites (S1, S2 or S4) were unmethylated in the AGS-SiMT1 cell line. The results indicated that DNMT1 knockdown changed the methylation status of certain CpG sites within the promoter of miR-30b-5p gene. Of note, methylation levels of CpG sites at the predicted binding sites for the STATx transcription factors was altered in AGS-cMT1 and AGS-SiMT1 cells. For the STATx transcription factors binding sites, the methylation ratio of the CpG sites was 100 and 60 % in AGS-cMT1 and AGS-SiMT1 cells, respectively. Collectively, these data suggested that miR-30b-5p expression is regulated in a methylation-dependent manner and that miR-30b-5p may be preferentially methylated by DNMT1.
DNMT1 suppresses miR-30b-5p expression via DNA methylation. a CpG island in the miR-30b-5p gene promoter (5,000 bp upstream of TSS). b The methylation status of the miR-30b-5p promoter was detected in AGS-SiMT1 and AGS-cMT1 cells by methylation-specific PCR. M, methylation-specific primer amplification; U, unmethylation-specific primer amplification. c Mapping of the methylation status of individual CpG sites in the miR-30b-5p promoter by bisulfite genomic sequencing in AGS-SiMT1 and AGS-cMT1 cells. The regions spanning the CpG island with 4 CpG sites were analyzed. Black represents the methylated CpG site; white represents the unmethylated CpG site; each circle represents bisulfite sequencing of 1 CpG site for 10 random clones from AGS-SiMT1 and AGS-cMT1 cells. The boxed regions show the schematic of transcription factor binding sites
miR-30b-5p inhibits migration of gastric cancer cell lines
To investigate the role of miR-30b-5p in gastric cancer metastasis, we transfected miR-30b-5p mimic into AGS and BGC-823 cells and performed in vitro cell migration assay. The in vitro migration assay showed that AGS cells migrated significantly slower after transfection of miR-30b-5p mimic at 24 h, compared to control cells (Fig. 6a); BGC-823 cells with miR-30b-5p overexpression also markedly inhibited cell migration ability at 24 h after transfection, in contrast to BGC-823 with control (Fig. 6b). Collectively, these results imply that miR-30b-5p inhibits the migration of gastric cancer cell lines.
Discussion
Studies have shown that miR-30b participated in tumorigenesis of several tumor types, including glioma [5], hepatocellular carcinoma [6], primary central nervous system lymphoma (PCNSL) [7], breast cancer patients [8] and Ehrlich ascites tumor [9], however, the expression profile is different varying the tumors. In present study, we detected miR-30b-5p expression in gastric cancer cases. The result demonstrated a markedly attenuation of miR-30b-5p expression in 62.75 % of gastric cancer tissues. While Fukushima R [17] et al. reported that miR-30b expression was up-regulated in pooled RNA samples of primary gastric cancer from 5 patients. Based on our findings, no consensus was reached may due to the differences in ethnic groups, geographical areas or sample size between individual studies.
Given that more than 90 % of cancer patients mortality is attributed to metastasis [18], miR-30b has been shown to play a role in metastasis and epithelial-to-mesenchymal transition (EMT) [19, 20]. Interestingly, we found down-regulation of miR-30b-5p expression significantly correlated with lymph node metastasis in gastric cancer patients. When we examined the metastatic ability of gastric cancer cells after miR-30b-5p mimic and negative control (NC) treatments, we found enhanced expression of miR-30b-5p can inhibit cancer metastasis. With the help of bioinformatics prediction (Target scan, miRanda, miRWalk and miRDB), Snail1 [19], MMP21 [21, 22], RECK [23], VIM, Bcl-9 [24], Bcl-10 [25] were identified as direct targets of miR-30b-5p. Thus, the function of miR-30b-5p in metastasis and EMT depends on its target regulation. However, future studies are required to validate the association between miR-30b-5p and targets. These results indicate that miR-30b-5p may serve as a potential predictor for prognosis of gastric cancer patients. Further studies with more clinical samples from different regions need to be warranted.
Expression of miR-30b-5p was down-regulated in gastric cancer, but the mechanisms underlying remain unknown. To further explore the reason of miR-30b-5p down-regulation in gastric cancer, we considered an alternative mechanism of miRNA down-regulation by epigenetic transcriptional silencing regulation [26]. We found that the level of miR-30b-5p could be induced with dose-dependent after treated with 5′-Aza. Thus, we suggest that decreased expression of miR-30b-5p in human gastric cancer cell lines are, at least in part, due to methylation of miR-30b-5p promoter.
The epigenetic regulation is essential for mammalian development and its abnormalization leads to a series of diseases including cancer [27–29]. Mammalian DNA is predominantly methylated at the C-5-position of CpG islands by concerted action of three DNA methyltransferases, namely, DNMT1, DNMT3A, and DNMT3B [30]. Several studies have suggested that 5′-Aza restores the expression of silenced genes by selective degradation or the partial influence of DNMT1 [15, 16], moreover, miRNAs were modulated by DNMT1 [31, 32]. We found DNMT1 knock-down induced miR-30b-5p expression. DNMT1 altered methylation status of specific transcription factor binding sites such as STATx. Methylation of STATx binding sites via DNMT1 maybe associated with impaired binding of STATx at the miR-30b-5p promoter and the inactivation transcription of miR-30b-5p. Up to date, there are no studies on the mechanisms of miR-30b-5p down-regulation in gastric cancer; the present study is the first report showing depletion of DNMT1 restored miR-30b-5p expression by demethylating the promoter in gastric cancer cell lines.
Conclusions
In conclusion, our study demonstrates for the first time that epigenetic mechanism of decreased expression of miR-30b-5p in gastric cancer. DNMT1 could suppress miR-30b-5p expression partly through methylation of its promoter. Diminished miR-30b-5p might be a poor prognostic factor for gastric cancer patients. Overexpression of miR-30b-5p inhibits cell metastasis.
Abbreviations
- qRT-PCR:
-
Quantitative reverse-transcription polymerase chain reaction
- DNMT1:
-
DNA methyltransferase 1
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. doi:10.3322/caac.20107
Ghafoor A, Jemal A, Cokkinides V, Cardinez C, Murray T, Samuels A, Thun MJ (2002) Cancer statistics for African Americans. CA Cancer J Clin 52(6):326–341
Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136(4):642–655. doi:10.1016/j.cell.2009.01.035
Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19(1):92–105. doi:10.1101/gr.082701.108
Quintavalle C, Donnarumma E, Iaboni M, Roscigno G, Garofalo M, Romano G, Fiore D, De Marinis P, Croce CM, Condorelli G (2012) Effect of miR-21 and miR-30b/c on TRAIL-induced apoptosis in glioma cells. Oncogene. doi:10.1038/onc.2012.410
Huang YH, Lin KH, Chen HC, Chang ML, Hsu CW, Lai MW, Chen TC, Lee WC, Tseng YH, Yeh CT (2012) Identification of postoperative prognostic microRNA predictors in hepatocellular carcinoma. PLoS ONE 7(5):e37188. doi:10.1371/journal.pone.0037188
Fischer L, Hummel M, Korfel A, Lenze D, Joehrens K, Thiel E (2011) Differential micro-RNA expression in primary CNS and nodal diffuse large B-cell lymphomas. Neuro-oncology 13(10):1090–1098. doi:10.1093/neuonc/nor107
Van der Auwera I, Limame R, van Dam P, Vermeulen PB, Dirix LY, Van Laere SJ (2010) Integrated miRNA and mRNA expression profiling of the inflammatory breast cancer subtype. Br J Cancer 103(4):532–541. doi:10.1038/sj.bjc.6605787
Husted S, Sokilde R, Rask L, Cirera S, Busk PK, Eriksen J, Litman T (2011) MicroRNA expression profiles associated with development of drug resistance in Ehrlich ascites tumor cells. Mol Pharm 8(6):2055–2062. doi:10.1021/mp200255d
Rahbari R, Holloway AK, He M, Khanafshar E, Clark OH, Kebebew E (2011) Identification of differentially expressed microRNA in parathyroid tumors. Ann Surg Oncol 18(4):1158–1165. doi:10.1245/s10434-010-1359-7
Yabushita S, Fukamachi K, Tanaka H, Sumida K, Deguchi Y, Sukata T, Kawamura S, Uwagawa S, Suzui M, Tsuda H (2012) Circulating microRNAs in serum of human K-ras oncogene transgenic rats with pancreatic ductal adenocarcinomas. Pancreas 41(7):1013–1018. doi:10.1097/MPA.0b013e31824ac3a5
Tao Q, Huang H, Geiman TM, Lim CY, Fu L, Qiu GH, Robertson KD (2002) Defective de novo methylation of viral and cellular DNA sequences in ICF syndrome cells. Hum Mol Genet 11(18):2091–2102
Fan H, Xu J, Wu S, Zhao Z, Zhang J, Xie W (2005) Construction of DNMT1 siRNA stable expressing vector and evaluation of its silenced efficiency in blocking gene expression (Zhonghua yi xue yi chuan xue za zhi = Zhonghua yixue yichuanxue zazhi). Chin J Med Genet 22 (2):142–145
Corcoran DL, Pandit KV, Gordon B, Bhattacharjee A, Kaminski N, Benos PV (2009) Features of mammalian microRNA promoters emerge from polymerase II chromatin immunoprecipitation data. PLoS ONE 4(4):e5279. doi:10.1371/journal.pone.0005279
Christman JK (2002) 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 21(35):5483–5495. doi:10.1038/sj.onc.1205699
Ghoshal K, Datta J, Majumder S, Bai S, Kutay H, Motiwala T, Jacob ST (2005) 5-Aza-deoxycytidine induces selective degradation of DNA methyltransferase 1 by a proteasomal pathway that requires the KEN box, bromo-adjacent homology domain, and nuclear localization signal. Mol Cell Biol 25(11):4727–4741. doi:10.1128/mcb.25.11.4727-4741.2005
Inoue T, Iinuma H, Ogawa E, Inaba T, Fukushima R (2012) Clinicopathological and prognostic significance of microRNA-107 and its relationship to DICER1 mRNA expression in gastric cancer. Oncol Rep 27(6):1759–1764. doi:10.3892/or.2012.1709
Barker HE, Chang J, Cox TR, Lang G, Bird D, Nicolau M, Evans HR, Gartland A, Erler JT (2011) LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res 71(5):1561–1572. doi:10.1158/0008-5472.can-10-2868
Zhang J, Zhang H, Liu J, Tu X, Zang Y, Zhu J, Chen J, Dong L (2012) miR-30 inhibits TGF-beta1-induced epithelial-to-mesenchymal transition in hepatocyte by targeting Snail1. Biochem Biophys Res Commun 417(3):1100–1105. doi:10.1016/j.bbrc.2011.12.121
Gaziel-Sovran A, Segura MF, Di Micco R, Collins MK, Hanniford D, Vega-Saenz de Miera E, Rakus JF, Dankert JF, Shang S, Kerbel RS, Bhardwaj N, Shao Y, Darvishian F, Zavadil J, Erlebacher A, Mahal LK, Osman I, Hernando E (2011) miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell 20(1):104–118. doi:10.1016/j.ccr.2011.05.027
Wu T, Li Y, Liu X, Lu J, He X, Wang Q, Li J, Du X (2011) Identification of high-risk stage II and stage III colorectal cancer by analysis of MMP-21 expression. J Surg Oncol 104(7):787–791. doi:10.1002/jso.21970
Huang Y, Li W, Chu D, Zheng J, Ji G, Li M, Zhang H, Wang W, Du J, Li J (2011) Overexpression of matrix metalloproteinase-21 is associated with poor overall survival of patients with colorectal cancer. J Gastrointest Surg 15(7):1188–1194. doi:10.1007/s11605-011-1519-5
Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu W, Xiao S, Lu H (2008) miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest 88(12):1358–1366. doi:10.1038/labinvest.2008.94
Mani M, Carrasco DE, Zhang Y, Takada K, Gatt ME, Dutta-Simmons J, Ikeda H, Diaz-Griffero F, Pena-Cruz V, Bertagnolli M, Myeroff LL, Markowitz SD, Anderson KC, Carrasco DR (2009) BCL9 promotes tumor progression by conferring enhanced proliferative, metastatic, and angiogenic properties to cancer cells. Cancer Res 69(19):7577–7586. doi:10.1158/0008-5472.can-09-0773
Rehman AO, Wang CY (2009) CXCL12/SDF-1 alpha activates NF-kappaB and promotes oral cancer invasion through the Carma3/Bcl10/Malt1 complex. Int J Oral Sci 1(3):105–118. doi:10.4248/ijos.09059
Baylin SB, Ohm JE (2006) Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer 6(2):107–116. doi:10.1038/nrc1799
Smith LT, Otterson GA, Plass C (2007) Unraveling the epigenetic code of cancer for therapy. Trends Genet 23(9):449–456. doi:10.1016/j.tig.2007.07.005
Feinberg AP (2007) Phenotypic plasticity and the epigenetics of human disease. Nature 447(7143):433–440. doi:10.1038/nature05919
Baylin SB (2005) DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol 2(Suppl 1):S4–S11. doi:10.1038/ncponc0354
Goll MG, Bestor TH (2005) Eukaryotic cytosine methyl transferases. Annu Rev Biochem 74:481–514. doi:10.1146/annurev.biochem.74.010904.153721
Zuo J, Xia J, Ju F, Yan J, Zhu A, Jin S, Shan T, Zhou H (2013) MicroRNA-148a can regulate runt-related transcription factor 3 gene expression via modulation of DNA methyltransferase 1 in gastric cancer. Mol Cells 35(4):313–319. doi:10.1007/s10059-013-2314-9
Xu Q, Jiang Y, Yin Y, Li Q, He J, Jing Y, Qi YT, Li W, Lu B, Peiper SS, Jiang BH, Liu LZ (2013) A regulatory circuit of miR-148a/152 and DNMT1 in modulating cell transformation and tumor angiogenesis through IGF-IR and IRS1. J Mol Cell Biol 5(1):3–13. doi:10.1093/jmcb/mjs049
Acknowledgments
This work was supported by the National Natural Science Foundation of China, No. 81171915 and 91229107, and the Fundamental Research Funds for the Central Universities (CXLX12_0074).
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Fengchang Qiao and Kun Zhang have equal contribution to this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qiao, F., Zhang, K., Gong, P. et al. Decreased miR-30b-5p expression by DNMT1 methylation regulation involved in gastric cancer metastasis. Mol Biol Rep 41, 5693–5700 (2014). https://doi.org/10.1007/s11033-014-3439-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3439-4