Abstract
The wheat aleurone is formed from surface endosperm cells, and its developmental status reflects its biogenesis, structural characteristics, and physiological functions. In this report, wheat caryopses at different development stages were embedded in Spurr’s low-viscosity embedding medium for observation of the development of aleurone cells (ACs) by light microscopy, scanning electron microscopy, and fluorescence microscopy, respectively. According to their structures and physiological characterization, the ACs development process was divided into five stages: endosperm cellulization, spherosome formation, aleurone grain formation, filling material proliferation, and maturation. Furthermore, ACs in different parts of the caryopsis formed differently. ACs near the vascular bundle developed earlier and formed transfer cells, but other ACs formed slowly and did not form transfer cells. ACs on the caryopsis backside were a regular square shape; however, ACs in the caryopsis abdomen were mainly irregular. There were also differences in development between wheat varieties. ACs were rectangular in hard wheat but square in soft wheat. ACs were larger and showed a greater degree of filling in hard compared to soft wheat. The storage materials in ACs were different compared to inner endosperm cells (IECs). The concentrations of minerals such as sodium, magnesium, silicon, phosphorus and potassium were higher in ACs than in IECs. ACs contained many aleurone grains and spherosomes, which store lipids and mineral nutrients, respectively. The cell nucleus did not disappear and the cells were still alive during aleurone maturation. However, IECs were dead and mainly contained amyloplast and protein bodies, which store starch and protein, respectively. Overall, the above results characterized major structural features of aleurone and revealed that the wheat aleurone has mainly four functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aleurone is the innermost layer of the wheat bran and it contains many nutrients, such as protein, lipid, minerals, vitamins and bioactive phytochemicals, which is the most beneficial dietary materials for human health [1–5]. However, a few starch lie in aleurone [6].
Wheat aleurone develops from surface endosperm cells and contains densely granular cytoplasms due to the accumulation of aleurone grains, and the aleurone cells (ACs) have regular cubical shapes and the patterns of cell division and subsequent behavior of daughter cells is highly organized. Buttrose [7] and Morrison et al. [8] group described the process of wheat aleurone development. Wheat aleurone initially develops 10 days after anthesis (DAA). After 14 DAA there were large vacuoles containing small, electron-dense inclusions, which indicated the beginnings of aleurone grains. After 28 DAA, the cell walls have greatly thickened and mature aleurone grains were formed. After 35 DAA, the aleurone grains are completely surrounded by lipid droplets. About 42 DAA, ACs changed little in shape and complex. The walls are distinctly two-layered and exhibit elastoplastic behavior [9, 10]. Their chemical composition was heterogeneous and contain the highest amount of β-glucan [11, 12]. Compared with rice aleurone, wheat aleurone had fewer cells but larger and thicker cell walls [6]. The aleurone development is not only regulated by the multiple levels of gene but also influenced by the hormones [13–16].
The aleurone layer has important functions in the accumulation of storage compounds during seed development and in the mobilization of storage compounds during germination [14, 17, 18]. The aleurone is not only a place where nutrients are absorbed, but also a place where lipids, minerals and proteins are stored [19]. In the seed germination stage, the aleurone perform digestive function, secreting hydrolases to break down the starch and proteins stored in the starchy endosperm cells, which undergo characteristics of programmed cell death [20–22].
Buttrose [7] and Morrison et al. [8] have observed and documented the structure of developing wheat ACs. However, the morphology, structural characteristics, and physiological functions are still unclear. Furthermore, aleurone at different position and varieties are not compared each other. Here, a variety of microscopy techniques were applied to investigate the aleurone development process and its functions were also observed and discussed in detail.
Materials and methods
Plant materials
The experiment was conducted at Yangzhou University farm, Yangzhou, China (32°30′N, 119°25′E) from November 2011 to June 2012. Two different protein cultivars of wheat (Triticum aestivum L.), Yangmai 13 (10.2 % protein content, soft wheat) and Yannong 19 (14.3 % protein, hard wheat), were grown in the farm. The sowing date was November 1. The field soil is sandy loam [Typic fluvaquents, entisols (US taxonomy)] which contains organic materials of 2.45 % and available nitrogen (N), phosphorus (P) and potassium (K) of 106, 33.8 and 66.4 mg/kg, respectively. Available N as urea at 75 kg/hm was applied into the soil on the day of sowing and at the jointing stage, respectively. Heads were tagged at flowering. Samples were harvested at 3, 5, 6, 7, 9, 10,12,13,15, 19, 23, 25, 27, 30, 31, and 33 DAA.
Identification of dehydrogenase in wheat aleurone
Wheat caryopses at different development stages were cut from seed back with a razor blade. Slices of wheat caryopses were placed on a block of white porcelain. They were then stained with 1 % 2,3,5-triphenyltetrazolium chloride (TTC) solution for 20 min at 30 °C and photographed under light microscopy (LM).
SEM observation of caryopsis structure and determination of mineral contents
Typical wheat caryopses were prepared by applying slight pressure on the middle of the caryopsis with a razor blade. The sample thickness was ~ 3 mm. Caryopses with the fractured surface facing upwards were mounted on a specimen stub and sputter-coated with gold before scanning electron microscopy (SEM) viewing (XL30 ESEM, Philips, Holland) at 20 kV. Mineral concentrations were determined for aleurone and endosperm in different parts of the caryopsis using an energy spectrometer attached to the SEM.
LM observation of caryopsis structure
Caryopses were harvested on different days after flowering for LM specimen preparation. Specimens were pre-fixed with 2 % glutaraldehyde, 1 % paraformaldehyde and 0.05 mol/L sodium methyl mercaptide and then fixed with 1 % osmium acid. Sample blocks were washed and dehydrated via an ethanol series of 30–100 %, and then embedded in Spurr’s low-viscosity embedding medium [23]. The semithin sections in 1 μm thickness were cut with a glass knife on a Leica Ultrathin Microtome (EM UC6, Germany), and stained with toluidine blue O for 3 min. The sections were visualized and photographed with Leica Dmls microscopy.
FM observation of caryopsis structure
Caryopses were cut by hand with a blade and slices were placed on a glass slide. The sections were then visualized and photographed by fluorescence microscopy (FM).
Results
Development of wheat aleurone
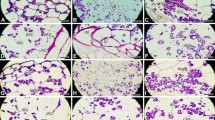
The aleurone development processes were observed by LM (Fig. 1; Supplementary Fig. S1), FM (Supplementary Fig. S2) and SCM (Supplementary Fig. S3), respectively. Aleurone gradually developed from surface endosperm cells, whose morphological structures changed markedly during caryopsis development.
Free nuclei were found in the endosperm nuclear layer by 1–3 DAA. By this time, cell nuclei were large and nucleoli were easily found (Fig. 1a). By 3–4 DAA, cell walls began to form in wheat endosperm. The pericarps were red autofluorescence, due to the abundant chlorophyll (Supplementary Fig. S2A). The vascular bundle in the pericarp was stained red by TCC (Supplementary Fig. S1A. By 5–7 DAA, surface endosperm cells were highly vacuolated (Fig. 1b) and a large number of spherosomes and plastids appeared. Fat began to be accumulated in spherosomes and lipid globules appeared around vacuoles. By 7–10 DAA, the cytoplasm and walls of superficial endosperm cells became thicker and the cells then gradually differentiated into ACs with a square (Fig. 1c). At the same time, aleurone grains began to form in cells. Lipid globules increased and gathered around aleurone grains. The AC walls were uniformly autofluorescent (Supplementary Fig. S2B). The presence of a ferulic acid–carbohydrate complex maybe leads the appearance [7–9]. By 11–27 DAA, aleurone grains and spherosomes greatly increase and the filling materials accumulate largely (Fig. 1d, e; Supplementary Fig. S3 B–F), and ACs were stained more deeply by toluidine blue (Fig. 1d, e), indicating that protein and lipid contents in surface-cell cytoplasm had increased. The autofluorescence of cell walls were further enhanced (Supplementary Fig. S2 C–E). The number of vacuoles decreased and many aleurone grains and lipid bodies were formed (Supplementary Fig. S3 D–F). In addition, ACs were intensely stained red by TTC (Supplementary Fig. S1 C–E), which demonstrated that the dehydrogenase activity in ACs was high, and that ACs had strong physiological activity and vigorous respiration metabolism [6, 19]. By 30–33 DAA, aleurone development was almost complete, ACs no longer showed changes, and the plasmodesmata joining adjacent ACs were obviously observed under LM (Fig. 3d). The cytoplasm of ACs was completely packed with aleurone grains and the cell walls significantly thickened (Fig. 1f). At the moment ACs were stained by TTC, indicating ACs were still alive.
According to their structures and physiological characterization, the ACs development process could be divided into five stages as follows: endosperm cellulization, spherosome formation, aleurone grain formation, filling material proliferation, and maturation. The corresponding characterization at each stage is shown in Table 1.
Differences in aleurone according to location in the caryopsis
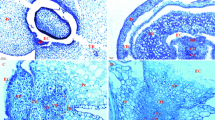
ACs in the caryopsis were respectively located in the backside (Fig. 2a), center (Fig. 2b) and the abdomen (Fig. 2c) of the cavity situated between vascular and endosperm tissue. ACs in the center of the cavity were of irregular shape, gaps between cells were larger (Fig. 2b), and had similar functions to transfer cells [19]. These cells developed ~2–3 days earlier than ACs in other parts of the caryopsis, and they died after filling. ACs in the caryopsis backside and abdomen differed in shape: cells in the backside were a regular square shape whereas those in the abdomen were mainly long and irregular.
Differences in aleurone according to wheat variety
There were aleurone differences between hard and soft wheat (Fig. 3). ACs in hard wheat variety Yannong 19 were larger in size and rectangular in shape (Fig. 3a, c) and they contained more aleurone grains and filling materials. By contrast, ACs in soft wheat variety Yangmai 13 were square in shape and smaller in size and had less filling material (Fig. 3b, d). Similar structures have been described in rice ACs by Chen [24].
Differences between ACs and IECs
It is well known that ACs and IECs both develop from a fertilized polar nucleus. However, they differ greatly in structure, physiology, chemical composition and development (Fig. 4; Table 2). Concentrations of Na, Mg, Si, P, K minerals were much higher in ACs than in endosperm cells (Fig. 4c), which is the similar to the results of Li [25].
Differences between ACs and IECs are listed in Table 2. In addition to differences in mineral contents, ACs contain spherosomes and aleurone grains and their major storage components are lipids and minerals, respectively. The mature AC contain larger nucleus and have thicker wall and higher physiological activity, thus, ACs are still alive cells. On the contrary, IECs contain starch and protein as major storage components. They have thinner walls and lack nucleus and activity. Therefore, they are dead cells.
Discussion
Division of aleurone development stages
Buttrose and Morrison et al. observed the structure of developing ACs of wheat grain. They focused on ACs ultrastructure [7, 8]. They illustrated the results agreed with ours. However, they just studied the aleurone development after 10 DAA. Here, aleurone development was observed during the whole process. Furthermore, we divide the whole process into five stages according to differences in morphological structure, physiological characteristics and the compounds present in ACs. At each stage aleurone exhibits a certain typical characterizations (Table 1). The stages follow on a continuous manner, it is difficult to define a specific time for aleurone development. Moreover, aleurone development is greatly affected by the external environment, especially temperature and light. The wheat used in Buttrose’s report was grown in a glasshouse, whereas wheat in this report was grown in the open field, the time of aleurone development they observed was later than that we observed. Thus, the stage of aleurone development might be classified differently by different environment.
Mechanism of aleurone development
The development of wheat aleurone is not only controlled by nuclear genes and environments [13–16], but also may be related to some filling materials such as minerals, proteins, lipids, and so on [18, 19]. Nutrients from the abdominal vasculature must first be transported via ACs and apoplasts. Then, they reach the inner endosperm via nucellar projections and the filling cavity, respectively [19]. Thus, there were two ways for nutrients to enter the inner endosperm. One is via apoplasts, which can transport nutrients into the surrounding endosperm, and then nutrients enter the inner endosperm via aleurone. The other is direct entry into the endosperm via aleurone near the filling cavity. When the peripheral layer of cells absorb the nutrients, the solute sugar and most of amino acid enter the inner endosperm. However, most of the minerals, lipids, fatty acid, and a few amino acids remain at the peripheral layer of cells. The minerals enter the vacuoles and give rise to the phytin globoids. Then, they form the aleurone grains in combination with protein. At the same time the lipid, which is synthesized by the fatty acid and phosphoglycerol, is accumulated in the spherosome. Therefore, the aleurone develops from the peripheral layer by accumulating rich mineral, lipid and protein.
The ACs morphological structure differed significantly at the different position. For example, ACs near the filling cavity have larger gaps between cells and their walls are thicker compared with other ACs. Most of them were transformed into transfer cells with powerful absorption activity. We found that the amount of filling materials were greater in the caryopsis backside than in the abdomen. There were more aleuronic layers in the backside endosperm than in the abdomen. Thus, the number of aleurone layers was closely related to the amount of filling material. In addition, we also found that the filling time was longer, more filling materials accumulated and AC size was larger for hard compared to soft wheat.
Physiological function of aleurone
According to differences in location, structural characteristics and physiological activity, we can infer that aleurone has four functions as follows. The first is as a nutrient transporter. AC walls are bonded to the outer cell wall of the degraded nucellus. The AC membrane is wrinkled and folded, which is beneficial for water and nutrient transport. Nutrient transport needs energy from respiration. Dehydrogenase plays an important role in driving the electron-transport-chain reactions of cell respiration. TTC is a proton receptor in the respiratory chain, and it can react with dehydrogenase, generating the red substances, which is generally used to represent the vitality of the cells. Here, we found ACs were stained red by TTC during aleurone development. This showed that dehydrogenase activity in ACs was high and ACs had strong physiological activity and vigorous respiration metabolism, and ACs were still alive until mature. Wang et al. also found there are many mitochondria in rice ACs, whose respiration provides energy for nutrient absorption [18]. Second, ACs can also accumulate nutrients and promote the seed germination. Soluble sugars and amino acids of filling materials that enter IECs are converted to starch and protein. Minerals, fatty acids and amino acids can accumulate in ACs, and these materials can provide the nutrient resource for embryo early germination. Third, ACs can decompose the storage materials of endosperm and accelerate the embryo growth. During the wheat seed germination the ACs can secretes hydrolases that release the internal reserves of starch and proteins stored in the starchy endosperm cells. At this point, the aleurone performs an important digestive function. Finally, ACs can maintain the activity of caryopsis and protect caryposis. The aleurone and inner endosperm cells store different types of materials, respectively. In addition, ACs are alive, arranged closely and have thicker walls and higher physiological activities (Table 2). The aleurone becomes a protective layer on the peripheral endosperm. It can keep the endosperm from water and gas, which make endosperm nutrient composition better and the seed preservation longer [26].
Abbreviations
- ACs:
-
Aleurone cells
- DAA:
-
Days after anthesis
- FM:
-
Fluorescence microscopy
- IECs:
-
Inner endosperm cells
- LM:
-
Light microscopy
- SEM:
-
Scanning electron microscopy
- TTC:
-
2,3,5-Triphenyltetrazolium chloride
References
Buri RC, von Reding W, Gavin MH (2004) Description and characterization of wheat aleurone. Cereal Foods World 49:274–282
Fardet A (2010) New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fiber? Nutr Res Rev 23:65–134
Graham SF, Hollis JH, Migaud M, Browne RA (2009) Analysis of betaine and choline contents of aleurone, bran, and flour fractions of wheat (Triticum aestivum L.) using 1H nuclear magnetic resonance (NMR) spectroscopy. J Agric Food Chem 57:1948–1951
Kumar A, Singh NK, Sinha PR, Kumar R (2010) Intervention of acidophilus-casei dahi and wheat bran against molecular alteration in colon carcinogenesis. Mol Biol Rep 37:621–627
Brouns F, Hemery Y, Price R, Anson NM (2012) Wheat aleurone: separation, composition, health aspects, and potential food use. Crit Rev Food Sci Nutr 52:553–568
Gu YJ, Xiong F, Wang Z, Chen G, Li WF (2001) A contrast of the endosperm development between rice and wheat. J Yang Zhou Univ Nat Sci Ed 24:65–74
Buttrose MS (1963) Ultrastructure of the developing aleurone cells of wheat grain. Aust. J. Biol. Sci. 16:768–774
Morrison IN, Kuo J, O’Brien TP (1975) Histochemistry and fine structure of developing wheat aleurone cells. Planta (Berl.) 123:105–116
Fulcher RG, O’Brien TP, Lee JW (1972) Studies on the aleurone layer. I. Conventional and fluorescence microscopy of the cell wall with emphasis on phenol–carbohydrate complexes in wheat. Aust. J. Biol. Sci. 25:23–34
Antoine C, Peyron S, Mabille F, Lapierre C, Bouchet B, Abecassis J, Rouau X (2003) Individual contribution of grain outer layers and their cell wall structure to the mechanical properties of wheat bran. J Agric Food Chem 51:2026–2033
Rhodes DI, Stone BA (2002) Proteins in walls of wheat aleurone cells. J Cereal Sci 36:83–101
Jamme F, Robert P, Bouchet B, Saulnier L, Dumas P, Guillon F (2008) Aleurone cell walls of wheat grain: high spatial resolution investigation using synchrotron infrared microspectroscopy. Appl Spectrosc 62:895–900
Manickavelu A, Koba T, Mishina K, Sassa H (2009) Identification of differential gene expression for Kr1 gene in bread wheat using annealing control primer system. Mol Biol Rep 36:2111–2118
Becraft PW, Yi G (2011) Regulation of aleurone development in cereal grains. J Exp Bot 62:1669–1675
Meziani S, Nadaud I, Benali M, Branlard G (2012) Proteomic analysis of the mature kernel aleurone layer in common and durum wheat. J Cereal Sci 55:323–330
Gillies SA, Futardo A, Henry RJ (2012) Gene expression in the developing aleurone and starchy endosperm of wheat. Plant Biotechnol J 10:668–679
Olsen OA, Lemmon B, Brown RA (1998) Model for aleurone development. Trends Plant sci 3:168–169
Wang Z, Gu YJ, Li WF, Huang S, Li KW (1998) Formation of rice aleurone layer and its changes during germination. J Yang Zhou Univ Nat Sci Ed 1:19–24
Wang Z, Gu YJ, Li WF, Chen G (1998) Development of wheat endosperm and the pathway of nutrient entering the endosperm. Acta Agron Sin 24:436–443
Amangeldy K, Alexander A, Sabira M, Murat K (2011) Presence of base excision repair enzymes in the wheat aleurone and their activation in cells undergoing programmed cell death. Plant Physiol Biochem 49:1155–1164
Wu MZ, Huang JJ, Xu S, Ling TF, Xie YJ, Shen WB (2011) Haem oxygenase delays programmed cell death in wheat aleurone layers by modulation of hydrogen peroxide metabolism. J Exp Bot 62:235–248
Young TE, Gallie DR (2000) Programmed cell death during endosperm development. Plant Mol Biol 44:283–301
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Chen YF, Gu YJ, Wang Z (2009) Difference in caryopsis development among four rice varieties differing in grain weight. Chin J Rice Sci 23(4):405–413
Li CY, Feng CN, Wang CYL, Zhang R, Guo WS, Zhu XK, Feng YX (2007) Differences of mineral element compositions and their contents among different positions of wheat grains. Plant Physiol Commun 43:1077–1081
Jesus VC, Pilar C (2005) Seed maturation: developing an intrusive phase to accomplish a quiescent state. Int J Dev Biol 49:645–651
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31171482), Jiangsu Natural Science Foundation (BK2011445), Jiangsu Graduate Innovation Project (CXLX12-0910), Priority Academic Program Development from Jiangsu Government, Program for New Century Excellent Talents in University (NCET-11-0670) and Jiangsu Shuangchuang Project.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiong, F., Yu, XR., Zhou, L. et al. Structural development of aleurone and its function in common wheat. Mol Biol Rep 40, 6785–6792 (2013). https://doi.org/10.1007/s11033-013-2795-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2795-9