Abstract
The pericarp and endosperm are two important wheat caryopsis sinks containing starch granules. In order to further clarify developmental characteristics of starch granules within wheat pericarp and endosperm, caryopses of hard and soft wheat at different days after pollination were used as experimental materials and their sections were observed with light and electron microscopy in this paper. The results indicated: (1) Pericarp parenchyma cells contained two types of starch granules: single-granule starch granules (ISG) and compound-granule starch granules (CSG). Pericarp parenchyma cells underwent degeneration from the part adjacent to cross cells to the part near the pericarp epidermis. Along with degeneration of pericarp parenchyma cells, CSG were transformed into ISG and all of ISG were gradually disintegrated. (2) There were denser chloroplasts present within the green pericarp cells around the vascular system in hard wheat than in soft wheat. (3) The tiny ISG appeared in aleurone cells at 9 days after pollination, but disappeared in the high-level differentiated aleurone cells. (4) ISG of the inner-layer endosperm transfer cells, sub-aleurone cells and the central starchy endosperm cells developed better in hard wheat than in soft wheat. This was probably because endosperm transfer cells had stronger nutrient transport function and the green pericarp cells provided more photosynthates for the endosperm in hard wheat than in soft wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat caryopsis commonly consists of several components including the pericarp, integuments, nucellar tissues, endosperm and embryo. The pericarp and endosperm are two important caryopsis sinks containing starch granules [3, 8].
The pericarp mainly includes three parts: the pericarp epidermis, pericarp parenchyma cells and the green pericarp cells. The green pericarp cells contain lots of chloroplasts. In addition to cross cells, the other green pericarp cells are around the vascular system. Pericarp parenchyma cells are the main pericarp cells containing starch granules [11].
The endosperm mainly differentiates into three types of cells: aleurone cells, endosperm transfer cells and starchy endosperm cells. Endosperm transfer cells develop wall ingrowths to facilitate nutrient uptake [4]. Aleurone cells contain most of caryopsis minerals [1]. Starchy endosperm cells mainly consist of sub-aleurone cells and the central starchy endosperm cells, and they contain starch granules and protein bodies [5]. According to the granule size, starch granules in mature wheat endosperm are sorted into two types: lenticular-shaped granules ranging from about 15–40 μm, and spherical granules ranging in size from approximately 1–10 μm [2].
Until now, developmental characteristics of starch granules within wheat pericarp and endosperm have not been entirely clarified. This paper used hard and soft wheat as experimental materials to further investigate starch granule developmental characteristics in wheat pericarp and endosperm. The results would provide some useful information for the future investigations on wheat caryopsis development.
Materials and Methods
Plant Material
Plants of two cultivars of wheat (Triticum aestivum L.), cv. Yannong-19 (hard wheat) and cv. Yangfu-2 (soft wheat) grew in the regularly managed experimental field. The growth period was from November 2012 to June 2013. Florets of wheat spike were marked with a permanent pen to determine the anthesis date. Caryopses at different days after pollination were collected.
Sample Observation with Scanning Electron Microscopy
Mature caryopses were cut transversely by hand into several sections of 2–3 mm thick. The caryopsis sections were mounted on aluminium stubs with conducting carbon paint and sputter-coated with gold. The samples could be observed under a scanning electron microscope (Philips XL-30 ESEM).
Sample Observation with Light Microscopy and Transmission Electron Microscopy
Caryopsis tissue slices were fixed in 2.5% glutaraldehyde (pH 7.2) and postfixed in 0.5% osmium tetroxide (pH 7.2).The tissue slices were dehydrated in a graded ethanol series, followed by propylene oxide. The tissue slices were infiltrated and embedded with the low glutinosity Spurr’s resin. The samples were ready for sectioning after the resin polymerized at 70 °C for 12 h in a thermostat. The samples were cut into 1-µm-thin sections and observed under the light microscope (Leica DMLS) after stained with 0.5% methyl violet. The samples were cut into 70-nm-thin sections and observed under the transmission electron microscope (Philips Tecnai 12) after stained with uranyl acetate and lead citrate [12].
Results and Discussion
Starch Granules of Pericarp Parenchyma Cells in Hard and Soft Wheat
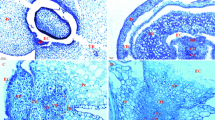
At 9 days after pollination (DAP), pericarp parenchyma cells near the pericarp epidermis contained sparse starch granules including single-granule starch granules (ISG) and compound-granule starch granules (CSG) in hard and soft wheat (Fig. 1a, g). There were mainly ISG present within the degenerated pericarp parenchyma cells adjacent to cross cells in hard and soft wheat (Figs. 1c, i, 2a, b). At 23 DAP, there were only two layers of pericarp cells in addition to the layer of cross cells in hard and soft wheat. The pericarp cells contained no starch granules (Fig. 3a, e). Pericarp parenchyma cells underwent degeneration from the part adjacent to cross cells to the part near the pericarp epidermis. Along with degeneration of pericarp parenchyma cells, CSG were transformed into ISG and all of ISG were gradually disintegrated. The degeneration of pericarp parenchyma cells not only provided space for the endosperm growth, but also formed gas cavity to store CO2 for photosynthesis of the green pericarp cells [11]. The pericarp starch granules were disintegrated into nutrients which were finally absorbed by the endosperm [7].
Microstructures of starch granules in endosperm and pericarp of hard and soft wheat at 9 days after pollination (bar 20 μm). a–f In caryopsis of hard wheat; g–l in caryopsis of soft wheat. AC aleurone cells, CC cross cells, Ch chloroplast, CSE central starchy endosperm, CSG compound-granule starch granules, dPPC degenerated pericarp parenchyma cells, GP green pericarp, ISG single-granule starch granules, ETC endosperm transfer cells, Pb protein body, PeP pericarp epidermis, PPC pericarp parenchyma cells, SAC sub-aleurone cells, VB vascular bundle, WI wall ingrowths
Ultrastructures of starch granules in wheat endosperm and pericarp at 9 days after pollination (a, b bar 5 μm; c, d 2 μm). a in caryopsis of hard wheat; b–d in caryopsis of soft wheat. AC aleurone cells, dPPC degenerated pericarp parenchyma cells, ISG single-granule starch granules, CSE central starchy endosperm
Microstructures of starch granules in endosperm and pericarp of hard and soft wheat at 23 days after pollination (bar 20 μm). a–d In caryopsis of hard wheat; e–h in caryopsis of soft wheat. AC aleurone cells, CC cross cell, CSE central starchy endosperm, ETC endosperm transfer cells, ISG single-granule starch granules, Pb protein body, Pe pericarp, SAC sub-aleurone cells, WI wall ingrowths
At 9 DAP, there were denser chloroplasts present within the green pericarp cells around the vascular system in hard wheat than in soft wheat (Fig. 1b, h). Photosynthates produced in the green pericarp cells got through maternal transport tissues including vascular system, chalaza and nucellar projection, and finally entered the endosperm [10, 11]. Thus, more photosynthates were probably transported into the endosperm from the green pericarp cells in hard wheat than in soft wheat.
Starch Granules of Different Endosperm Cells in Hard and Soft Wheat
At 9 DAP, aleurone cells contained a few tiny ISG and sub-aleurone cells contained a few lenticular-shaped ISG in hard wheat (Fig. 1d). The central starchy endosperm cells contained more and larger lenticular-shaped ISG than sub-aleurone cells (Fig. 1d, f). In soft wheat, aleurone cells contained much smaller ISG than central starchy endosperm cells (Fig. 2c, d). Aleurone cells contained fewer tiny ISG and sub-aleurone cells contained slimmer lenticular-shaped ISG in soft wheat than in hard wheat (Fig. 1d, j). Endosperm transfer cells had thinner wall ingrowths in soft wheat than in hard wheat (Fig. 1e, k). Central starchy endosperm cells contained smaller lenticular-shaped ISG in soft wheat than in hard wheat (Fig. 1f, l). At 23 DAP, there were no starch granules in the high-level differentiated aleurone cells. Sub-aleurone cells contained larger lenticular-shaped ISG in hard wheat than in soft wheat (Fig. 3b, f). Starch granules were much smaller in the inner-layer endosperm transfer cells than in the central starchy endosperm cells (Fig. 3c, d, g, h). Endosperm transfer cells had thicker wall ingrowths and cells of the inner layers contained more ISG in hard wheat than in soft wheat (Fig. 3c, g). The central starchy endosperm cells contained larger lenticular-shaped ISG in hard wheat than in soft wheat (Fig. 3d, h). At 30 DAP, the central starchy endosperm cells in hard wheat were almost filled with lenticular-shaped ISG, among which there were a few spherical ISG (Fig. 4a). The central starchy endosperm cells contained more spherical ISG and fewer lenticular-shaped ISG in soft wheat than in hard wheat (Fig. 4a, b). In mature caryopsis, the central starchy endosperm cells were filled with very dense lenticular-shaped ISG in hard wheat (Fig. 4c). There were dense spherical ISG among lenticular-shaped ISG in the central starchy endosperm cells of soft wheat (Fig. 4d).
Microstructures of starch granules in the central starchy endosperm of hard and soft wheat (bar 20 μm). a, b At 30 days after pollination, c, d in mature caryopses. a, c In hard wheat, b, d in soft wheat. The arrowheads indicated the spherical starch granules of single-granule. CSE central starchy endosperm, ISG single-granule starch granules, PM protein matrix
Starch granule development differed in different endosperm tissues. In order to make nutrient uptake convenient, endosperm transfer cell-specific factors promote wall ingrowth formation and limit starch granule growth at certain degree. Thus, starch granules developed more slowly in the inner-layer endosperm transfer cells than in the central starchy endosperm cells. Aleurone cell-specific factors promote aleurone granule formation and limit starch granule growth [9]. Thus, starch granules disappeared in the high-level differentiated aleurone cells. ISG of the inner-layer endosperm transfer cells, sub-aleurone cells and central starchy endosperm cells developed better in hard wheat than in soft wheat. This probably because endosperm transfer cells had thicker wall ingrowths to facilitate nutrient uptake in hard wheat than in soft wheat. Moreover, more photosynthates were probably transported into the endosperm from the green pericarp cells in hard wheat than in soft wheat.
Conclusions
The pericarp development is coordinated with the endosperm development. Pericarp cells finish proliferation around the time of fertilization and undergo expansion and differentiation at the time of endosperm nuclei proliferation and cellularization [6, 11]. The pericarp is mainly differentiated into the pericarp epidermis, pericarp parenchyma cells and the green pericarp cells. The green pericarp cells perhaps provide photosynthates for the endosperm. The degeneration of pericarp parenchyma cells provides space for the endosperm growth, and starch granules in pericarp parenchyma cells are disintegrated into nutrients which are finally absorbed by the endosperm.
Starch granule development differs in different endosperm cells. Development of endosperm starch granules differs in soft and hard wheat, probably because endosperm transfer cells have stronger nutrient transport function and the green pericarp cells provide more photosynthates for the endosperm in hard wheat than in soft wheat.
The results and conclusions of this paper will further illuminate developmental characteristics of starch granules within wheat pericarp and endosperm, further clarify the relationship between wheat pericarp and endosperm and enrich morphological and physiological knowledge on wheat caryopsis development.
Abbreviations
- DAP:
-
Days after pollination
- CSG:
-
Compound-granule starch granules
- ISG:
-
Single-granule starch granules
References
Brouns F, Hemery Y, Price R, Anson NM (2012) Wheat aleurone: separation, composition, health aspects, and potential food use. Crit Rev Food Sci Nutr 52:553–568
Copeland L, Blazek J, Salman H, Tang MC (2009) Form and functionality of starch. Food Hydrocolloid 23:1527–1534
Fei X, Yu X-R, Zhou L, Wang F, Xiong A-S (2013) Structural and physiological characterization during wheat pericarp development. Plant Cell Rep 32:1309–1320
Offler CE, McCurdy DW, Patrick JW, Talbot MJ (2003) Transfer cells: cells specialized for a special purpose. Annu Rev Plant Biol 54:431–454
Olsen OA (2004) Nuclear endosperm development in cereals and Arabidopsis thaliana. Plant Cell (Suppl) 16:S214–S227
Radchuk V, Weier D, Radchuk R, Weschke W, Weber H (2011) Development of maternal seed tissue in barley is mediated by regulated cell expansion and cell disintegration and coordinated with endosperm growth. J Exp Bot 62:1217–1227
Wang Z, Gu YJ, Li WF, Chen G, Shi HY, Chen XH (1998) Development of wheat endosperm and pathway of nutrient entering the endosperm. Acta Agron Sin 24:536–545
Yu X-R, Zhou L, Zhang J, Yu H, Xiong F, Wang Z (2015) Comparison of starch granule development and physicochemical properties of starches in wheat pericarp and endosperm. J Sci Food Agric 95:148–157
Zheng Y-K, Wang Z (2014) Differentiation mechanism and function of the cereal aleurone cells and hormone effects on them. Plant Cell Rep 33:1779–1787
Zheng Y-K, Wang Z, Gu Y-J (2014) Development and function of caryopsis transport tissues in maize, sorghum and wheat. Plant Cell Rep 33:1023–1031
Zheng Y-K, Wang Z, Yang J-C, Gu Y-J (2015) Observation and comparison of structure changes in wheat caryopsis maternal tissues and endosperm. Braz J Bot 38(2):417–427
Zheng Y-K, Wang Z, Zeng D-E (2016) Developmental characteristics of starch granule occurrence center in sorghum central starchy endosperm. Ind J Plant Physiol 22(1):34–39
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zheng, Y., Fei, X. & Yu, X. Observation and Investigation of Starch Granules Within Wheat Pericarp and Endosperm. Agric Res 6, 320–325 (2017). https://doi.org/10.1007/s40003-017-0270-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-017-0270-x