Abstract
The chromosomal region 10p13 has been linked to paucibacillary leprosy in two independent studies. The MRC1 gene, encoding the human mannose receptor (MR), is located in the 10p13 region and non-synonymous SNPs in exon 7 of the gene have been suggested as leprosy susceptibility factors. We determined that G396S is the only non-synonymous exon 7-encoded polymorphism in 396 unrelated Vietnamese subjects. This SNP was genotyped in 490 simplex and 90 multiplex leprosy families comprising 704 patients (47% paucibacillary; 53% multibacillary). We observed significant under-transmission of the serine allele of the G396S polymorphism with leprosy per se (P = 0.036) and multibacillary leprosy (P = 0.034). In a sample of 384 Brazilian leprosy cases (51% paucibacillary; 49% multibacillary) and 399 healthy controls, we observed significant association of the glycine allele of the G396S polymorphism with leprosy per se (P = 0.016) and multibacillary leprosy (P = 0.023). In addition, we observed a significant association of exon 7 encoded amino acid haplotypes with leprosy per se (P = 0.012) and multibacillary leprosy (P = 0.004). Next, we tested HEK293 cells over-expressing MR constructs (293-MR) with three exon 7 haplotypes of MRC1 for their ability to bind and internalize ovalbumin and zymosan, two classical MR ligands. No difference in uptake was measured between the variants. In addition, 293-MR failed to bind and internalize viable Mycobacterium leprae and BCG. We propose that the MR–M. leprae interaction is modulated by an accessory host molecule of unknown identity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leprosy, caused by the human pathogenic bacterium Mycobacterium leprae (M. leprae) is a chronic infectious disease that has been recognized for centuries. Leprosy is primarily a disease of the skin and nerves but the mechanisms of pathogenesis that lead to cutaneous spread of bacilli and irreversible peripheral nerve damage are poorly understood. The disease manifests itself in two clinical forms that have been termed “paucibacillary” and “multibacillary” leprosy depending on the number of skin lesions and the number of bacilli found in these lesions. The development of leprosy is strongly influenced by host genetics as illustrated by the high concordance rates among monozygotic twins (Chakravartti and Vogel 1973). Consequently, a strategy to better understand the disease pathogenesis is to uncover genes that modulate leprosy susceptibility. Past genome-wide linkage analyses have identified several susceptibility loci in chromosome regions 6q25–q26 (Mira et al. 2003), 6p21 (Miller et al. 2004; Mira et al. 2003), 10p13 (Siddiqui et al. 2001), 17q22 (Miller et al. 2004), 20p12–13 (Miller et al. 2004; Tosh et al. 2002) and 21q22 (Wallace et al. 2004). So far, high density linkage disequilibrium (LD) mapping of the linkage peaks on chromosome regions 6q25–q26 and 6p21 has successfully identified two susceptibility loci by positional cloning, PARK2/PACRG (Mira et al. 2004) and LTA (Alcais et al. 2007), respectively.
The 10p13 chromosomal region carries a replicated susceptibility locus for paucibacillary leprosy (Mira et al. 2003; Siddiqui et al. 2001). The MRC1 gene underlies the corresponding linkage peak and three closely spaced amino acid changes encoded by exon 7 of MRC1 have been suggested to be associated with altered susceptibility to paucibacillary leprosy (Cooke and Hill 2008; Hill 2006). MRC1 encodes the mannose receptor C-type lectin (MR), a cell surface protein that belongs to a family of receptors for pathogen-associated molecular patterns, or PAMPs, and is part of the innate arm of the immune system. MR is a receptor for mannose, fucose and N-acetyl-glucosamine-containing molecules and binds to yeast cell walls (Ezekowitz et al. 1990), to capsular polysaccharides of Klebsiella pneumonia (Kabha et al. 1995) as well as to mycobacterial components such as mannose-capped lipoarabinomannans (ManLAM) (Schlesinger et al. 1996). MR has been shown to be an important mediator between M. tuberculosis and the host immune system (Kang et al. 2005; Nigou et al. 2001; Schlesinger 1993). Most notably, cellular entry through MR has been correlated with M. tuberculosis virulence (Schlesinger 1993; Schlesinger et al. 1996). Taken together, these data prompted us to study MRC1 as a possible leprosy susceptibility gene.
We analyzed the role of MRC1 exon 7 non-synonymous coding polymorphisms in leprosy susceptibility in a familial sample from Vietnam and a case–control sample from Brazil. We observed in both populations that the G396S polymorphism was significantly associated with leprosy per se and multibacillary leprosy. However, we found in the Brazilian sample that the risk effect of the glycine residue at position 396 depended on the exon 7 haplotype. In functional analysis, we failed to observe an impact of the exon 7 polymorphisms on MR mediated uptake of zymosan and ovalbumin. In addition, HEK293 cells over-expressing MR (293-MR) were unable to bind and ingest viable M. leprae and Bacille Calmette Guerin (BCG) bacteria. We concluded that exon 7 polymorphisms mediated the interaction of MR with an unknown accessory molecule that is required for MR-mediated uptake of M. leprae.
Methods
Patients and controls
In Vietnam, 490 simplex (53% multibacillary) and 90 multiplex (55% multibacillary) leprosy families were identified from the records available at the Dermato-Venereology Hospital in Ho Chi Minh City (Alcais et al. 2007; Mira et al. 2003). The criterion for enrollment was the availability of both parents for genetic analysis. In Brazil, 384 leprosy patients were recruited from the Leprosy Outpatient Clinic at the Oswaldo Cruz Institute in Rio de Janeiro. The 399 healthy controls (i.e., no reported infectious or inflammatory disease) were recruited from the same geographic area of Rio de Janeiro. Cases and controls were matched based on self-reported ethnicity. In previous experiments, using genomic controls, we failed to observe significant evidence for population stratification in case–control samples matched on self-reported ethnicity from the same geographic area (Mira et al. 2004). The diagnosis of all leprosy patients and the definition of clinical sub-forms were based on clinical and histological criteria (Ridley and Jopling 1966). Informed consent was obtained from all study participants. The study was approved by institutional review boards and health authorities in Ho Chi Minh City, Vietnam, the Oswaldo Cruz Institute, Rio de Janeiro, Brazil, and the Research Institute of the McGill University Health Centre, Montreal, Canada.
SNP selection
The identification of informative coding SNPs (i.e., minor allele frequency (MAF) >5%) was done by sequencing 23 unrelated Vietnamese individuals for all 30 MRC1 exons, including both untranslated (UTR) regions, using an ABI PRISM® 3100 genetic analyzer. In addition, exon 7 was sequenced in 396 unrelated Vietnamese individuals (a subset of parents from the 490 Vietnamese simplex families). For subsequent analysis, the six identified informative coding SNPs were designated by their corresponding ‘rs’ numbers. In total, 75 SNPs spanning MRC1 (101.8 kb) on chromosome region 10p12.33 were selected based on allelic frequencies publicly available from the NCBI EntrezSNP database (http://www.ncbi.nlm.nih.gov/sites/entrez?db=snp) and the International HapMap project (http://www.hapmap.org/) (Table S1). Conflicting annotations between physical maps regarding gene localization and supposed gene duplication (i.e., MRC1 and MRC1L1) posed technical challenges to the genetic analysis of MRC1, particularly the resolution of SNP positions. For consistency, SNPs with two positions were mapped to MRC1 spanning chr10:17,891,368–17,993,183 (HapMap Data Rel 27) and were referred to by their MRC1L1 ‘rs’ numbers (NCBI EntrezSNP database Build 130). We analyzed MRC1 exon 7 chromatograms in 2,423 unrelated individuals (from our Vietnamese and Brazilian samples and the HGDP-CEPH Human Genome Diversity Cell Line Panel) and found no evidence for a common gene duplication event (i.e., biallelic ratio for SNPs was 1:1). The data argues against the duplication of MRC1 and suggests that MRC1L1 is an erroneous annotation caused by the presence of a sequence gap and the incorrect assignment of a polymorphic haplotype.
Genotyping methods
The SNPs were genotyped on one or several of the following platforms (1) genotyping by direct sequencing on an ABI PRISM® 3100 genetic analyzer; (2) genotyping on the high-throughput GenomeLabΒ™ SNPstream® platform (formerly Orchid SNPstream UHT), which uses a single-base pair extension (SBE) method to incorporate fluorescently labeled terminal nucleotides, which are then detected by a specialized imager (Bell et al. 2002); (3) genotyping on the high-throughput SEQUENOM® MassARRAY® platform, which uses the iPLEX™ assay to incorporate mass-modified terminal nucleotides in the SBE step, which are then detected by MALDI-TOF MS (Griffin and Smith 2000); (4) genotyping on the ultra-high throughput Illumina® platform. This platform uses the GoldenGate™ assay followed by a bead-based technology to resolve individual SNP genotypes (Fan et al. 2003). Three SNPs, including rs1926736, were genotyped by two independent methods and the few individuals (<1.7%) with discrepant genotypes were eliminated from subsequent analyses. A total of 15 SNPs could not be successfully genotyped and one SNP could not be placed unambiguously on the sequence map. We excluded 12 of the remaining 59 SNPs from the analysis for the following reasons: 1 showed deviations (P < 0.01) from Hardy–Weinberg equilibrium among parents and 11 were non-informative or had a MAF < 5%.
Reagents and antibodies
Dulbecco’s modified Eagle Medium, Minimum Essential Medium (MEM) alpha, RPMI-1640, GlutaMAX, penicillin, streptomycin, fetal bovine serum (FBS), AlexaFluor 488-conjugated ovalbumin (OVA488), FITC-conjugated zymosan, rabbit anti-FITC, AlexaFluor 488-conjugated goat anti-mouse antibody and 4’,6-diamidino-2-phenylindole (DAPI) were purchased from Invitrogen (Carlsbad, CA, USA). Endothelial cell medium (ECM) was from Sciencell (Carlsbad, CA, USA). Puromycin, yeast mannan, digitonin, paraformaldehyde, polybrene and porcine gelatin were purchased from Sigma (St. Louis, MO, USA). Anti-MR monoclonal antibody (clone 15-2) was purchased from Cell Sciences (Canton, MA, USA). Cy3-conjugated goat anti-mouse and Cy5-conjugated goat anti-rabbit antibodies were from Jackson ImmunoResearch (West Grove, PA, USA). Permafluor mounting medium was purchased from Thermo Scientific (Waltham, MA, USA). YG fluorescent 1 μm polystyrene microspheres were obtained from Polysciences Inc. (Warrington, PA, USA).
Cell culture
The HEK293 cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in MEM-alpha. Phoenix-Ampho cells were purchased from ATCC with permission of Gary Nolan (Stanford University, Palo Alto, CA, USA). These media were supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin and 100 U/ml streptomycin and 2 mM GlutaMAX. Human dermal microvascular endothelial cells (HDMECs) were purchased from Sciencell and cultured in ECM containing 5% FBS, 100 penicillin and 100 U/ml streptomycin, in gelatin-coated flasks. All cells were cultured in a humidified incubator at 37°C and 5% CO2. When mycobacteria were added, cells were cultured under the same conditions except that antibiotics were omitted and in the case of M. leprae, the temperature was set to 33°C.
Expression vector cloning
The cDNA encoding the human mannose receptor and cloned in pCDNA3 was a generous gift of Dr. J. J. He (Indiana University, IN, USA). The MR cDNA was amplified by proofreading PCR using primers MR_fwd_SalI, 5′-ATGCGTCGACATGAGGCTACCCCTGCTCC-3′, and MR_rev_MfeI, 5′-GCATCAATTGCTAGATGACCGAGTGTTCA-3′. The PCR product was purified, digested with SalI and MfeI and ligated in the pMSCV-puro vector (Clontech, Mountain View, CA) previously cut with XhoI and EcoRI. A total of 2 μl of the ligation was used to transform STLB2 competent cells (Invitrogen). A positive clone was grown overnight at 30°C in 50 ml of culture broth and the plasmid DNA was extracted using a Perfectprep plasmid midi kit (Eppendorf, Hamburg, Germany). The nucleotide sequence was verified by cycle sequencing in an ABI PRISM® 3100 genetic analyzer (Applied Biosystems, Foster City, CA, USA).
Site-directed mutagenesis
Two polymorphic variants of MRC1 were generated using the original hMR cDNA as a template for site-directed mutagenesis by overlap extension PCR (Boskovic et al. 2006). MR_fwd_SalI and MR_rev_MfeI were used as the flanking universal primers. Primers 5′-GCAGGAAGGAAGGCAGTGACCTCGCGAGTATCC-3′ (sense) and 5′-GGATACTCGCGAGGTCACTGCCTTCCTTCCTGC-3′ (antisense) were used for the generation of the MR(SAF) haplotype. Primers 5′-AAGTATCCACACCATCGAGGAATTGGACTTT-3′ (sense) and 5′-ATGGTGTGGATACTTGTGAGGTCACCGCCTT-3′ were used for the generation of the MR (GTL) haplotype. All constructs were cloned in pMSCV-puro and their sequence was verified.
Transduction
Amphotropic retroviruses were produced by transfecting the Phoenix-Ampho cell line with pMSCV-puro (empty) or pMSCV-MR constructs using Fugene6 (Roche Diagnostics, Laval, QC, Canada) according to the manufacturer’s instructions. Forty-eight hours after transfection, the culture supernatant was harvested, filtered, aliquoted and stored at −80°C. Aliquots showed similar (within margin of error) titers when tested on HEK293 cells. For transductions, HEK293 cells were seeded and grown to 50% confluence, then infected with Phoenix-Ampho supernatants supplemented with 8 μg/ml of polybrene for 24 h. The medium was then removed and fresh medium containing 1 μg/ml of puromycin was added. Stable pools of transductants (293-puro and 293-MR) were collected after 2 weeks of selection.
Immunostaining
Expression of MR was analyzed by indirect immunofluorescence. For microscopy, cells were seeded on gelatin-coated coverslips and fixed with 4% paraformaldehyde (PFA) diluted in PBS for 10 min. Alternatively, single cell suspensions were prepared for cytometry and fixed in 4% PFA. Cells were then incubated for 30 min in SuperBlock blocking buffer (Pierce, Rockford, IL, USA) containing 0.1% Tween-20, then incubated with 1 μg/ml of anti-MR mouse monoclonal antibody for 16 h at 4°C in the same buffer. Cells were rinsed twice in PBS and incubated with either Alexa Fluor 488- or Cy3-conjugated secondary antibodies (1:2,000 dilution in SuperBlock) for 60 min at 24°C. For microscopy, coverslips were rinsed twice then mounted onto slides in Permafluor mounting medium containing DAPI. For cytometry, suspensions were processed in a FACSCalibur flow cytometer (BD Biosciences).
Ovalbumin uptake assay
Cell lines were seeded in 12-well plates at a density of 5 × 105 cells/well and left to attach overnight. They were then treated for 1 h with 5 μg/ml of OVA488 in fresh complete medium. Cells were washed twice in PBS, detached in PBS containing 0.2 g/L EDTA and processed for flow cytometry. Fluorescence was measured in the FL1 channel.
Zymosan uptake assay
Cell lines were seeded in 12-well plates at a density of 5 × 105 cells/well and left to attach overnight. They were treated with zymosan-FITC at a ratio of five particles per cell for 16 h in fresh complete medium. Cells were washed twice in PBS then fresh complete medium containing 1 μg/ml anti-FITC antibody was added. Cells were kept at 4°C for 30 min to stop phagocytosis and allow binding of antibodies to surface exposed zymosans. Cells were washed twice in PBS and lysed for 10 min in 400 μl lysis buffer (0.01% digitonin in PBS) containing 106 YG 1 μm beads (about 1 bead per cell) and anti-rabbit Cy5 diluted 1:200. The lysate was further homogenized in a bath sonicator for 5 min. Samples were processed by flow cytometry, surface-bound particles were positive for both the FL1 (green) and FL4 (far red) channels whereas internalized zymosans were scored negative in FL4.
Mycobacteria binding assay
PKH67-stained viable M. leprae bacilli grown in nude mouse footpads were obtained from Dr R. Truman and Dr L. Adams (Hansen’s Disease Program; Baton Rouge, LA, USA). GFP-expressing M. bovis BCG strain Pasteur was cultured in Middlebrook 7H9 medium containing ADC enrichment and used fresh to make single-cell suspensions. The 293-puro or 293-MR cells were seeded on coverslips at a density of 2.5 × 105 cells/well, in complete medium without antibiotics then treated with mycobacteria at a multiplicity of infection (MOI) of five for 16 h. In some experiments, cells were pre-incubated for 30 min with yeast mannan or anti-MR monoclonal antibody. Cells were washed three times in PBS then fixed in 4% paraformaldehyde and briefly permeabilized in cold methanol. Nuclei were counterstained with DAPI, then coverslips were mounted and examined by fluorescence microscopy. Images of both green particles and nuclei were acquired using the Northern Eclipse software (Mississauga, ON, USA). Bound bacteria were manually counted in at least 4 fields containing a minimum of 500 cells total.
Statistical methods
We estimated population allelic frequencies from parental data using the algorithm implemented in Haploview 4.1 software (Barrett et al. 2005). Family based association studies were performed using principally a classical transmission disequilibrium test, as implemented in FBAT v2.0.3 software (Horvath et al. 2001). For the analysis of the multi-case families and the combined sample, we used the empirical variance–covariance estimator previously advocated (Lake et al. 2000). We carried out a population-based association study in the Brazilian sample, using classical multivariate logistic regression techniques as implemented in the LOGISTIC procedure of SAS software v.9.1. Haplotype analyses were further carried out using the THESIAS software (Tregouet and Garelle 2007). All population-based analyses were adjusted for sex, which has been identified as a classical risk factor for leprosy. We did not correct for multiple testing given the context of a positive replication utilizing an alternative study design in an ethnically different population, and the reported association of the MRC1 exon 7 SNPs with leprosy in two reviews (Cooke and Hill 2008; Hill 2006).
Results
Analysis of exon 7 SNPs in Vietnamese leprosy families
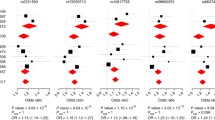
Three non-synonymous exon 7 SNPs were of particular interest since they had been suggested as risk factors for paucibacillary leprosy in a South Indian population (Cooke and Hill 2008). To investigate the impact of MRC1 exon 7 SNPs on leprosy susceptibility, we first sequenced exon 7 in 396 unrelated healthy individuals (792 chromosomes) to identify any common population-specific variants. We found two polymorphic SNPs: the non-synonymous rs1926736 [G396S; allele A (S396) MAF = 0.35] and the synonymous rs2437256 [I404I; allele C (I404) MAF = 0.21]. Two additional previously described non-synonymous SNPs, rs2478577 (T399A) and rs2437257 (L407F), were found to be non-polymorphic in the Vietnamese population (MAF = 0). Since the focus of the study was on non-synonymous SNPs, we genotyped the single non-synonymous SNP rs1926736 in 490 simplex families and 90 multiplex families comprising a total of 704 leprosy patients for which both parents were available. These families included approximately even numbers of paucibacillary (n = 325) and multibacillary (n = 374) cases. Under a best dominant model, we observed significant evidence for the association of rs1926736 with leprosy per se [P dom = 0.035, allele A (S396) is protective, OR = 0.76 (95% CI 0.60–0.96)] and multibacillary leprosy [P dom = 0.034, allele A (S396) is protective, OR = 0.71 (0.51–0.99)] (Table 1).
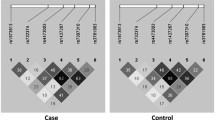
To delineate the extent of LD between rs1926736 and additional MRC1 SNPs, we excluded rare SNPs (i.e., MAF < 5%) given that they cannot be highly correlated with rs1926736 (MAF = 0.35). We selected 69 SNPs from the NCBI EntrezSNP database and the International HapMap project that span the entire 101.8 kb of the MRC1 gene. To assure inclusion of all common coding SNPs, we sequenced the remaining 29 MRC1 exons including the 5′- and 3′-UTR regions in 23 unrelated Vietnamese individuals. According to our criterion, four identified coding SNPs, all previously described in the NCBI EntrezSNP database, were included: rs2253120 (exon 2, T81T), rs2296414 (exon 3, T167I), rs2985837 (exon 4, Q242Q) and rs2477664 (exon 4, I260I) (Table S2). Including the two previously described exon 7 SNPs, a total of 75 MRC1 SNPs were genotyped in 198 simplex families to establish the LD pattern of the MRC1 gene. Among the 75 MRC1 SNPs genotyped, 47 were suitable for analysis (Table S1). The LD pattern of the entire MRC1 gene was plotted and revealed that rs1926736 was highly correlated with only two intronic SNPs (rs525830, r 2 = 0.89 and rs493862, r 2 = 0.86) with no obvious functional implication (Fig. 1; Table S3). In addition, none of the additional SNPs showed significant evidence for association with leprosy or its sub-forms in the 198 families (data not shown). Finally, we utilized the International HapMap Project database (Data Rel 24) to screen a 2-Mb region around rs1926736 in the Han Chinese in Beijing, China (CHB) samples. No SNPs were correlated with rs1926736 using an r 2 = 0.5 cut-off. From these results, we concluded that rs1926736 (G396S) was the most plausible cause of the observed association and that S396 of MR was a leprosy protective factor.
Pairwise linkage disequilibrium (LD) as defined by r 2 across the MRC1 gene. The chromosome 10p location of 47 SNPs from rs2947600 (MRC1 intron 1) to rs941 (MRC1 3′-UTR) is indicated above the LD plot. The r 2 value for each SNP pair is indicated within the corresponding diamond. Increasing depth of black color indicates higher r 2 values. D’-defined haplotype blocks are also indicated at the top of the LD plot. There is no evidence for long range LD extending over the entire MRC1 gene
Analysis of exon 7 SNPs in Brazilian leprosy cases
Given that the evidence for association of rs1926736 with leprosy in the Vietnamese samples was weak, we decided to study MRC1 exon 7 SNPs in a leprosy sample from Brazil. We sequenced exon 7 in 399 healthy controls and observed four SNPs: rs1926736 [G396S; allele A (S396) MAF = 0.32], rs2478577 [T399A; allele A (T399) MAF = 0.21], rs2437256 [I404I; allele C (I404) MAF = 0.21], and rs2437257 [L407F; allele G (L407) MAF = 0.21]. The three non-synonymous exon 7 SNPs could be unambiguously assigned to three amino acid haplotypes, two of which had been observed in the Vietnamese sample (Fig. 2). Next, we sequenced MRC1 exon 7 in an additional 384 Brazilian leprosy cases and conducted a case–control association analysis of the three non-synonymous SNPs with leprosy. LD analysis showed that SNPs rs2478577 (T399A) and rs2437257 (L407F) were in complete LD (r 2 = 1) and therefore statistically redundant. Allele G (G396) of SNP rs1926736 showed evidence for association with susceptibility to leprosy per se [P add = 0.016; OR = 1.34 (95% CI 1.06–1.70)], and the multibacillary sub-form [P add = 0.023; OR = 1.42 (1.05–1.93)] in a best additive model. Similarly, allele G (L407) of SNP rs2437257 showed borderline evidence for association with resistance to leprosy per se [P dom = 0.09; OR = 0.75 (0.54–1.05)] and the multibacillary sub-form [P dom = 0.04; OR = 0.63 (0.41–0.97)] in a best dominant model.
Human MRC1 exon 7 haplotypes. In the Brazilian sample, four common MRC1 exon7 polymorphisms were found, three of which were non-synonymous (boxed). Due to strong LD between SNPs two (rs2478577), three (rs2437256) and four (rs2437257), only three amino acid haplotypes were observed and termed MR (GAF), MR (SAF) and MR (GTL). SNP 1 (rs1926736) gives rise to a G/S amino acid polymorphism that was only observed on the MR(AF) background. Among these three haplotypes, only two–MR(GAF) and MR(SAF)–were observed in the Vietnamese sample
Multivariate analysis of the three non-synonymous variants confirmed the correlation between SNPs rs2478577 and rs2437257. The best sex-adjusted model (P = 0.002) as determined by multivariate analysis included only rs1926736 and rs2437257. Haplotype analysis of rs1926736 (G396S) and rs2437257 (L407F) identified three haplotypes with G396-F407 being the most common haplotype (Table 2). Haplotype G396-F407 had a risk effect while haplotype S396-F407 was protective in the overall sample (Table 2). Considering rs2478577 (T399A) and rs2437257 (L407F) are in complete LD, MRC1 exon 7 therefore encodes two protective amino acid haplotypes: the borderline protective G396-T399-L407 haplotype and the strongly protective S396-A399-F407 haplotype (Table 2). Interestingly, while the G396 residue is a susceptibility factor overall, on the T399-L407 haplotype background, this susceptibility can be compensated and may even be protective. This is concurrent with the result in the Vietnamese leprosy families. Given that in the Vietnamese population rs2478577 (T399A) and rs2437257 (L407F) are non-polymorphic, the protective S396 allele corresponds unambiguously to the S396-A399-F407 haplotype (Fig. 2).
Establishment of an HEK293 cellular model for MR function
The three MRC1 exon 7 encoded amino acid haplotypes observed in Vietnamese and Brazilian samples were termed MR(GAF), MR(SAF) and MR(GTL) (Fig. 2). The underlying three non-synonymous amino acid changes G396S, T399A and L407F map to the CTLD2 segment of the mannose receptor protein, a domain of unknown function. To better understand the functional basis of the observed genetic effect of exon 7 haplotypes, we decided to compare the biological activity of the corresponding MR genetic variants by ectopically expressing the receptor in a non-expressing cell line. A cDNA copy of human MRC1 in pcDNA3 (a generous gift of Dr. J. J. He, Indiana University, IN, USA) was obtained and subcloned in a MSCV-puro retroviral backbone. Packaged viruses were used to infect the HEK293 cell line, which does not express MR. The resulting pool of transductants (293-MR) was compared to an empty-vector control HEK293 line as well as to HDMEC, which are known to express MR (Groger et al. 2000). In addition to MR protein expression, the capacity to internalize fluid-phase (ovalbumin) and particulate (zymosan) ligands was evaluated by microscopy. Immunofluorescence showed that 293-MR and endothelial cells mostly expressed the mannose receptor in an intracellular endocytic compartment as well as on their surface, which overlapped the pattern of ovalbumin uptake (Fig. 3a). The control cell line did not stain for MR and was unable to take up ovalbumin. In addition, both 293-MR and HDMECs could bind and internalize yeast particles (zymosans) (Fig. 3b). Hence, the 293-MR cell line was considered a good model for the study of ectopically expressed MR since it mirrored the natural activity of the receptor.
Ectopic MR is functional in HEK293 cells. A MR cDNA was transduced into HEK293 cells. The resulting cells were compared to vector-transduced cells and microvascular endothelial cells (HDMEC) which express endogenous MR. a Cells were treated with Alexa-488-conjugated ovalbumin (an MR ligand, shown in green) for 1 h then immunostained with monoclonal anti-MR followed by Cy3-conjugated anti-mouse (red). b Cells were treated with fluorescein-conjugated zymosan for 16 h, then washed and stained with rabbit anti-fluorescein followed by Cy3 anti-rabbit antibodies. Ingested zymosans are green while non ingested particles are stained red and appear yellow in the merged images. Nuclei are stained blue with DAPI
Cloning and expression of human mannose receptor variants
The original MRC1 cDNA sequence used in the generation of the 293-MR cell line corresponded to the MR(GAF) haplotype. This sequence was modified by site-directed mutagenesis at two sites to yield the MR(SAF) and MR(GTL) variants (Fig. 2). The DNAs were cloned in a retroviral expression vector, packaged and used to infect HEK293 cells. The entire pools of puromycin-resistant transduced cells were used at passage 3 for further experiments to avoid artifacts due to clonal variations. The proportion and level of MR expression were measured by immunocytometry (Fig. 4a). MR(GAF) and MR(SAF) pools had very similar expression levels whereas the MR(GTL) pool was slightly lower, possibly due to a somewhat lower retroviral transduction efficiency during the generation of this cell line. Expression levels were monitored throughout the experiments and were found to be stable. Vector-transduced control cells showed no expression.
Absence of biological differences among MR variants. HEK293 transductants were analyzed for MR function by flow cytometry. a Transduced stable populations of HEK293 cells were immunostained for MR. b Populations were incubated for 1 h with 5 μg/ml Alexa488-conjugated ovalbumin and uptake was measured by FACS. Percentage of positive cells is given as well as median fluorescence value (MFV) of the positive population. c Populations were incubated with 106 zymosan-FITC particles (ratio zymosan:cell 5:1) for 16 h. Extracellular particles were then stained with anti-FITC and Cy5-conjugated secondary antibodies. Cells were lysed and intracellular and extracellular zymosans were gated in FL1 and counted in FL4 channels. Data is expressed as percent of ingested zymosans over total bound, and as number of bound zymosan per cell. Results shown here are representative of three independent experiments
MR variants do not differ in their capacity to take up ligands
The three pools of MR-expressing transductants were then used to compare the functional activity of their corresponding MR proteins by employing ovalbumin and zymosan, two classical mannose-containing ligands. Fluid-phase endocytosis of ovalbumin was measured by flow cytometry. There was no difference in endocytosis between MR(GAF) and MR(SAF) variants and only a slightly lower activity for MR(GTL) that was consistent with a somewhat lower expression (Fig. 4b). Vector control cells showed only background level uptake. It has been suggested that the processes of MR-dependent binding and endocytosis of soluble molecules might differ from MR-dependent phagocytosis of particles (Le Cabec et al. 2005). Therefore, we simultaneously measured binding and internalization of fluorescinated zymosans by flow cytometry. Ingestion efficiency was determined by the ratio of internalized particles over total cell-associated particles. Again, MR(GAF) and MR(SAF) variants were indistinguishable while the lower expressing MR(GTL) variant ingested slightly lower numbers (Fig. 4b). Overall, the MR-expressing cells took up 64–83% of bound particles after 16 h of incubation. Vector control cells did not bind sufficient numbers of particles to allow quantification of phagocytosis.
MR expressed by HEK293 cells does not bind to M. leprae or BCG
Mycobacteria are thought to bind to MR because of the presence of mannosylated molecules such as ManLAM on their surface (Kang et al. 2005; Schlesinger et al. 1996). We therefore evaluated the impact of MR polymorphisms on the interaction with whole viable M. leprae and M. bovis BCG. Fluorescent mycobacteria were incubated with 293-puro and 293-MR cells for 16 h. Binding was determined by microscopic examination of the monolayer after repeated washing. The presence of MR had no impact on binding of either mycobacteria (Fig. 5). Control cells could bind bacteria to the same extent as MR+ cells, regardless of the variant expressed. These results suggested that 293-MR cells lacked accessory molecules required for efficient uptake of mycobacteria by MR.
Absence of impact of ectopic MR expression on binding to mycobacteria. HEK293 transductants were incubated for 16 h with PKH67-stained fluorescent Mycobacterium leprae (a) and GFP-expressing Mycobacterium bovis BCG strain Pasteur (b) at a MOI of 5. Measurement of bacterial binding was performed by microscopic examination, counting at least 500 cells in four different fields. Results per field were averaged per cell and are given on the left of the graph. Results are given for HEK293 control cells (PURO) and HEK293 cells over-expressing the MR(GAF), MR(SAF) and MR(GTL) expression constructs
Discussion
Linkage of the 10p13 chromosomal region to the paucibacillary sub-form was observed independently in two ethnically different populations from South India and Vietnam (Mira et al. 2003; Siddiqui et al. 2001). In several reviews (Cooke and Hill 2008; Hill 2006), the MRC1 gene was suggested to be the underlying cause of this linkage signal. While experimental details are not yet publicly available, three exon 7 encoded amino acid polymorphisms were reportedly associated with paucibacillary leprosy in South Indian patients. Based on these reports, we decided to study the role of MRC1 in susceptibility to leprosy and its clinical sub-forms. In a large sample of Vietnamese patients, we detected weak but significant evidence that serine at amino acid position 396 was a protective factor for leprosy per se and multibacillary leprosy. These results strongly argued that MRC1 is indeed a leprosy susceptibility gene but not the paucibacillary susceptibility gene detected in the genome-wide scan in Vietnamese families (Mira et al. 2003). The results obtained in the Brazilian sample were more complex. In the Brazilian population, we detected three non-synonymous exon 7 SNPs. As suggested for the South Indian patients, an exon 7 encoded S396-A399-F407 haplotype was significantly protective for leprosy. Similar to the results in the Vietnamese families, the protective effect of this haplotype was significant in the overall sample and in the subset of multibacillary patients. Importantly, in both the Vietnamese and Brazilian samples, the effect of the exon 7 polymorphisms is on leprosy per se. Although in subset analysis formal significance of association was only obtained for multibacillary leprosy, there was no significant heterogeneity between paucibacillary and multibacillary cases. The limited effect of MRC1 detected by our association study is consistent with the absence of linkage (i.e., a major gene effect) detected between the 10p13 region and leprosy per se. These results strongly argue that MRC1 is indeed a leprosy susceptibility gene but does not explain the observed linkage peak in the South Indian patients (Siddiqui et al. 2001) or Vietnamese families (Mira et al. 2003). A high-density association scan of the underlying interval is necessary to identify the paucibacillary risk factor(s).
Although our data pinpoint G396S (rs1926736) as the causal variant, we cannot exclude the possibility that multiple rare coding variants in exons 1–30 contribute to leprosy susceptibility (i.e., allelic heterogeneity). However, the practicality and value of sequencing 30 exons in a large sample of individuals in order to identify these variants is uncertain. Unless a sufficient number of rare variants are identified, a ‘meta-SNP’ analysis combining the information at each loci would not have sufficient power to detect a genetic effect in the expected range of odds-ratios. Supporting G396S as the causal variant, a recent genetic study found rs1926736 to be associated with asthma risk in 446 Japanese cases (P = 0.011, OR = 0.61 [0.41–0.89]) (Hattori et al. 2009). Moreover, the most significant haplotype association was observed for a five-SNP risk haplotype carrying allele G396 (P = 0.00047). The authors concluded that the haplotype association was seemingly driven by rs1926736 in the Japanese population. This study not only substantiates our results but suggests that MRC1—particularly G396S—participates in the immune response to mycobacterial infection and the inflammatory process, two highly related immunological pathways.
The results obtained from the Brazilian sample help to better understand the mechanism of susceptibility mediated by the glycine residue at position 396. Closer inspection of the exon 7 haplotype frequencies in Brazilian patients revealed that the G396-A399-F407 haplotype is enriched in cases while the S396-A399-F407 haplotype is enriched in controls (Table 2). In contrast, G396 in the context of the G396-T399-L407 haplotype appears neutral and may even be slightly protective (Table 2). Hence, G396 was a risk factor only on the G396-A399-F407 haplotype background. The question is then why G396 was a susceptibility factor on the A399-F407 background, while on the T399-L407 background it was not? Exon 7 polymorphisms in MRC1 map to the second C-type lectin domain (CTLD2) of the MR protein. CTLD2 has been structurally assigned to a hinge domain linking the C-type lectin region to the fibronectin type II and cysteine-rich domains (Boskovic et al. 2006). While CTLD2 is homologous to C-type lectins, it does not bind to ligands (Taylor et al. 1992). Of course, it is possible that changes at CTLD2 indirectly impact on MR ligand affinities. However, our results obtained in the 293-MR cells suggest a direct interaction of CTLD2 with an accessory receptor molecule in the MR–M. leprae interaction. In this view, interaction of the CTLD2 domain with the accessory molecule would only be sensitive to G396 in the context of the A399-F407 haplotype.
The importance of accessory molecules for MR function was highlighted by the results of our studies in a cellular model of MR function. To assay MR function, we used the non-professional phagocytic human cell line HEK293 as a platform for expression of MR variants. This cellular model mimicked critical features of MR as seen in HDMECs, previously shown to express this receptor (Groger et al. 2000). Although HEK293 cells are not professional phagocytes, expression of MR bestowed on these cells the ability to ingest zymosan particles. We then showed that polymorphisms in the MRC1 associated with leprosy risk did not impact on the core MR protein functions as measured by internalization of soluble or particulate ligands. All genetic variants analyzed showed no deviation from their expected endocytic or phagocytic activity. This implies that the amino acid polymorphisms encoded by exon 7 of MRC1 have no impact on the ability to directly bind and internalize mannose-containing ligands. Since mycobacterial ManLAM is known to interact with MR through its terminal mannooligosaccharide in a similar fashion to other ligands (Schlesinger et al. 1994), we expect that the binding of this bacterial product would not be affected by the polymorphism under study. Furthermore, 293-MR cells failed to bind and ingest viable BCG and M. leprae indicating that MR alone is not sufficient to mediate mycobacterial phagocytosis. These results are consistent with the hypothesis that the interaction of MR with M. leprae involves additional molecules and that differential activity of the MR alleles can only be revealed in the context of this partner host molecule. Such an accessory molecule may be present in macrophages, which are considerably more efficient at internalizing zymosans than 293-MR cells despite a much lower expression of the receptor (data not shown). Unfortunately, macrophages cannot be used easily in a study of MR variants because of their endogenous expression of MRC1.
Whether the impact of the leprosy-associated MRC1 alleles is via the alteration of MR ligand interactions or by changed receptor signaling or trafficking is not known. Hence, it is possible that aspects of MR function not directly related to ligand binding and internalization are affected by genetic CTLD2 variations. For instance, MR delivers an intracellular signal that affects the immune status of cells as well as endosome–lysosome fusion (Kang et al. 2005; Nigou et al. 2001; Shimada et al. 2006). However, this signaling is mediated by the cytoplasmic domain of MR and would not be expected to be affected by a remote extracellular site. Our data strongly suggest that exon 7 variants have an indirect effect on overall structure and/or stability of the CTLD2 domain, likely by modulating the interaction with an additional host molecule(s). This heteromeric complex may then impact on M. leprae phagocytosis, MR trafficking and/or MR signaling. Clearly, if our hypothesis is correct, identification of the MR-interacting host cell molecule(s) is of highest importance.
References
Alcais A, Alter A, Antoni G, Orlova M, Van Thuc N, Singh M, Vanderborght PR, Katoch K, Mira MT, Thai VH, Huong NT, Ba NN, Moraes M, Mehra N, Schurr E, Abel L (2007) Stepwise replication identifies a low-producing lymphotoxin-[alpha] allele as a major risk factor for early-onset leprosy. Nat Genet 39:517–522
Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265
Bell P, Chaturvedi S, Gelfand C, Huang C, Kochersperger M, Kopla R, Modica F, Pohl M, Varde S, Zhao R, Zhao X, Boyce-Jacino M, Yassen A (2002) SNPstream UHT: ultra-high throughput SNP genotyping for pharmacogenomics and drug discovery. Biotechniques (Suppl 70–2, 74, 76–7)
Boskovic J, Arnold JN, Stilion R, Gordon S, Sim RB, Rivera-Calzada A, Wienke D, Isacke CM, Martinez-Pomares L, Llorca O (2006) Structural model for the mannose receptor family uncovered by electron microscopy of Endo180 and the mannose receptor. J Biol Chem 281:8780–8787
Chakravartti M, Vogel F (1973) A twin study on leprosy. In: Becker PE, Lenz W, Vogel F, Wendt GG (eds) Topics in human genetics, vol 1. Georg Thieme, Stuttgart, pp 1–123
Cooke G, Hill A (2008) Tuberculosis, leprosy and other mycobacterial diseases. In: Kaslow RA, McNicholl JM, Hill AVS (eds) Genetic susceptibility to infectious diseases. Oxford University Press, New York, pp 333–351
Ezekowitz RA, Sastry K, Bailly P, Warner A (1990) Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med 172:1785–1794
Fan J, Oliphant A, Shen R, Kermani B, Garcia F, Gunderson K, Hansen M, Steemers F, Butler S, Deloukas P, Galver L, Hunt S, McBride C, Bibikova M, Rubano T, Chen J, Wickham E, Doucet D, Chang W, Campbell D, Zhang B, Kruglyak S, Bentley D, Haas J, Rigault P, Zhou L, Stuelpnagel J, Chee M (2003) Highly parallel SNP genotyping. Cold Spring Harb Symp Quant Biol 68:69–78
Griffin TJ, Smith LM (2000) Single-nucleotide polymorphism analysis by MALDI-TOF mass spectrometry. Trends Biotechnol 18:77–84
Groger M, Holnthoner W, Maurer D, Lechleitner S, Wolff K, Mayr BB, Lubitz W, Petzelbauer P (2000) Dermal microvascular endothelial cells express the 180-kDa macrophage mannose receptor in situ and in vitro. J Immunol 165:5428–5434
Hattori T, Konno S, Hizawa N, Isada A, Takahashi A, Shimizu K, Shimizu K, Gao P, Beaty TH, Barnes KC, Huang SK, Nishimura M (2009) Genetic variants in the mannose receptor gene (MRC1) are associated with asthma in two independent populations. Immunogenetics. doi:10.1007/s00251-009-0403-x
Hill AV (2006) Aspects of genetic susceptibility to human infectious diseases. Annu Rev Genet 40:469–486
Horvath S, Xu X, Laird NM (2001) The family based association test method: strategies for studying general genotype–phenotype associations. Eur J Hum Genet 9:301–306
Kabha K, Nissimov L, Athamna A, Keisari Y, Parolis H, Parolis LA, Grue RM, Schlepper-Schafer J, Ezekowitz AR, Ohman DE (1995) Relationships among capsular structure, phagocytosis, and mouse virulence in Klebsiella pneumoniae. Infect Immun 63:847–852
Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS (2005) The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med 202:987–999
Lake SL, Blacker D, Laird NM (2000) Family-based tests of association in the presence of linkage. Am J Hum Genet 67:1515–1525
Le Cabec V, Emorine LJ, Toesca I, Cougoule C, Maridonneau-Parini I (2005) The human macrophage mannose receptor is not a professional phagocytic receptor. J Leukoc Biol 77:934–943
Miller EN, Jamieson SE, Joberty C, Fakiola M, Hudson D, Peacock CS, Cordell HJ, Shaw MA, Lins-Lainson Z, Shaw JJ, Ramos F, Silveira F, Blackwell JM (2004) Genome-wide scans for leprosy and tuberculosis susceptibility genes in Brazilians. Genes Immun 5:63–67
Mira MT, Alcais A, Van Thuc N, Thai VH, Huong NT, Ba NN, Verner A, Hudson TJ, Abel L, Schurr E (2003) Chromosome 6q25 is linked to susceptibility to leprosy in a Vietnamese population. Nat Genet 33:412–415
Mira MT, Alcais A, Van Thuc N, Moraes MO, Di Flumeri C, Hong Thai V, Chi Phuong M, Thu Huong N, Ngoc Ba N, Xuan Khoa P, Sarno EN, Alter A, Montpetit A, Moraes ME, Moraes JR, Dore C, Gallant CJ, Lepage P, Verner A, van de Vosse E, Hudson TJ, Abel L, Schurr E (2004) Susceptibility to leprosy is associated with PARK2 and PACRG. Nature 427:636–640
Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G (2001) Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol 166:7477–7485
Ridley DS, Jopling WH (1966) Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis 34:255–273
Schlesinger LS (1993) Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol 150:2920–2930
Schlesinger LS, Hull SR, Kaufman TM (1994) Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol 152:4070–4079
Schlesinger LS, Kaufman TM, Iyer S, Hull SR, Marchiando LK (1996) Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol 157:4568–4575
Shimada K, Takimoto H, Yano I, Kumazawa Y (2006) Involvement of mannose receptor in glycopeptidolipid-mediated inhibition of phagosome-lysosome fusion. Microbiol Immunol 50:243–251
Siddiqui MR, Meisner S, Tosh K, Balakrishnan K, Ghei S, Fisher SE, Golding M, Shanker Narayan NP, Sitaraman T, Sengupta U, Pitchappan R, Hill AVS (2001) A major susceptibility locus for leprosy in India maps to chromosome 10p13. Nat Genet 27:439–441
Taylor ME, Bezouska K, Drickamer K (1992) Contribution to ligand binding by multiple carbohydrate-recognition domains in the macrophage mannose receptor. J Biol Chem 267:1719–1726
Tosh K, Meisner S, Siddiqui MÂR, Balakrishnan K, Ghei S, Golding M, Sengupta U, Pitchappan Ramasamy ÂM, Hill Adrian ÂVÂS (2002) A region of chromosome 20 is linked to leprosy susceptibility in a south Indian population. J Infect Dis 186:1190–1193
Tregouet DA, Garelle V (2007) A new JAVA interface implementation of THESIAS: testing haplotype effects in association studies. Bioinformatics 23:1038–1039
Wallace C, Fitness J, Hennig B, Sichali L, Mwaungulu L, Ponnighaus JM, Warndorff DK, Clayton D, Fine PEM, Hill AVS (2004) Linkage analysis of susceptibility to leprosy type using an IBD regression method. Genes Immun 5:221–225
Acknowledgments
We are grateful to all patients and their families who participated in this study. We thank J.L. Casanova, M. Behr and K. Morgan for helpful discussions and A. Montpetit and A. Bélisle for assistance with high-throughput genotyping. Fresh nu/nu mouse derived M. leprae were supplied by Dr. R. Truman and Dr. L. Adams of the National Hansen’s Disease Program Laboratory Research Branch, Baton Rouge, LA. Funding for this service was provided by the American Leprosy Missions. We thank Dr. J.J. He (Indiana University, IN) for providing the MRC1 cDNA. This study was supported by a grant from the Canadian Institutes of Health Research (CIHR) to E.S. and MAGRALEPRE from l’Ordre de Malte to Al.A. The Laboratory of Human Genetics of Infectious Diseases is supported by grants from The Rockefeller University Center for Clinical and Translational Science grant number 5UL1RR024143-03 and The Rockefeller University. An.A. holds a graduate studentship from the Natural Science and Engineering Research Council of Canada (NSERC). Al.A. and L.A. are supported by the Assistance Publique-Hôpitaux de Paris, Programme de Recherche Fondamentale en Microbiologie Maladies Infectieuses et Parasitaires (PRFMMIP), and the Agence Nationale de la Recherche (ANR) of the Ministère Français de l’Éducation Nationale de la Recherche et de la Technologie. E.S. is a Chercheur National du Fonds de la Recherche en Santé du Québec and an International Research Scholar of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Alter, L. de Léséleuc contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
439_2009_775_MOESM2_ESM.doc
Identification of coding SNPs with minor allele frequencies >5% in MRC1 by direct sequencing in 23 unrelated Vietnamese individuals (DOC 36 kb)
Rights and permissions
About this article
Cite this article
Alter, A., de Léséleuc, L., Van Thuc, N. et al. Genetic and functional analysis of common MRC1 exon 7 polymorphisms in leprosy susceptibility. Hum Genet 127, 337–348 (2010). https://doi.org/10.1007/s00439-009-0775-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-009-0775-x