Abstract
Basic leucine zipper transcription factor (bZIP) is involved in signaling transduction for various stress responses. Here we reported a bZIP transcription factor (accession: JX887153) isolated from a salt-resistant lotus root using cDNA-AFLP approach with RT-PCR and RACE-PCR method. Full-length cDNA which consisted of a single open reading frame encoded a putative polypeptide of 488 amino acids. On the basis of 78, 76, and 75 % sequence similarity with the bZIPs from Medicago truncatula (XP_003596814.1), Carica papaya (ABS01351.1) and Arabidopsis thaliana (NP_563810.2), we designed it as LrbZIP. Semi quantitative RT-PCR results, performed on the total RNA extracted from tips of lotus root, showed that LrbZIP expression was increased with 250 mM NaCl treatment for 18 h. Effects of low temperature on the expression of LrbZIP was also studied, and its expression was significantly enhanced with a 4 °C treatment for 12 h. In addition, LrbZIP expression was strongly induced by treatment with exogenous 100 μM ABA. To evaluate its function across the species, tobacco (Nicotiana tabacum L.) was transformed with LrbZIP in a binary vector construct. Transgenic plants exhibited higher resistance as compared with the control according to the results of the root growth, chlorophyll content and electrolyte leakage when exposed to NaCl treatment. In addition, LrCDPK2, LrLEA, and TPP also showed enhanced expression in the transgenic plants. Overall, expression of LrbZIP was probably very important for salt-resistant lotus root to survive through salt stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salinity affects about 800 million hectares of land worldwide, comprising nearly 7 % of the world’s total land area and one-third of irrigated land [1]. Most crop plants are sensitive to saline concentrations that raise the electrical conductivity of saturated soil extracts above 4 dS m−1 [2]. NaCl, the dominant salt in nature, elicits two primary effects on plants: osmotic stress and ionic toxicity [3]. Osmotic stress reduces the ability of plants to take up water and minerals. It not only reduces the growth rate in proportion to the salinity level, but also the tiller numbers in plants [4, 5]. Ion toxicity inhibits a variety of processes such as K+ absorption, vital enzyme reactions, protein synthesis and photosynthesis [6, 7]. Secondary effects include the production of reactive oxygen intermediates (ROSs) [3].
Recently, it was shown that salinity affected plant growth through various pathways, with the involvement at the level of genome, transcriptome, proteome, metabolome, and ionome [8–10]. It is reported that the responses to salt stress at physiological and biochemical levels are different in cultivated species and wild species [11]. Therefore, it is imperative to have a clear understanding of this tolerance mechanism at molecular level [3], especially cloning the genes involved in salt tolerance, to improve salt resistance in plants, through molecular engineering strategies [12]. Many genes responding to salt stress have been documented from different plants, and most of them are also involved in various stress responses [13–18]. Transcription factors, which regulate the down-stream gene expression, are important in almost all biological processes. Basic region/leucine zipper motif (bZIP) transcription factors are involved in various metabolisms, such as defense response, signaling transduction and flower development [19, 20].
Constitutive expression of maize ABP9, which enodes a bZIP transcription factor in Arabidopsis leads to remarkably enhanced tolerance to multiple stresses [21]. SlAREB, a member of the ABF/AREB subfamily, encodes a bZIP transcription factor in Solanum lycopersicum. Overexpression of this gene improves the survival rate of tobacco plants in drought stress [22]. Evidence shows that bZIP can enhance survival in salt stress in transgenic plants through mediating physiological and biochemical metabolism. Constitutive expression of ThbZIP1 in tobacco improves the activity of both peroxidase (POD) and superoxide dismutase (SOD), and increases the content of soluble sugars and soluble proteins [23]. However, overexpression of OsABI5 (a bZIP transcription factor) in rice enhances the sensitivity to salt stress, suggesting that different expression profiles of bZIP regulates plant adaptation to stress response. At present, most reports on stress responses show that bZIP transcription factors regulate stress response through mediating endogenous abscisic acid [24, 25]. Further study testifies that bZIP transcription factors may interact with specific ABA-responsive cis-acting elements (ABRE) and promotes transcription of down-stream genes [26]. The ABA adaptive responses leading to stress adaptation can be divided into two broad categories: ABA-dependent and ABA-independent pathways [27]. Therefore, it can conclude that some bZIPs should be involved into ABA-dependent pathway to respond to stresses [28, 29].
Lotus root (Nelumbo nucifera Gaertn), which originated from India and China, is an aquatic herb vegetable and a member of the family Nymphaeaceae [30]. It is one of the oldest dicot plants in the world with many features of monocot plants, and has been widely grown in China, Japan, and other Southeast Asian counties for multiple purposes [31]. The products of lotus root such as fresh, salted and boiled rhizomes, lotus root starch, drinks, teas, and lotus seeds are very popular in the daily diet because of its richness in nutrients including starch, proteins, vitamins and mineral substances [32, 33]. Therefore, China is already exporting the processed products of lotus root to Japan, Korea, Europe, and the United States as a kind of off-season vegetable. In addition, nodus nelumbinis rhizomatis, germ, stamens and lotus root stems are also used as important ingredients in the traditional medicine [34–36].
Production of lotus root has already been affected with increasing concentration of salinity in soil. Commonly cultivated species of lotus root are sensitive to salt stress, while wild type species show more resistance with unknown mechanisms at molecular level. Therefore, isolation and functional identification of salt-tolerance genes from wild type species is expected to improve salt resistance of cultivated species. RNA fingerprinting method, cDNA-AFLP provides detailed characterization of gene expression in a wide range of biological processes without prior sequence information. This technique helps to carry out a comprehensive and systematic analysis on the transcriptome of organism, and successfully separates differentially expressed genes [37]. Using cDNA-AFLP approach, we identified a bZIP gene from a salt-tolerant species of lotus root. Because its sequence showed high similarity with bZIPs from other organisms, we named it LrbZIP. The expression profiles and possible functions of the gene were also investigated.
Materials and methods
Plant material and growth conditions
Almost all cultivated species of lotus root are sensitive to salt stress, therefore, in this experiment, a salt tolerant wild species (W08124) of lotus root was used to isolate salt tolerant genes. The lotus root was planted 10 cm deep in water in the pots in spring, with average temperature 25 °C/day and 17 °C/night during the whole growth season. Several stolons developed and elongated in proper order in each plant about 20 days of plantation, and then plants were treated with 250 mM NaCl for 24 h with the light regime set at the normal growth conditions, and the tips of stolon were used as sample to extract RNA.
cDNA-AFLP
Total RNA was extracted from tips of lotus root treated with NaCl for 24 h for cDNA-AFLP. M-MLV RTase cDNA synthesis kit (Takara, Japan) was used to synthesize cDNA. cDNA-AFLP was performed according to Lang et al. and Cheng et al. [38, 39]. 50 ng of each ds-cDNA sample was incubated with EcoRI and MseI enzymes for 24 h, and the fragments were ligated to adapters for amplification (EcoRI-F: 5′-CTCGTAGACTGCGTACC-3′ and EcoRI-R: 5′-AATTGGTACGCAG TCTAC-3′; MseI-F: 5′-GACGATGAGTCCTGAG-3′ and MseI-R: 5′-TACTCAG GACTCAT-3′). A primer pair (forward 5′-GACTGCGTACCAATTC-3′ and reverse 5′-GATGAGTCCTGAGTAA-3′) was designed for pre-amplification. Sequences of the selective primers used were: forward 5′-GACTGCGTACCAATTCACC-3′ and reverse 5′-GATGAGTCCTGAGTAACTT-3′. Pre-amplification was performed with 20 μl reaction mixture including 0.2 mM dNTPs, 0.2 μM non-selective primers, 1 mM MgCl2, 0.8 U Taq polymerase (Tiangen, China), and 2 μl ligated cDNA fragments. The PCR reaction consisted of 30 cycles: 94 °C for 5 min; 94 °C for 1 min; 56 °C for 1 min; 72 °C for 1 min, and a final extension at 72 °C for10 min. For the selection of gene, 4 μl pre-amplification products were used as template in the reaction, and concentrations of all the other reagents used were same as mentioned above. The PCR program consisted of 35 cycles: 94 °C for 2 min; 94 °C for 1 min; 58 °C for 1 min; 72 °C for 1 min, and the final extension at 72 °C for 10 min. The PCR products were separated on a 6 % polyacrylamide sequencing gels (40 cm-long, 0.25-mm spacer thickness) containing 6 % polyacrylamide gel solution (BMA, Rockland, ME, USA), 7.0 M urea and 1.0 TBE (10 TBE: 89 mM Tris, 89 mM boric acid and 2 mM EDTA). Final AFLP reaction products were mixed with a 2 μl of loading buffer (95 % deionized formamide, 20 mM EDTA, pH 7.5, 1 % bromophenol blue), denatured for 10 min in boiling water, and then transferred to ice before loading. The mix solution was run at 80 W until the bromophenol blue reached the bottom. DNA fragments were visualized by silver staining according to the Silver Sequence™ DNA Sequencing System Technical Manual (Promega, USA) [40].
Full length amplification by RACE-PCR
The PAGE gel was treated with diluted water two times. All differentially expressed bands were cut from the gel with a surgical blade and eluted into centrifuge tube with 40 μl sterile distilled water. The centrifuge tube was placed into boiling water for 10 min, and then centrifuged for 5 min. The supernatant was used as template for following sub-cloning. 2 μl from the above supernatant was used as template for re-amplification using selective amplification primers (forward 5′-GACTGCGTACC AATTCA CC-3′ and reverse 5′-GATGAGTCCTGAGTA ACTT-3′.). PCR product was purified with a PCR purification kit (Tiangen, China) and cloned into PMD18-T vector and sequenced.
To amplify full length gene, RACE-PCR was performed using Clontech SMART ™ RACE mix. RNA was extracted from the tips of lotus root by using plant RNA extract mix (Tiangen, China). DNase was added to remove any DNA contaminations. For the first cDNA strand synthesis, ~2–3 μg RNA was used with RNA first strand mix (Promega, USA). 20 μl PCR reaction mixture consisted of 0.2 mM dNTP, 0.2 μM of forward and reverse primers, 1 mM MgCl2, 0.5 U of Taq polymerase (Tiangen, Beijing, China), and 2 ng cDNA fragments. Primer sequences used include: forward 5′- ATGGGGAATACATGTGTTG -3′ and reverse 5′-TCAGCCAAGCTTTAAGGC -3′. The PCR program consisted of 35 cycles: 94 °C for 2 min; 94 °C for 30 s; 52 °C for 30 s; 72 °C for 60 s and the final extension at 72 °C for 10 min.
Expression analysis of LrbZIP
Four-leaf old seedlings of wild type lotus root in pots were exposed to 250 mM NaCl, low temperature (4 °C), and then total RNA was extracted from 100 mg tips after 0, 6, 12, 18, 24, and 30 h time intervals treatment. Semi quantitative qRT-PCR was performed to determine the expression of LrbZIP. To ensure the reproducibility of the results, these experiments were repeated three times. For plant hormone treatments, 100 μM ABA was applied on 4-leaf old seedlings under normal conditions. Total RNA was extracted from the tips of treated seedlings after time intervals of 0, 6, 12, 18, 24, and 30 h, respectively. Semi quantitative RT-PCR was carried out to study the gene expression. In all these Semi quantitative RT-PCR experiments, β-actin was used as internal standard (forward 5′-ACGCGTATGAAGTCAGTTGT-3′ and reverse 5′-TTTATGGGGAT CAGCTGGT-3′ primers). The PCR program consisted of 30 cycles: 94 °C for 2 min; 94 °C for 30 s; 56 °C for 30 s; 72 °C for 60 s and the final extension at 72 °C for 10 min. the PCR product was identified by 1 % agro gel.
Functional study in transgenic tobacco
Tobacco (Nicotiana tabacum L.) was used to study the function of LrbZIP. Tobacco seeds were surface-sterilized in 30 % sodium hypochlorite for 20 min and rinsed six times with sterile water before putting on the medium for germination. Plant growth conditions were set at 25 °C with a diurnal cycle of 16 h light/8 h darkness and a light intensity of 150 m−2s−2. Full-length LrbZIP cDNA was ligated into a binary vector (pSN1301) under the control of CaMV 35S promoter, and then inserted into Agrobacterium tumefaciens strain GV3101. Tobacco transformation was carried out by A. tumefaciens-mediated leaf disc method [41]. Leaf segments were co-cultivated with A. tumefaciens harboring the binary vector pSN1301, followed by regeneration under the selection medium containing kanamycin (100 μg ml−1). Timentin (400 mg l−1, Agri-Bio) was added in the regeneration/selection medium in the early process to eliminate Agrobacteria. Regenerated leaves were excised and transferred to MS agar medium for root development, containing the same concentration of kanamycin as used in the regeneration medium for root development.
Screening for transgenic plants
Plants from transgenic tobacco were transferred in the pots and grown in a greenhouse to obtain the seeds of self-pollinated T 0 progeny. Transgenic plants were identified by screening on a medium containing hygromycin B (20 μg ml−1) and PCR method. T 2 plants were used for further stress treatment. T 2 plants (per pot with the same soil moisture) including transgenic plants and wild type plants at the two-leaf stage were first applied to 250 mM NaCl. Fresh and dry weight of the treated plants (20 plants each) was measured after 20 days of treatment. Root growth was also studied after exposing the two-leaf old plants of both types of plants to different concentrations of NaCl (50 mM, 100 mM, and 250 mM) for 20 days, and then the root length was measured.
Analysis of chlorophyll content and electrolyte leakage in transgenic and wild type plants
Transgenic tobacco and wild type plants treated with 250 mM NaCl for 20 days, were used to study the content of chlorophyll. Tobacco seeds were surface-sterilized in 30 % sodium hypochlorite for 20 min and rinsed six times with sterile water before putting on the medium for germination, and then transferred into pots. Plant growth conditions were set at 28 °C with a diurnal cycle of 16 h light/8 h darkness and a light intensity of 150 m−2s−2. About 10 plants (per pot with the same soil moisture) including the transgenic line 1 and line 2, and wild type plants at the four-leaf stage were treated with 250 mM NaCl. The leaves of these plants were collected three weeks after NaCl exposure. Chlorophyll were isolated and determined using the methods of Hiscox and Israelstam (1979) [42].
For electrolyte leakage measurements, seedlings of four-leaf stage transgenic as well as wild type plants were harvested after salt treatment and washed with deionized water to remove surface-adhered electrolytes. The tubers containing three plantlets were incubated at 25 °C by in the growth chambers and set the thermostat at dark for the first 24 h. The deionized water was added into the tubes and kept at room temperature for 2 h, and then Solution conductivity of about 20 ml of solution was measured using a conductivity meter (Value A). Samples were boiled for 20 min and the final electrical conductivity (Value B) was obtained. Electrolyte leakage was calculated as following: Electrolyte leakage (%) = (A/B) × 100. Above experiments were repeated three times.
Expression study of stress related genes by RT-PCR
Semi-RT-PCR analysis was performed to study the expression of five novel genes associated with stress response with four leaf old seedlings. Total RNA was extracted using RNA extraction mini kit (QIAGEN, Germany) from wild type and transgenic plants (line 1 and line 2). DNaseI was used to digest DNA during the RNA extraction process to eliminate DNA contamination. A total of 1–2 μg of RNA was used in cDNA synthesis according to the manufacturer’s instructions (Promega, USA). Possible targeted genes were selected from bibliographical information [22]. According to the cDNA sequences in NCBI databases, primers used for five genes relevant to stress response were designed which are as follows: NtCDPK1: forward primer 5′-CGTTGAGGA ATTAGCACAGG-3′, reverse primer; 5′-CGAATAGTCACACCATGCAA-3′; NtCDPK2: forward primer 5′-CACGATCGGGAAGTTGTTG-3′, reverse primer 5′-TGACCTCTCGCTTAACATCCT-3′, NtCDPK3: forward primer 5′-AGGAAGTA GGAAGAGGGCATT-3′, reverse primer 5′-TCTTCACCTCCCTTCTCACA-3′. NtLEA: forward primer 5′-CTTTCTCTAACTCCAAACTCATC-3′, reverse primer 5′-AAATTTAACTTTATTAGAAGGTCA-3′; trehalose-6-phosphate phosphatase (TPP): forward primer 5′-AAGACATCACGGGAGCAAAG-3′, reverse primer: 5′-CCCTTGTCCCAGTTAAGCAC-3′; Tobacco actin gene was used as internal standard with the primer sequences; forward primer 5′-GCCGTGACCTAAC TGATAACC-3′, reverse primer 5′-GCTCCTGCTCGTAGTCAAGA-3′. The PCR reaction consisted of 30 cycles: 94 °C for 5 min; 94 °C for 1 min; 56–59 °C for 1 min; 72 °C for 1 min, and a final extension at 72 °C for10 min.
Results
Several differentially expressed genes were isolated by cDNA-AFLP method from lotus root after exposure to NaCl for 24 h (Fig. 1a). All these gene fragments were cloned and sequenced, and most of which were ribosomal protein gene (data not shown). Only one band containing 725 bp DNA fragment was identified to show high similarity to bZIP of other species. Semi quantitative RT-PCR was used to confirm the respective gene through the study of its expression. Analysis of expression profile testified that this gene fragment was obviously induced by salt (Fig. 1b, c). Primary analysis of this fragment showed high sequence similarity with some previously reported bZIP genes from other organs.
Isolation of LrbZIP from lotus root. a A differentially expressed gene was identified through cDNA-AFLP method from the total RNA extracted from the tips of salt-tolerant lotus root after exposure to 250 mM for 24 h (pointed with an arrow in the figure). b Semi quantitative RT-PCR was performed to ascertain that the gene was induced by salt stress (C: control; T: sample treated with salt stress). c Quantitation of RT-PCR product was determined by IOD value through a UVP Bioimaging system. The level of expression of LrbZIP treated with salt stress (T) was five times higher as compared with that of control (C)
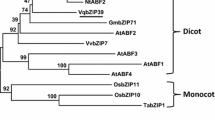
Full length of this gene was amplified with RACE-PCR method, which was found to be 1,464 bp consisting of a single open reading frame, encoding a putative polypeptide of 488 amino acids. The deduced protein contained a conserved domain of bZIP1 super family and DOG1 super family. When compared with the existing protein sequences in NCBI database, this gene showed 78, 76, 75 % sequence similarity with the bZIPs from Medicago truncatula (XP_003596814.1), Carica papaya (ABS01351.1), and Arabidopsis thaliana (NP_563810.2) (Fig. 2). On the basis of this similarity, we named it as LrbZIP. In addition, molecular evolutionary analysis also indicated that the origin of LrbZIP was very close to the bZIP of maize and rice (Fig. 3).
Phylogenetic tree was obtained by using ClustlWand Mega 4.0. Ten bZIPs were selected from plants which are as follows: bZIP (NP.563810) from Arabidopsis; bZIP (ABS01351) from Carica; bZIP (XP_003606112) from Medicago; bZIP (XP_002450285.1) from Sorghum; bZIP (NP 0011104893.1) from Zea; bZIP (AAF06696.1) from Nacotiana; bZIP (BAB72064.1) from oryza; bZIP (ABP8823.1) from Glycine; bZIP (ADK74993.1) from Phaseolus
Expression of LrbZIP
To study the pattern of LrbZIP mRNA accumulation in lotus root, total RNA was extracted from tips at different time intervals after NaCl treatment. Semi quantitative RT-PCR was performed to evaluate the expression of LrbZIP. Results showed that LrbZIP expression was induced by NaCl treatment and its expression was increased after exposure to NaCl for 18 h (Fig. 4a). The expression of LrbZIP to low temperature (4 °C) and ABA (100 μM) was also analyzed. LrbZIP was responsive to low temperature, and the expression was strongly enhanced after treatment of 12 h, and then declined at 30 h (Fig. 4b). Exogenous ABA significantly induced the LrbZIP expression after 24 h of treatment under normal growth conditions (Fig. 4c).
Expression of LrbZIP in tips of lotus root. Total RNA was extracted from tips after 0, 6, 12, 18, 24, and 30 h time intervals of stresses and hormone treatment. DNaseI was added to eliminate DNA contamination. Semi quantitative RT-PCR was used to study the gene expression. Three experiments were replicated and actin was used as internal standard. a LrbZIP expression in tips with 250 mM salt treatment; b LrbZIP expression with low temperature treatment (4 °C). c LrbZIP expression with exogenous 100 μm ABA treatment
Functional study in tobacco
The transgenic tobacco plants carrying LrbZIP were confirmed by RT-PCR method with primer designed in low homologous region of nucleotide sequence between LrbZIP and NtbZIP. The expression of LrbZIP was detected in transgenic plants when grown at 25 °C, whereas no expression was observed in the wild type plants (Fig. 5a). After selection, the transgenic tobacco plants were exposed to 250 mM NaCl for 20 days, and wild type plants with the same treatment were used as control. Results clearly demonstrated that transgenic plants with overexpression of LrZIP showed better growth as compared with wild type plants (Fig. 5b). For further confirmation, fresh and dry weight of salt treated transgenic plants and control plants were measured, which showed that transgenic plants had higher fresh and dry weights than the control (Fig. 5c).
Study of LrbZIP function in the transgenic and wild type plants, when exposed to salt stress. Transformed tobacco plants with LrbZIP, inserted in a binary vector construct under a 35S promoter. Two-leaf old seedlings of transgenic and wild type plants were treated with 250 mM NaCl, and measured the dry and fresh weight of plants as survival index under salt stress
Effects of different concentrations (50, 100, and 250 mM) of NaCl on roots of transgenic tobacco plants and wild type plants were also investigated. No significant effects were observed on the root growth of both transgenic and wild type plants under normal growth condition. But inhibition of root metabolism and growth was observed in the wild type plants exposed to salt concentrations of 50, 100, and 250 mM of NaCl concentrations (Fig. 6). Both transgenic and wild type plants were used to study the chlorophyll and electrolyte leakage after NaCl treatment. It was observed that transgenic plants (line 1 and line 2) had significantly higher chlorophyll as compared with the wild type seedlings when exposed to NaCl stress (Fig. 7a). However, the electrolyte leakage was lower in transgenic plants as compared with that of wild type plants (Fig 7b). Above experiments were further confirmed that transgenic plants with overexpression of LrbZIP enhanced the tolerance to salt stress.
Growth of transgenic and wild type plants subjected to salt stress. Four-leaf old seedlings of transgenic and wild type plants were treated with different concentrations of NaCl for a period of 20 days, and then growth conditions were investigated. a Treated with 0 mM NaCl; b treated with 50 mM NaCl; c treated with 100 mM NaCl and d treated with 250 mM NaCl
Chlorophyll content and electrolyte leakage in transgenic plants and wild type plants after salt streatmen. Transgenic tobacco and wild type plants were treated with 250 mM NaCl for 20 days. The chlorophyll content and electrolyte leakage were measured in both type of plants. a Chlorophyll content in transgenic plants and wild type plants; b electrolyte leakage in transgenic plants and wild type plants
Expression of stress related genes in transgenic and wild type plants
A few stress-responsive genes in tobacco were selected as candidate target genes for this transcription factor, and homologous sequences (LEA, CDPKs, and TPP) were searched in the databases for tobacco. Most of these genes have been reported to be induced by different stresses in the literature. After 24 h of salt treatment, total RNA was extracted from leaves and the expression of these genes was analyzed with semi RT-PCR method. The expression of three genes (LEA, CDPK2, and TPP) in wild type plants was lower or undetectable, whereas the expression level was enhanced in transgenic plants transformed with 35S-LrbZIP after exposure to salt stress. In addition, we observed that the expression of CDPK1 and CDPK2 did not have any significant change after NaCl treatment. These results indicate that the expression of LrbZIP in tobacco could improve the expression of certain genes, which further provided evidence for the involvement of this transcription factor in stress responses in the salt stress-resistant species of lotus root.
Discussion
Isolation of LrbZIP with cDNA-AFLP approach
Salt tolerance of crops must be increased to sustain food production in many regions of the world. If all the other agronomic constraints are overcome, even then subsoil salinity remains a major limitation to agriculture in all semi-arid regions. Various approaches have been used to improve the salt tolerance of plants by introducing the genes for salt tolerance into adapted cultivars [14]. Research has been carried out with the aims to exploit variation in salt tolerance within lotus root and its progenitors or close relatives to produce new cultivars with more tolerance than common lotus root cultivars. Therefore, isolation of salt resistant genes becomes very important for engineering salt-tolerant species to enhance agricultural production.
cDNA-AFLP approach is a powerful gel-based genome-scale transcript profiling technique to generate gene expression profiles [37]. Many stress-induced genes, for example, cold acclimated genes in leaves of Citrus unshiu and salt-induced gene in soybean, have been discovered using this method [38, 43]. In this study, a transcription factor, LrbZIP was isolated from salt-tolerated lotus species by cDNA-AFLP (Fig. 1), which showed 78, 76, and 75 % sequence similarity with the bZIPs from M. truncatula, C. papaya, and A. thaliana (Fig. 2).
bZIPs are normally classified into 19 groups of homologues according to their highly conserved domains [44], and their diversity probably results from their independent ways from different ancients [45]. Although there is a large variation in amino acid sequence within a subfamily among organisms, these changes usually occur outside the conserved domains of bZIP, which indicates that this kind of changes might facilitate new interactions with signal transduction and coactivator–corepressor proteins [46]. Our molecular phylogenetic analysis showed that the origin of LrbZIP lies very close to the bZIPs of M. truncatula, C. papaya, and A. thaliana. (Fig. 3). Functions of these bZIPs in response to different stresses have already been identified [47]. In addtition, The LrbZIP belongs to bZIP1 superfamily (the S-group bZIPs) according to the conserved domain of deduced protein. bZIP1 was shown to be linked to the SnRK1 (The sucrose non-fermenting 1-related protein kinase) signal cascade. SnRK is homologous of SNF1 and AMP-activated protein kinase (AMPK), which widely exists in plant and involves in a variety of signaling pathways, and is believed as a switch in plant response to stress and other metabolism [48]. Evidence shows that both rice (Oryza sativa) and Arabidopsis (A. thaliana) SnRK1 activities critically influence the expression of stress-inducible and lead to the induction of stress tolerance [49, 50]. Thus LrbZIP might be playing a critical role in lotus root to adapt to different stresses.
Expression of LrbZIP
Plant growth substances or hormones are involved in signal transduction [51]. To elaborate the role of plant hormones in the induction of LrbZIP expression, we exogenously exposed lotus root to the ABA. ABA is a small, lipophilic plant hormone that modulates plant growth, seed maturation, dormancy, and adaptive responses to environmental stresses [52]. It is ubiquitous in lower and higher plants and its biosynthetic and catabolic pathways have been elucidated. Physiological responses to ABA are brought about by changes in gene expression.
Hundreds of genes in various species have been shown to be responsive to ABA [53]. Recent genome-wide expression profiles have revealed that over a thousand genes are either up- or down regulated by ABA in Arabidopsis [54]. It has been reported that application of the plant hormone-ABA has resulted in increasing stress tolerance both in herbaceous and woody species [55]. ABA plays an important role in adaptive responses to various abiotic stresses [52]. Depending on its involvement, the ABA adaptive responses can be divided into two broad categories: ABA-dependent and ABA-independent pathways [27]. Here in this study, ABA induced the expression of LrbZIP (Fig 4c), which showed that expression of this gene belonged to ABA dependent pathways. Many bZIP subfamilies are found to interact with G-ABRE or G-box sequence [56]. However, until now only the ABA-responsive element binding protein subfamily of bZIP transcription factors has been documented to be involved in ABA and stress responses [57]. From the expression characteristic to salt, low temperature and ABA (Fig. 4a, b, c), we conclude that LrbZIP should belong to AREB subfamily of bZIP transcription factors.
Functional analysis of LrbZIP
We transformed tobacco plants with LrbZIP under a 35S promoter to study its response to stresses. After selection, the transgenic tobacco plants were exposed to NaCl treatment. Wild type plants with the same treatment were used as control. Results demonstrate that transgenic plants with overexpression of LrbZIP showed better growth as compared with the control (Fig. 5). In addition, transgenic tobacco plants treated with salt showed better root growth than that of wild type plants (Fig. 6), suggesting that expression of LrbZIP probably helps salt-tolerant species of lotus root to adapt to salt stress.
Overexpression of transcription factors enhances the tolerance of transgenic plants to various abiotic stresses [58]. LrbZIP has been testified to belong to AREB/ABF bZIP transcription factor according to expression profile, because in plant kingdom, only the AREB/ABF bZIP transcription factor is ABA-responsive gene which regulates plant abiotic stress responses [57, 59]. Until now, four types of AREB/ABF bZIP transcription factors are found to be induced by exogenous ABA and other stresses [34]. StABF1, a potato AREB/ABF bZIP transcription factor, is induced by ABA and salt stress [60]. The expression of OsABF1 in rice shoots and roots is also observed to be enhanced by anoxia, salinity, drought and oxidative stress, cold and ABA. Osabf1-1 shows more sensitivity to drought and salt stress as compared with that of wild type plants, suggesting that OsABF1 is involved in abiotic stress responses and ABA-dependent pathways in rice [52]. In addition, ABF2 over expression in plants enhanced their resistance to drought, salt, heat, and oxidative stress [62].
Uno et al. [24] found that AREB2 is responsive to exogenous ABA, drought and salinity stresses. Further study testifies that AREB2 and AREB1 have largely overlapping functions to stress conditions according to expression patterns and subcellular localization [63]. For ABF3 and ABF4, constitutive expression of these two genes in plant confers positive regulatory roles in ABA and stress responses [64], and down-regulated ABF3 or ABF4 lead to insensitive to ABA and drought stress [62]. Unlike other ABFs, plants with constitutive expression of ABF3 leads to reprogramming of the drought response through enhancing expression of some drought response genes without growth inhibition or visible phenotypic alterations [65, 66]. We found that transgenic tobacco plants overexpressing LrbZIP showed enhanced survival to salt stress. However, transgenic and wild type plants showed no obvious difference after recovery from low temperature treatment (data not shown), suggesting that the roles of AREB/ABF bZIP transcription factors might be different in different stresses.
Aside from the LrbZIP, several salt-resistance genes have already been identified in different organs in literature. Transgenic Arabidopsis plants with AtSOS1 display enhanced salt tolerance [67]. An antiporter AtNHX1 induced into Arabidopsis leads to increase both Na+ accumulation and Na+ tolerance [68], giving similar results in tomato and brassica [69, 70]. At the same time, grain yield is also enhanced when AtNHX1 is overexpressed in wheat in moderately saline soils [71]. Wu et al. [72] found that constitutive expression of a cotton orthologue, GhNHX1, markedly increased salt tolerance in tobacco. Transgenic rice with wheat LEA genes PMA80 and PMA1959 shows enhanced salt tolerance in glasshouse tests [73]. Above reports raise the exciting possibility that salt tolerance can be engineered into important crop plants through the transfer and appropriate expression of a single gene. Therefore, improvement in the salt resistance of cultivated species of lotus root with our identified LrbZIP will be possible in near future.
Expression of stress related genes in transgenic plants
Several classes of calcium-sensing proteins have been identified in plants and many extracellular signals elicit changes in the cellular Ca2+ concentrations in plants [73, 74]. Decoding of these calcium signals is performed by protein kinases, such as the CDPKs, that mediate cellular responses either directly by changing enzymatic activities via protein phosphorylation or indirectly by changing gene expression patterns [75]. NtCDPK1, 2, and 3 are membrane associated protein kinases in tobacco. Although calcium binding is a necessary prerequisite for CDPK activation, our data indicate that kinase phosphorylation is also mechanistically involved for the CDPK to become biologically functional. The phosphorylation of the NtCDPKs by unidentified upstream kinase(s) was observed to be stimulus-dependent, suggesting that these kinases will also be activated during a biotic or abiotic stimulation [76]. Expression of NtCDPK2 is enhanced and it is phosphorylated when treated with osmotic stress [74]. NtCDPK3, which shares high similarity with NtCDPK2, has also been found to be involved in the plant defence responses [77]. Therefore it is very important to study the expression of Ca2+ dependent proteins CDPK1, CDPK2, and CDPK3, in transgenic tobacco plants transformed with LrbZIP when exposed to salt stress. Results show that there was no significant change in the expression of NtCDPK1 and NtCDPK3 in transgenic or control plants. However, the expression of NtCDPK2 was enhanced in transgenic plants as compared with the wild type plants (Fig. 8). Therefore it can be concluded that overexpression of LrbZIP in transgenic plants induced the expression of NtCDPK2, which contributed in their survival in salt stress.
Expression of stress-related genes in transgnic plants and wild type plant after salt stress treatment. The expression of five novel genes (CDPK1, CDPK2, CDPK3, LEA and TPP) associated with stress response was studied by semi RT-PCR method with four leaf old tobacco seedlings. Tobacco actin gene was used as internal standard
Late-embryogenesis abundant (LEA) proteins are a family of hydrophilic proteins which are involved in stress tolerance in plant kingdom [78]. LEA proteins not only accumulate in seeds but also in vegetative tissues after exogenous ABA treatment or environmental stresses such as chilling, freezing, drought, and salinity stimulation [79]. A series of experiments with recombinant Saccharomyces cerevisiae illustrated that increased stress tolerance is directly attributable to the accumulation of LEA proteins. Xiao found that Overexpression of a LEA gene in rice improves drought resistance in transgenic plants compared with wild type plants under the field conditions. At the same time, plants transformed with a Thellungiella LEA gene can improve salt-tolerance in Arabidopsis, which suggests that LEA may participate in response to stresses [80]. Yeasts harbouring tomato Le4 (LEA II), barley HVA1 (LEA III) or tomato Le25 (LEA II) genes were studied under various stresses, and over-expression of these proteins increases abiotic stress tolerance [81–83]. Blackman et al. (1991) [84] demonstrated that the increased LEA protein level might reduce the electrolyte leakage after desiccation and subsequent rehydration. Over-expression of barley HVA1 (a late embryogenesis abundant protein gene) confers tolerance to salt stress in transgenic rice [85]. In addition, transgenic plants overexpressing Rab16A, a Group 2 lea gene families, exhibited significantly increased tolerance to salinity, and the transgenic plants show normal growth, morphology, seed production, delayed development of damage symptoms, lesser chlorophyll loss under stress conditions [86].
The above reports suggest that different LEA proteins may have unique contributions to cellular protection against stresses, and may increase plant adaptability under extreme environments. We found that expression of TPP was enhanced in transgenic plants (Fig. 8). TPP encodes an enzyme involved in the synthesis of disaccharide trehalose, which is believed to play an important role in synthesis of sugar, regulation of various metabolic reactions and stress protectant in a variety of organisms [87]. A mutant deficient in the trehalose-6-phosphaste was sensitive to salinity, suggesting that a role of trehalose as a secondary solute involved in plant adaptation to salt stress [88]. Wingler (2002) [89] reports that TPP provides a protective role when plant is in osmotic stress situations. Further study found that overexpression of TPP gene in rice can improve the tolerance to salt stress and low temperatures [90, 91]. In this experiment, improvement in the expression of NtLEA and NtTPP in transgenic plants containing LrbZIP, might aid tobacco to adapt to salt stress.
References
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plant 133:651–669
Maas E, Grieve C (1990) Spike and leaf development in salt-stressed wheat. Crop Sci 30:1309–1313
Kader MA, Lindberg S (2010) Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signal Behav 5:233–238
Husain S, Munns R, Condon A (2003) Effect of sodium exclusion trait on chlorophyll retention and growth of durum wheat in saline soil. Aus J of Agri Res 54:589–598
Munns R, James R, Läuchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57:1025–1043
Hall J, Flowers T (1973) The effect of salt on protein synthesis in the halophyte Suaeda maritima. Planta 110:361–368
Murguia JR, Belles JM, Serrano R (1995) A salt-sensitive 3′(2′),5′-bisphosphate nucleotidase involved in sulfate activation. Science 267:232–234
Salt DE (2004) Update on plant ionomics. Plant Physiol 136:2451–2456
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Lahner B, Gong J, Mahmoudian M, Smith EL, Abid KB, Rogers EE, Guerinot ML, Harper JF, Ward JM, McIntyre L (2003) Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nat Biotech 21:1215–1221
Santa-Cruz A, Acosta M, Rus A, Bolarin MC (1999) Short-term salt tolerance mechanisms in differentially salt tolerant tomato species. Plant Physiol Biochem 37:65–71
Seki M, Kamei A, Yamaguchi-Shinozaki K, Shinozaki K (2003) Molecular responses to drought, salinity and frost: common and different paths for plant protection. Curr Opin Biotechnol 14:194–199
Wang X, Li Y, Ji W, Bai X, Cai H, Zhu D, Sun XL, Chen LJ, Zhu YM (2011) A novel Glycine soja tonoplast intrinsic protein gene responds to abiotic stress and depresses salt and dehydration tolerance in transgenic Arabidopsis thaliana. J Plant Physiol 168(11):1241–1248. doi:10.1016/j.jplph.2011.01.016
Zhang H, Mao X, Jing R, Chang X, Xie H (2011) Characterization of a common wheat (Triticum aestivum L.) TaSnRK2. 7 gene involved in abiotic stress responses. J Exp Bot 62:975–988
Yokotani N, Higuchi M, Kondou Y, Ichikawa T, Iwabuchi M, Hirochika H, Matsui M, Oda K (2011) A novel chloroplast protein, CEST induces tolerance to multiple environmental stresses and reduces photooxidative damage in transgenic Arabidopsis. J Exp Bot 62:557–569
Kang HG, Kim J, Kim B, Jeong H, Choi SH, Kim EK, Lee HY, Lim PO (2011) Overexpression of FTL1/DDF1, an AP2 transcription factor, enhances tolerance to cold, drought, and heat stresses in Arabidopsis thaliana. Plant Sci 180:634–641
Xu GY, Rocha PSCF, Wang ML, Xu ML, Cui YC, Li LY, Zhu YX, Xia X (2011) A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 234(1):47–59. doi:10.1007/s00425-011-1386-z
Hu HH, You J, Fang YJ, Zhu XY, Qi ZY, Xiong LH (2010) Erratum to: characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol Bio 72:567–568
Rodriguez-Uribe L, Connell MAO (2006) A root-specific bZIP transcription factor is responsive to water deficit stress in tepary bean (Phaseolus acutifolius) and common bean (P. vulgaris). J Exp Bot 5:1391–1398
Jakoby M, Weisshaar B, Droge-Laser W, Carbajosa JV, Tiedeman J, Kroj T (2002) bZIP transcription factors in Arabidopsis. Trends Plant Sci 7:106–111
Zhang X, Wang L, Meng H, Wen H, Fan Y, Zhao J (2011) Maize ABP9 enhances tolerance to multiple stresses in transgenic Arabidopsis by modulating ABA signaling and cellular levels of reactive oxygen species. Plant Mol Biol 75:365–378
Yanez M, Caceres S, Orellana S, Bastıas A, Verdugo I, Ruiz-Lara S, Casaretto JA (2009) An abiotic stress-responsive bZIP transcription factor from wild and cultivated tomatoes regulates stress-related genes. Plant Cell Rep 28:1497–1507
Wang YC, Gao CQ, Liang YN, Wang C, Yang CP, Liu GF (2010) A novel bZIP gene from Tamarix hispida mediates physiological responses to salt stress into tobacco plants. J Plant Physiol 167:222–230
Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2000) Arabidospsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high salinity conditions. Pro Natl Acad Sci USA 97:11632–11637
Xue GP, Loveridge CW (2003) HvDRF1 is involved in abscisic acid-mediated gene regulation in barley and produces two forms of AP2 transcriptional activators, interacting preferably with a CT-rich element. Plant J 37:326–339
Zou MJ, Guan YC, Ren HB, Zhang F, Chen F (2008) A bZIP transcription factor, OsABI5, is involved in rice fertility and stress tolerance. Plant Mol Biol 66:675–683
Kim S (2005) The role of ABF family bZIP class transcription factors in stress response. Physiol Plant 126:519–527
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Kobayashi Y, Murata M, Minami H, Yamamoto S, Kagaya Y, Hobo T, Yamamoto A, Hattori T (2005) Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA-response element-binding factors. Plant J 44:939–949
Du H, Zhao X, You JS, Park JY, Kim SH, Chang KJ (2010) Antioxidant and hepatic protective effects of lotus root hot water extract with taurine supplementation in rats fed a high fat diet. J Biomed Sci 17(Suppl 1):S39. doi:10.1186/1423-0127-17-S1-S39
Sakamoto Y (1977) Lotus. Hosei University Press, Tokyo
Liu J, Zhang M, Wang S (2010) Processing characteristics and flavour of full lotus root powder beverage. J Sci Food Agric 90:2482–2489
Slocum PD, Robinson P (1996) Water gardening, water lilies and lotuses. Timber, Portland, OR
Borgi W, Ghedira K, Chouchane N (2007) Antiinflammatory and analgesic activities of zizyphus lotus root barks. Fitoterapia 78:16–19
Renato BRAZ, Hechenleitner AAW, Cavalcanti OA (2007) Extraction, structural modification and characterization of lotus roots polysaccharides (Nelumbo nucifera Gaertn). Excipient with potential application in modified drug delivery systems. Lat Am J Pharm 26:706–710
Terashima M, Awano K, Honda Y, Yoshino N, Mori T, Fujita H, Ohashi Y, Seguchi O, Kobayashi K, Yamagishi M, Fitzgerald PJ, Yock PG, Maeda K (2011) Arteries within the artery after kawasaki diease-A lotus root appearance by intravascular ultrasound. Circulation 106(7):887. doi:10.1161/01.CIR.0000030708.86783.92
Vos P, Hogers R, Bleeker M, Reijans M, Van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M (1995) AFLP: a new technique for DNA fingerprinting. Nuc Acids Res 23:4407–4414
Lang P, Zhang C, Ebel R, Dane F, Dozier W (2005) Identification of cold acclimated genes in leaves of Citrus unshiu by mRNA differential display. Gene 359:111–118
Cheng LB, Huan ST, Sheng YD, Hua XJ, Song SQ, Jing XM (2009) GMCHI, cloned from soybean [Glycine max (L.) Meer.], enhances survival in transgenic Arabidopsis under abiotic stress. Plant Cell Rep 28:145–153
Vuylsteke M, Daele HVD, Vercauteren A, Zabeau M, Kuiper M (2006) Genetic dissection of transcriptional regulation by cDNAAFLP. Plant J 45:439–446
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Hiscox JD, Israelstam GF (1979) A method for extraction of chlorophyll from leaf tissue without maceration. Can J Bot 59:463–469
Umezawa T, Mizumo K, Fujimura T (2002) Discrimination of genes expressed in response to the ionic or osmotic effect of salt stress in soybean with cDNAa-AFLP. Plant Cell Environ 25:1617–1625
Hurst HC (1994) Transcription factors 1: bZIP proteins. Protein Profile 1:123–168
Correa LGG, Riano-Pachon DM, Schrago CG, Santos RV, Mueller-Roeber B, Vincentz M (2008) The role of bZIP transcription factors in green plant evolution: adaptive features emerging from four founder genes. PLoS One 3(8):e2944
Amoutzias GD, Veron AS, Weiner J, Robinson-Rechavi M, Bornberg-Bauer E, Oliver SG, Robertson DL (2007) One billion years of bZIP transcription factor evolution: conservation and change in dimerization and DNA-Binding site specificity. Mol Biol Evol 24:827–835
Huang XS, Liu JH, Chen XJ (2010) Overexpression of PtrABF gene, a bzip transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Bio 10:230–248
Dong XF, Cui N, Wang L, Zhao XC, Qu B, Li TL, Zhang GL (2012) The SnRK protein kinase family and the function of SnRK1 protein kinase. Int J Agric Biol 14:575–579
Cho YH, Hong JW, Kim EC, Yoo SD (2012) Regulatory functions of SnRK1 in stress-responsive gene expression and in plant growth and development. Plant Physiol 158:1955–1964
Cheng C, Yun KY, Ressom HW, Mohanty B, Bajic VB, Jia YL, Yun SJ, de los Reyes BG (2007) An early response regulatory cluster induced by low temperature and hydrogen peroxide in seedlings of chilling-tolerant japonica rice. BMC Genomics 8:175
Sembdner G, Parthie B (1993) The biochemistry and the physiological and molecular actions of jasmonates. Ann Rev Plant Biol 44:569–589
Yang S, Zeevaart J (2006) Expression of ABA 8′-hydroxylases in relation to leaf water relations and seed development in bean. Plant J 47:675–686
Finkelstein R, Gampala S, Rock C (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14:15–45
Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nakajima M, Enju A, Sakurai T (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J 31:279–292
Li C, Junttila O, Heino P, Palva E (2003) Different responses of northern and southern ecotypes of Betula pendula to exogenous ABA application. Tree Physiol 23:481–487
Menkens AE, Schindler U, Cashmore AR (1995) The G-box: a ubiquitous regulatory DNA element in plants bound by the GBF family of bZIPs protein. Trends Biochem Sci 20:506–512
Kim SY (2006) The role of ABF family bZIP class transcription factors in stress response. Physiol Plant 126:519–527
Jaglo K, Kleff S, Amundsen K, Zhang X, Haake V, Zhang J, Deits T, Thomashow M (2001) Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species. Plant Physiol 127:910–917
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Ann Rev Plant Biol 57:781–803
García MNM, Giammaria V, Grandellis C, Téllez-Iñón MT, Ulloa RM, Capiati DA (2012) Characterization of StABF1, a stress-responsive bZIP transcription factor from Solanum tuberosum L. that is phosphorylated by StCDPK2 in vitro. Planta 235:761–778
Hossain MA, Lee YJ, Cho JI, Ahn CH, Lee SK, Jeon JS, Kang H, Lee CH, An G, Park PB (2010) The bZIP transcription factor OsABF1 is an ABA responsive element binding factor that enhances abiotic stress signaling in rice. Plant Mol Biol 72:557–566
Kim S, Kang JY, Cho D-I, Park JH, Kim SY (2004) ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J 40:75–87
Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2010) AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA forfull activation. Plant J 61:672–685
Kang J, Choi H, Im M, Kim SY (2002) Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell 14:343–357
Abdeen A, Schnell J, Miki B (2010) Transcriptome analysis reveals absence of unintended effects in drought-tolerant transgenic plants overexpressing the transcription factor ABF3. BMC Genomics 211:69–90
Oh SJ, Song SI, Kim YS, Jang HJ, Kim SY, Kim M, Kim YK, Nahm BH, Kim JK (2005) Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol 138:341–351
Shi H, Lee B, Wu S, Zhu J (2003) Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotech 21:81–85
Apse MP, Blumwald E (2002) Engineering salt tolerance in plants. Cur Opin Biotechnol 13:146–150
Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768
Zhang HX, Hodson JN, Williams JP, Blumwald E (2001) Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc Natl Acad Sci USA 98:12832–12836
Xue Z, Zhi D, Xue G, Zhang H, Zhao Y, Xia G (2004) Enhanced salt tolerance of transgenic wheat (Tritivum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Sci 167:849–859
Wu CA, Yang GD, Meng QW, Zheng CC (2004) The cotton GhNHX1 gene encoding a novel putative tonoplast Na(+)/H(+) antiporter plays an important role in salt stress. Plant Cell Physiol 45:600–607
Cheng SH, Willmann MR, Chen HC, Sheen J (2002) Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol 129:469–485
Ludwig AA, Romeis T, Jones JDG (2004) CDPK mediated signalling pathways: specificity and cross talk. J Exp Bot 55:181–188
Sathyanarayanan P, Poovaiah B (2004) Decoding Ca(2+) signals in plants. CRC Crit Rev Plant Sci 23:1–11
Witte CP, Keinath N, Dubiella U, Demoulière R, Seal A, Romeis T (2010) Tobacco calcium-dependent protein kinases are differentially phosphorylated in vivo as part of a kinase cascade that regulates stress response. J Biological Chem 285:9740–9748
Romeis T (2001) Protein kinases in the plant defence response. Curr Opin Plant Biol 4:407–414
Dalal M, Tayal D, Chinnusamy V, Bansal KC (2009) Abiotic stress and ABA-inducible Group 4 LEA from Brassica napus plays a key role in salt and drought tolerance. J Biotechnol 139:137–145
Ginger A, Swire-Clark WR, Marcotte JR (1999) The wheat LEA protein Em functions as an osmoprotective molecule in Saccharomyces cerevisia. Plant Mol Biol 39:117–128
Zhang Y, Li Y, Lai J, Zhang H, Liu Y, Liang L, Xie Q (2012) Ectopic expression of a LEA protein gene TsLEA1 from Thellungiella salsuginea confers salt-tolerance in yeast and Arabidopsis. Mol Biol Rep 39:4627–4633
Imai L, Chang A, Otha EA, Bray TM (1996) A lea-class gene of tomato confers salt and freezing tolerance when expressed in Saccharomyces cerevisiae. Gene 170:243–248
Zhang L, Ohta A, Takagi M, Imai R (2000) Expression of plant group 2 and group 3 lea genes in Saccharomyces cerevisiae revealed functional divergence among LEA proteins. J Biochem 127:611–616
Lai SLN, Grlyani VH, Khurana PJ (2008) Overexpression of HVA1 gene from barley generates tolerance to salinity and water stress in transgenic mulberry (Morus indica). Transgenic Res 17:651–663
Blackman SA, Wettlaufer SH, Obendorf RL, Leopold AC (1991) Maturation proteins associated with desiccation tolerance in soybean. Plant Physiol 96:868–874
Xu D, Duan X, Wang B, Hong B, Ho THD, Wu R (1996) Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol 1:249–257
RoyChoudhury A, Roy C, Sengupta DN (2007) Transgenic tobacco plants overexpressing the heterologous lea gene Rab16A from rice during high salt and water deficit display enhanced tolerance to salinity stress. Plant Cell Rep 26:1839–1859
Kondrak M, Marincs F, Antal F, Juhasz Z, Banfalvi Z (2012) Effects of yeast trehalose-6-phosphate synthase 1 on gene expression and carbohydrate contents of potato leaves under drought stress conditions. BMC Plant Biol 12:74
Reina-Bueno M, Argandoña M, Salvador M, Rodríguez-Moya J, Iglesias-Guerra F, Csonka LN, Nieto JJ, Vargas C (2012) Role of trehalose in salinity and temperature tolerance in the model halophilic bacterium Chromohalobacter salexigens. PLoS One 7:e33587
Wingler A (2002) The function of trehalose biosynthesis in plants. Phytochemistry 60:437–440
Miranda JA, Avonce N, Suárez R, Thevelein JM, Van Dijck P, Iturriaga G (2007) A bifunctional TPS-TPP enzyme from yeast confers tolerance to multiple and extreme abiotic-stress conditions in transgenic Arabidopsis. Planta 226:1411–1421
Ge L, Chao D, Shi M et al (2008) Overexpression of the trehalose-6-phosphatase gene OsTPP1 confers stress tolerance in rice and results in the activation of stress-responsive genes. Planta 228:191–201
Acknowledgments
Authors thank Xiong Liben for her suggestions and language check. This work was supported by Special Fund for Agro-scientific Research in the Public Interest (200903017-02), China Postdoctoral Science Foundation (2012M511805) and Jiangsu Postdoctoral Science Foundation (1102144C).
Author information
Authors and Affiliations
Corresponding author
Additional information
Libao Cheng and Shuyan Li have contribution equally to this research work.
Rights and permissions
About this article
Cite this article
Cheng, L., Li, S., Hussain, J. et al. Isolation and functional characterization of a salt responsive transcriptional factor, LrbZIP from lotus root (Nelumbo nucifera Gaertn). Mol Biol Rep 40, 4033–4045 (2013). https://doi.org/10.1007/s11033-012-2481-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2481-3