Abstract
MicroRNAs (miRNAs) are a class of non-coding small RNAs representing one of the most exciting areas of modern medical science. miRNAs modulate a large and complex regulatory network of gene expression of the majority of the protein-coding genes. Currently, evidences suggest that miRNAs play a crucial role in the pathogenesis of heart failure. Some miRNAs as miR-1, miR-133 and miR-208a are highly expressed in the heart and strongly associated with the development of cardiac hypertrophy. Recent data indicate that these miRNAs as well as miR-206 change their expression quickly in response to physical activity. The differential regulation of miRNAs in response to exercise suggests a potential value of circulating miRNAs (c-miRNAs) as biomarkers of physiological mediators of the cardiovascular adaptation induced by exercise. Likewise, serum levels of c-miRNAs such as miR-423-5p have been evaluated as potential biomarkers in the diagnosis and prognosis of heart failure. On the other hand, the manipulation of miRNAs levels using techniques such as ‘miR mimics’ and ‘antagomiRs’ is becoming evident the enormous potential of miRNAs as promising therapeutic strategies in heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last two decades, one family of regulatory RNAs called microRNAs (miRNAs), because of their short length (~22 nucleotides), has fundamentally transformed our understanding of how gene networks are regulated representing one of the most exciting areas of the modern cardiology research. Unlike the wide range of RNAs encoded by the human genome this RNA variety has been noted for its unique ability to modulate an enormous and complex regulatory network of gene expression.
To date, more than 1,500 miRNAs have been identified in the human genome (www.mirbase.org) and this number is expected to grow further due to the recent development of sequencing technologies and computational prediction methods [1]. Recently, several studies have shown that miRNAs modulate a wide spectrum of cardiac functions not only in heart development but also in pathogenic conditions such as heart failure (HF).
MicroRNAs in cardiac and skeletal muscles
Although the biological functions of miRNAs are not fully understood, studies have shown that some miRNAs are specifically expressed in certain tissues or cell types [1]. Four miRNAs are highly expressed in the heart: miR-1, miR-133, miR-208 and miR-499. The miR-1 family is the most expressed miRNA in the heart while miR-208 is the only cardio-specific miRNA known until now. However, some miRNAs such as miR-128, miR-302, miR-367 and miR-499 are potentially cardio-specific, but more studies are needed to confirm.
In skeletal muscle, miR-1, miR-133a, miR-133b and miR-206 together account for approximately 25 % of the expression of miRNAs and are sometimes referred to as myomiRs [2]. These miRNAs are not only expressed in skeletal muscle but also in the heart and induced during muscle differentiation playing a critical role in this process [3]. Moreover, miRNAs whose expression is not restricted to the heart may have important cardio-specific functions.
To date, many cardiovascular miRNAs were described as dynamically regulated in response to acute cardiac stress and in some cases during long-term compensatory response of the heart to a chronic injury or hemodynamic overload [4, 5]. Thus, there is growing evidence that the expression of miRNAs is an important part of the response mechanism of the heart to acute stress and contributes to the homeostasis and cardiac pathology. The analyses of deregulated miRNAs in distinct disease conditions may help the diagnosis and prognosis as well as guide the development of drugs targeting the normalization of miRNAs levels [6].
MicroRNAs in myocardial hypertrophy and heart failure
In recent years, many miRNAs were shown to be deregulated in specific tissues playing critical roles in pathogenesis and progression of the HF (Table 1). During cardiac hypertrophy, it seems to be an exchange of the genetic program leading to a reactivation of cardiac genes normally expressed in fetal heart during embryonic development. In 2007, an impressive similarity was observed between the expression pattern of miRNAs in the adult hearts with HF and fetus hearts at 12–14 weeks of gestation. About 80 % of miRNAs analyzed were found similarly regulated in both hearts. The most notable changes were associated to an increased expression of miR-21, miR-29b, miR-129, miR-210, miR-211, miR-212, miR-423, and reduced expression of miR-30, miR-182 and miR-526 [7].
Experiment in mice showed that one of the earliest changes observed after a pressure overload to the heart is the reduction of miR-1, even before the increase in cardiac mass [4]. This result suggests that the reduction in the expression level of miR-1 may be cause rather than consequence of the underlying pathogenesis. Thus, both in vitro [8] and in vivo [9] data suggest that reduced expression of miR-1 is required for increased cell mass. On the other hand, the behavior of miR-1 in HF has divergent findings. While some authors [10, 11] observed a reduced expression of miR-1 in dilated and ischemic cardiomyopathy, others [7, 12] reported an increase. Han et al. [13] suggest the possibility that the expression of miR-1 is reduced in hypertrophy, but it returns to baseline or above when it progresses to heart failure.
In addition to miR-1, another muscle-specific miRNA, miR-133, has also reduced expression during cardiac hypertrophy [9, 14]. Mice with reduced expression of miR-133 showed cardiomyopathy, heart failure and an abnormal proliferation of cardiomyocytes [15]. In a recent study, the expression of miR-133 was induced in a mice model subjected to acute hypertrophic stimulus. Although the weight of the heart has not been standardized, other aspects of hypertrophy such as apoptosis and fibrosis were restored to baseline levels [5].
Some miRNAs may be differentially expressed in specific types of disease. In a study by Ikeda et al., the expression patterns of miRNAs in samples of myocardium of patients with ischemic cardiomyopathy, idiopathic cardiomyopathy and aortic stenosis was analyzed. Interestingly, their results show that subsets of miRNAs are differentially regulated in each of etiologies [10]. Similar results were also found by Sucharov et al. [11]. These data show that differences in expression patterns of miRNAs may be clinically important if used for diagnosis and/or prognosis purposes.
On the other hand, not only the miRNAs subsets have influence on the phenotype, but also some specific miRNAs appear to be key regulators. In 2006, van Rooij et al. showed that increased expression of miR-195 in the mice myocardium was sufficient to induce a pathological cardiac growth and heart failure within several weeks after birth. Moreover, while no phenotype was obtained by increasing expression of miR-214, miR-24 resulted in embryonic lethality. This study indicates that some specific miRNAs can play crucial roles in the cardiac hypertrophy program [14].
Cardiac contractility depends on the expression of the two MHC isoforms α- and β-MHC and changes in their proportions may lead to hypertrophy, fibrosis and serious effects on the contractile function of the heart. Indeed, increased expression of β-MHC in the myocardium, a common feature of cardiac hypertrophy and heart failure, may decreases power output and can contributes to depressed systolic function in end-stage heart failure [16]. Recently, the increase of β-MHC was associated to an overexpression of miR-208a (encoded by a intronic region of the same β-MHC encoding gene) in the heart leading to arrhythmia, fibrosis and hypertrophic growth in mice and poor clinical outcomes in human dilated cardiomyopathy [17, 18].
The miR-21 is one of the few miRNAs that has shown a regular overexpression pattern in HF. The expression of miR-21 seems to be induced in endothelial cells by shear stress and regulate the function of vascular smooth muscle cells by modulating the nitric oxide synthase (eNOS) activity [7, 19]. MiR-21 is also highly expressed in many cancers and cell lines, suggesting that this miRNA have a common behavior in response to stress and pathological cells growth.
MicroRNAs in the diagnosis and prognosis of heart failure
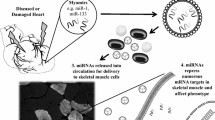
Due to the fact of many miRNAs are tissue-specific, most clinical studies have based on the measurement of miRNAs expression levels in samples of origin tissue. However, numerous studies have demonstrated that some miRNAs are not only found intracellularly, but also detectable outside cells, including various body fluids such as blood, saliva, urine and etc. [20]. These miRNAs were named circulating miRNA or c-miRNAs. Interestingly, ~90 % of extracellular miRNAs are packed with proteins (i.e. Ago2, HDL, and other RNA-binding proteins) and ~10 % are wrapped in small membranous particles (i.e. exosomes, microvesicles, and apoptotic bodies) [20]. Recent evidences reveal that some proportion of c-miRNAs is secreted from normal healthy or damaged cells as microvesicles. The fact that these c-miRNAs can be detected in peripheral blood make them potentially useful to aid diagnosis or to guide therapy through rapid and easy tests eliminating the necessity of performing an invasive procedure such as biopsies [6].
The first study in mice has shown that the plasma level of the cardio-specific miR-208a is increased after myocardial injury induction [21]. In humans, miR-208a [22] and miR-499 [23] were proposed as good biomarkers of acute myocardial infarction with plasma levels significantly higher when compared to control group. Plasma level of miR-208a could detect with 90.9 % sensitivity at 100 % specificity, although the level of miR-499 was less sensitive (36.4 %) for acute myocardial infarction diagnosis [22]. In addition, miR-208a presented a similar diagnostic accuracy to troponin with the advantage of detected early in plasma [22].
In a study conducted by Widera et al. [24], the plasma levels of miR-1, miR-133a/b, miR-208a/b, and miR-499 were measured in a large acute coronary syndrome (ACS) cohort. As result, miR-1, miR-133a/b and miR-208b were independently associated with high-sensitivity troponin T (hsTnT) levels (all P < 0.001). Patients with myocardial infarction presented higher levels of miR-1, miR-133a and miR-208b compared with patients with unstable angina. However, all six investigated miRNAs showed a large overlap between patients with unstable angina or myocardial infarction. miR-133a and miR-208b levels were significantly associated with the risk of death in univariate and age- and gender-adjusted analyses. Both miRNAs lost their independent association with outcome upon further adjustment for hsTnT. However, contrasting results are observed comparing the results of three studies. In the Widera et al. study [24], the plasma levels of miR-208a were not elevated in ACS patients and Fichtlscherer et al. [25] have shown that plasma levels of miR-208a tend to be high in patients with coronary artery disease (CAD). However, De Rosa et al. [26] have shown that the plasma levels of miR-208a, as well as miR-499 and miR-133a, were significantly elevated in ACS patients compared with CAD patients.
In 2009, Matkovich et al. evaluated the miRNAs expression profile in HF patients before and after treatment with left ventricular assist devices. Interestingly, 71.4 % of miRNAs differentially regulated in HF were normalized after treatment [12]. These results suggest that miRNAs may be useful as myocardial recovery markers in patients with advanced HF. However, the need for an invasive procedure to obtain myocardium samples makes the clinical application very limited. Recent evidence has shown that the c-miRNA miR-423-5p displays increased levels during the HF and can be used as a biomarker. Tijsen et al. [27] evaluated the plasma levels of several miRNAs in patients with acute HF. It was observed that the increase of miR-423-5p was a strong diagnosis predictor of HF. The mechanisms involved in increasing circulating levels of miR-423-5p in patients with HF are unknown.
The use of some miRNAs in the fight against heart failure is summarized in Table 2.
MicroRNAs in the treatment of heart failure
Currently, two therapeutic strategies involving the knowledge of miRNAs have been studied: the use of antagomirs and miR mimics. These strategies are based on the normalization of the tissue-specific miRNAs levels in pathological processes inhibiting miRNAs upregulated or replacing miRNAs downregulated.
In a pathological process where certain miRNAs are overexpressed the first thing we ponder is how to intervene in the effect caused by the excessive increase in the expression of these miRNAs. For this purpose it was developed a class of anti-miRNAs called antagomirs.
The antagomirs are small single-strands antagonistic nucleotide sequences artificially synthesized to be perfectly complementary to a specific mature miRNA. When injected systemically or locally, antagomirs interact with miRNAs in the cytoplasm and hybridize specifically with the mature miRNA target hindering the binding of miRNA with their corresponding mRNA. Thus, antagomirs act as competitive inhibitors of miRNA and lead to a decrease in the effect caused by the excessive increase in the expression of certain miRNAs [28].
Far from utopian, this therapeutic strategy has already been studied by many researchers. In a pioneering study, Thum et al. used as model cardiac hypertrophy induced by pressure overload in mice. After 3 weeks, it was administered an antagomir functionally designed to inhibit miR-21 (miRNA upregulated in fibroblasts during cardiac hypertrophy). As a result, it was observed that the mice showed a significant regression of cardiac hypertrophy and fibrosis and attenuation of impairment of cardiac function [29]. Another successful approach was published in 2011 by Montgomery et al. where the antimiR-208a was systemically administered during the HF-induced hypertension in mice leading to a potent miR-208a silencing in the heart. The therapeutic inhibition of miR-208a avoided the pathological myosin changes and cardiac remodeling, improving cardiac function and increasing their survival [30].
These results demonstrate the use of antagomirs can be useful in preventing and/or reversing cardiac hypertrophy. However, most studies to date focused on “silence” only isolated miRNAs. Then given that more than one miRNA may be involved in the disease process probably several miRNAs must be silenced to obtain an effective therapy.
As the increased expression of some miRNAs may be related to the triggering of pathogenic processes, decreased expression of specific miRNAs can also lead to a pathological development. In these cases, the intervention to normalize their expression levels is based on the administration of molecules that will functionally mimic natural miRNAs.
The miR-mimics are artificial small nucleotide sequences, double-strand, similar to miRNA precursors (pre-miRNA). When introduced into the cells the miR-mimics are recognized by the miRNA biogenesis machinery and processed by the enzyme Dicer and subsequently incorporated into the RISC enzyme complex. Thus, the miR-mimics will function as a replacement of some miRNAs downregulated by setting the mRNA-target as endogenous miRNAs.
On the other hand, the miRNAs replacement is subject to an additional obstacle: the specificity. The miR-mimics should act only on the tissue-target. Otherwise, if they are administered systemically, it could result in one or more miRNAs carrying regulatory function in tissues which they are not normally expressed. This erroneous adjustment probably would lead to the onset of side effects.
To overcome this obstacle, management systems more complex and accurate are needed. To this end, the use of viral vectors has been promising. These vectors are produced by bioengineering from non-pathogenic viruses belonging to the family Parvoviridae that have a high affinity for the myocardium [31].
Likewise antagomirs, the therapeutic efficacy of miR-mimics has also been studied. In a study by Suckau et al., a viral vector optimized with miR-mimics has been used successfully in mice with pressure overload. As a result, the authors observed a normalization of cardiac dilation and a significant reduction of cardiac hypertrophy and cardiac fibrosis [32].
MicroRNAs and physical exercise
In a relatively recent past, bed rest and restricted physical activity was recommended to patients with HF. Currently, exercise training has been formally recommended by major guidelines as an important and safe treatment for patients with HF [33, 34]. There is evidence that physiological adaptability to exercise has a parallel with gene expression [35]. However, the complexity of genetic interaction limits the identification of individual genes that may explain this variability. Moreover, its expression changes according to the stimulus that the organism receives and, in this setting, the study of miRNAs has a promising role [36].
Until now, there are no studies linking miRNAs, physical exercise and HF. Future miRNAs studies may generate hypotheses about the mechanisms by which exercise affects the pathophysiology of HF. The signaling pathways leading to hypertrophy resulting from physiological stimuli, such as exercise, are different from those that cause pathological hypertrophy and therefore can trigger different miRNAs expressions [37]. The ischemic preconditioning and its relationship with the miRNA have already been evaluated in experimental studies [38].
Skeletal muscle is an organ that has a high plasticity, capable of altering the phenotype in response to mechanical overload. Experimental studies have identified changes in the profile of specific miRNAs involved in aerobic exercise and strength training in skeletal muscle (Table 3).
In healthy people miR-1, miR-133a, miR-133b and miR-206 have significantly reduced their expression in skeletal muscle after 12 weeks of aerobic exercise [39]. Interestingly, the expression levels of these miRNAs return to baseline 14 days after cessation of physical training. These results indicate that these miRNAs adjust quickly to the level of physical activity.
The muscle mass gain of individuals subjected to resistance exercise training is also highly variable. This variability is accompanied by differences in the miRNAs behavior. In Davidsen et al. study [40], 56 men were submitted to resistance training for 12 weeks. By analyzing the vastus lateralis muscle biopsies, it was observed significantly reduction on miR-378 expression level and increasing of miR-478 only in those individuals who less responded to the training. In addition, there was a strong correlation between the variation in the miR-378 expression and the gain in lean body mass. There were no changes in miR-1 and miR-133 expression levels.
In a recent study, Baggish et al. [41] evaluated the behavior of circulating miRNAs involved in angiogenesis (miR-20a, miR-210, miR-221, miR-222, miR-328), inflammation (miR-21, miR-146a), cardiac and skeletal muscle contractility (miR-21, miR-133a) and muscular adaptation to hypoxia and ischemia (miR-21, miR-146a, and miR-210) in response to exercise. As a result, various expression patterns were observed (summarized in Table 3) indicating a potential value of c-miRNAs as exercise biomarkers and physiological mediators of the cardiovascular adaptation induced by exercise.
The miRNAs study may also contribute to the understanding of the exercise effects on immune response. By studying the behavior of neutrophils in peripheral blood after an exercise session, it was possible to observe changes in the miRNAs expression profile. The results suggest that miRNAs contribute to changes in neutrophils gene expression in response to physical activity [42].
By the time of publication of this review, we have no knowledge of studies that have evaluated the behavior of miRNAs in response to physical training specifically in patients with HF.
Considerations
The understanding of the miRNAs biology and their role in pathogenic processes is an exciting new frontier in cardiovascular medicine. The differential regulation of miRNAs in response to physical exercise has an important role in elucidating the mechanisms that govern the adaptation to exercise and may in the near future be used as therapeutic intervention. It is increasingly evident the potential of miRNAs as new tools in the diagnosis and prognosis as well as promising therapeutic strategies in many sub-areas of cardiology such as HF. In fact, the involvement in various pathological processes that lead to HF makes it clear that the miRNAs are new players in HF.
References
Beuvink I, Kolb FA, Budach W, Garnier A, Lange J, Natt F, Dengler U, Hall J, Filipowicz W, Weiler J (2007) A novel microarray approach reveals new tissue-specific signatures of known and predicted mammalian microRNAs. Nucleic Acids Res 35(7):e52
McCarthy JJ, Esser KA, Dupont-Versteegden EE, Peterson CA (2009) Evidence of MyomiR network regulation of beta-myosin heavy chain gene expression during skeletal muscle atrophy. Physiol Genomics 39(3):219–226
Townley-Tilson WH, Callis TE, Wang D (2010) MicroRNAs 1, 133, and 206: critical factors of skeletal and cardiac muscle development, function, and disease. Int J Biochem Cell Biol 42(8):1252–1255
Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M (2007) MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res 100(3):416–424
Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, LE Dorn, Condorelli G, Diwan A, Nerbonne JM, Dorn GW 2nd (2010) MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res 106(1):166–175
Carvalho VO, Carvalho VO, Silva MMF, Guimarães GV, Bocchi EA (2012) MicroRNAs: a new paradigm in the treatment and diagnosis of heart failure? Arq Bras Cardiol 98(4):362–370
Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J (2007) MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 116(3):258–267
Elia L, Contu R, Quintavalle M, Varrone F, Chimenti C, Russo MA, Cimino V, De Marinis L, Frustaci A, Catalucci D, Condorelli G (2009) Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation 120(23):2377–2385
Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G (2007) MicroRNA-133 controls cardiac hypertrophy. Nat Med 13(5):613–618
Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, Golub TR, Pieske B, Pu WT (2007) Altered microRNA expression in human heart disease. Physiol Genomics 31(3):367–373
Sucharov C, Bristow MR, Port JD (2008) miRNA expression in the failing human heart: functional correlates. J Mol Cell Cardiol 45(2):185–192
Matkovich SJ, Van Booven DJ, Youker KA, Torre-Amione G, Diwan A, Eschenbacher WH, Dorn LE, Watson MA, Margulies KB, Dorn GW 2nd (2009) Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation 119(9):1263–1271
Han M, Toli J, Abdellatif M (2011) MicroRNAs in the cardiovascular system. Curr Opin Cardiol 26(3):181–189
van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN (2006) A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 103(48):18255–18260
Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN (2008) microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev 22(23):3242–3254
Stelzer JE, Brickson SL, Locher MR, Moss RL (2007) Role of myosin heavy chain composition in the stretch activation response of rat myocardium. J Physiol 579:161–173
Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP et al (2009) MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest 119:2772–2786
van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA et al (2009) A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 17(5):662–673
Weber M, Baker MB, Moore JP, Searles CD (2010) MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun 393(4):643–648
Zhu H, Fan GC (2011) Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am J Cardiovasc Dis 1(2):138–149
Ji X, Takahashi R, Hiura Y, Hirokawa G, Fukushima Y, Iwai N (2009) Plasma miR-208 as a biomarker of myocardial injury. Clin Chem 55(11):1944–1949
Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q (2010) Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J 31(6):659–666
Adachi T, Nakanishi M, Otsuka Y, Nishimura K, Hirokawa G, Goto Y, Nonogi H, Iwai N (2010) Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem 56(7):1183–1185
Widera C, Gupta SK, Lorenzen JM, Bang C, Bauersachs J, Bethmann K, Kempf T, Wollert KC, Thum T (2011) Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol 51(5):872–875
Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S (2010) Circulating microRNAs in patients with coronary artery disease. Circ Res 107(5):677–684
De Rosa S, Fichtlscherer S, Lehmann R, Assmus B, Dimmeler S, Zeiher AM (2011) Transcoronary concentration gradients of circulating microRNAs. Circulation 124(18):1936–1944
Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, Pinto YM (2010) MiR423-5p as a circulating biomarker for heart failure. Circ Res 106(6):1035–1039
Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M (2005) Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438(7068):685–689
Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S (2008) MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456(7224):980–984
Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E (2011) Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation 124(14):1537–1547
Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, Russell DW, Chamberlain JS (2004) Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 10(8):828–834
Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskämper J, Westermann D, Bisping E, Ly H, Wang X, Kawase Y, Chen J, Liang L, Sipo I, Vetter R, Weger S, Kurreck J, Erdmann V, Tschope C, Pieske B, Lebeche D, Schultheiss HP, Hajjar RJ, Poller WC (2009) Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation 119(9):1241–1252
Piña IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ (2003) Exercise and heart failure. A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 107:1210–1225
Downing J, Balady GJ (2011) The role of exercise training in heart failure. J Am Coll Cardiol 58(6):561–569
Timmons JA, Jansson E, Fischer H, Gustafsson T, Greenhaff PL, Ridden J, Rachman J, Sundberg CJ (2005) Modulation of extracellular matrix genes reflects the magnitude of physiological adaptation to aerobic exercise training in humans. BMC Biol 3:19
Silva MMF, Carvalho VO, Guimarães GV, Bocchi EA, Bacal F (2012) Physical exercise and microRNAs: new frontiers in heart failure. Arq Bras Cardiol 98(5):459–466
Hill JA, Olson EN (2008) Cardiac plasticity. N Engl J Med 358(13):1370–1380
Kim HW, Haider HK, Jiang S, Ashraf M (2009) Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem 284(48):33161–33168
Nielsen S, Scheele C, Yfanti C, Akerstrom T, Nielsen AR, Pedersen BK, Laye M (2010) Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J Physiol 588(20):4029–4037
Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM (2011) High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol 110(2):309–317
Baggish AL, Hale A, Weiner RB, Lewis GD, Systrom D, Wang F, Wang TJ, Chan SY (2011) Dynamic regulation of circulating MicroRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol 589(16):3983–3994
Radom-Aizik S, Zaldivar F Jr, Oliver S, Galassetti P, Cooper DM (2010) Evidence for microRNA involvement in exercise-associated neutrophil gene expression changes. J Appl Physiol 109(1):252–261
Fang Y, Shi C, Manduchi E, Civelek M, Davies PF (2010) MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci USA 107(30):13450–13455
Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D (2009) miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460(7256):705–710
Zhao T, Li J, Chen AF (2010) MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am J Physiol Endocrinol Metab 299(1):110–116
Smits M, Mir SE, Nilsson RJ, van der Stoop PM, Niers JM, Marquez VE, Cloos J, Breakefield XO, Krichevsky AM, Noske DP, Tannous BA, Würdinger T (2011) Down-regulation of miR-101 in endothelial cells promotes blood vessel formation through reduced repression of EZH2. PLoS ONE 6(1):e16282
Li D, Yang P, Xiong Q, Song X, Yang X, Liu L, Yuan W, Rui YC (2010) MicroRNA-125a/b-5p inhibits endothelin-1 expression in vascular endothelial cells. J Hypertens 28(8):1646–1654
Kim GH, Samant SA, Earley JU, Svensson EC (2009) Translational control of FOG-2 expression in cardiomyocytes by MicroRNA-130a. PLoS ONE 4(7):e6161
Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami AP, Angelini G, Emanueli C, Madeddu P (2011) Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving Micro-RNA-132. Circ Res 109(8):894–906
Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, Guo Z, Liu J, Wang Y, Yuan W, Qin Y (2011) Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis 215(2):286–293
Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, Nicolaou P, Pritchard TJ, Fan GC (2009) MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation 119(17):2357–2366
Zhang X, Wang X, Zhu H, Zhu C, Wang Y, Pu WT, Jegga AG, Fan GC (2010) Synergistic effects of the GATA-4-mediated miR-144/451 cluster in protection against simulated ischemia/reperfusion-induced cardiomyocyte death. J Mol Cell Cardiol 49(5):841–850
Ringholm S, Bienso RS, Kiilerich K, Guadalupe-Grau A, Aachmann-Andersen NJ, Saltin B, Plomgaard P, Lundby C, Wojtaszewski JF, Calbet JA, Pilegaard H (2011) Bed rest reduces metabolic protein content and abolishes exercise-induced mRNA responses in human skeletal muscle. Am J Physiol Endocrinol Metab 301(4):E649–E658
Drummond MJ, McCarthy JJ, Fry CS, Esser KA, Rasmussen BB (2008) Aging differentially affects human skeletal muscle microRNA expression at rest and after an anabolic stimulus of resistance exercise and essential amino acids. Am J Physiol Endocrinol Metab 295(6):E1333–E1340
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliveira-Carvalho, V., Silva, M.M.F.d., Guimarães, G.V. et al. MicroRNAs: new players in heart failure. Mol Biol Rep 40, 2663–2670 (2013). https://doi.org/10.1007/s11033-012-2352-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2352-y