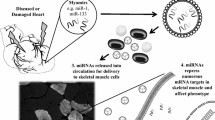
Abstract
MicroRNAs (miRNAs, miRs), a group of small non-coding RNAs, repress gene expressions at posttranscriptional level in most cases and are involved in cardiovascular physiology and disease pathogenesis. Increasing evidence has proved that miRNAs are potential regulators of exercise induced cardiac growth and mediate the benefits of exercise in a variety of cardiovascular diseases. In this chapter, we will review the regulatory effects of miRNAs in cardiac adaptations to exercise, and summarize their cardioprotective effects against myocardial infarction, ischemia/reperfusion injury, heart failure, diabetic cardiomyopathy, atherosclerosis, hypertension, and pulmonary hypertension. Also, we will introduce circulating miRNAs in response to acute and chronic exercise. Therefore, miRNAs may serve as novel therapeutic targets and potential biomarkers for cardiovascular diseases.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cardiovascular diseases (CVDs) are major causes of morbidity and mortality worldwide [1]. Currently, despite continuous advances achieved in clinical treatments including medical and surgical therapies, CVDs are still considered to be major diseases and exert a considerable emotional and economic burden [2]. In light of these, development of innovative therapeutic strategies for CVDs are urgently needed.
Physical exercise, as well as pregnancy and postnatal cardiac growth, are major stimuli for physiological cardiac hypertrophy [3]. For many years, cardiologists advocated prolonged rest for patients with CVDs especially ischemic heart diseases [4]. However, increasing number of studies have validated the multiple benefits of physical exercise in comparison to the detrimental effects of a sedentary lifestyle, making physical exercise a therapeutic modality for patients with a variety of chronic diseases, such as CVDs, type II diabetes, fatty liver, stroke, disseminated sclerosis, and malignant tumor [5,6,7,8,9,10,11]. Among them, further studies have demonstrated that individuals with proper level of physical exercise have lower prevalence and death rate for CVDs [12]. Thus, physical exercise has been established not only as a mean to maintain a healthy lifestyle but also as a safe and important nonpharmacological way for prevention and treatment of CVDs.
Non-coding RNAs (ncRNAs) are a diverse group of functional RNA molecules without protein-coding functions, which may range from short microRNAs (~22 nucleotides) to long non-coding RNAs (>200 nucleotides) [13, 14]. MicroRNAs (miRNAs, miRs) repress gene expressions at posttranscriptional level in most cases and are involved in various cellular processes, including differentiation, proliferation, apoptosis, migration, angiogenesis, and so on [15]. Importantly, mounting data have suggested that ncRNAs, especially miRNAs, could lead to a profound regulation of target genes and related signaling pathways, thus engaging in a variety of beneficial effects of exercise in the heart [16, 17]. This may raise a hope that miRNAs may serve as potential therapeutic targets mediating the benefits of physical exercise to combat CVDs.
In this chapter, we will provide an overview of the protective effects of physical exercise on diverse CVDs and the involvement of miRNAs in this process.
2 Cardiac Adaptations to Physical Exercise
2.1 Cardiac Growth: Cardiac Hypertrophy and Cardiomyocyte Renewal
Cardiac hypertrophy is an adaptation to increased cardiac workload including a variety of mechanical, hemodynamic, and hormonal factors [18]. There are two different forms of ventricular hypertrophy, namely physiological hypertrophy and pathological hypertrophy [19]. Both hypertrophic processes involve increased cardiomyocyte size, enhanced protein synthesis, and recombination of sarcomere structure. However, cardiac physiological hypertrophy differs from pathological hypertrophy in its stimuli and its structural and functional adaptations [4, 20,21,22]. As for stimuli, physiological hypertrophy occurs in healthy individuals following exercise training, pregnancy, or postnatal growth [23, 24], while pathological hypertrophy is associated with hypertension, or loss of myocytes due to ischemic or hypoxic myocardium damages [21]. As for structural and functional adaptations, physiological hypertrophy caused by endurance exercise training mainly exhibits ventricular hypertrophy with addition of sarcomeres and increase of cell length and cardiac mass, leading to preserved even enhanced left ventricular function, reduced collagen content, and improved myocardial antioxidant capacity and mitochondrial function [22, 25]. However, besides addition of sarcomeres, pathological hypertrophy is also characterized by increased cell thickness, enhanced apoptosis, and impaired cardiomyocyte metabolism switching from fatty acid to glucose metabolism, which could ultimately lead to increased cardiac fibrosis and stiffness and progressive reduction in cardiac output [26,27,28].
Over the past decades, the adult mammalian heart has been considered as a post-mitotic organ without any regenerative capacity [29]. However, more recent evidence has contradicted the long established belief, indicating that the adult mammalian heart sustains certain endogenous growth and regenerative capacity under some physiological or pathological conditions [30]. Actually, nearly half of cardiomyocytes are replaced during a whole human lifespan [30]. In a normal mouse heart, the turnover rate of cardiomyocytes is nearly 1.3–4.0% per year, while after myocardial injury, the rate of cardiomyocyte renewal is significantly increased, especially in the infarct border zone [31]. Importantly, physical exercise is shown as a novel strategy to endogenously enhance the limited capacity of cardiomyocytes for proliferation [32]. The potential sources of newly formed cardiomyocytes could be originated from division of pre-existing cardiomyocytes or differentiation of cardiac stem/progenitor cells [33, 34].
2.2 Angiogenesis
In cardiac physiological hypertrophy, the coordinated growth of myocardium and vasculature is an important adaptation of heart to deliver enough oxygen to the myocardium [35]. It was reported that endogenous cardiac stem cells (eCSCs) can be activated upon exercise [34]. Interestingly, these c-kit positive eCSCs were also committed to Nkx2.5 positive or Ets-1 positive cell lineages, indicating their potential to differentiate into both cardiomyocytes and vascular cells [34]. In addition, endothelial progenitor cells (EPCs), a type of circulating monocytes derived from bone marrow, can also be activated in response to exercise [36]. Acute exercise leads to a rapid increase in circulating EPCs that can maintain for up to 2–3 days after exercise termination. Furthermore, systematic and chronic exercise is able to trigger the mobilization of EPCs into the circulation from the bone marrow in both healthy or diseased individuals [37]. Given the capacity of EPCs to proliferate, migrate, and differentiate into mature endothelial cells which contributes to neo-vascularization, exercise via promoting angiogenesis, may act as an important physical strategy or compensatory mechanism for cardiac regeneration and repair.
Taken together, the physiological adaptation of adult heart to exercise has three main components: (1) physiological hypertrophy of cardiomyocytes; (2) renewal of cardiomyocytes originated from pre-existing cardiomyocytes or cardiac stem/progenitor cells; (3) the accumulation of new microvasculature (Fig. 15.1). These cardiac physiological adaptations to physical exercise can lead to increased cardiac mass and even enhanced cardiac function.
3 miRNAs Responsible for Cardiac Adaptations to Exercise
Currently, miRNAs are emerging as pivotal modulators of cardiovascular development and disease [38, 39]. miRNAs have also been reported to participate in the beneficial adaptations promoted by exercise including physiological cardiac hypertrophy (Table 15.1).
3.1 Cardiac Growth
miR-1 and miR-133 were firstly reported to be decreased in both physiological hypertrophy induced by treadmill exercise and pathological hypertrophy induced by pressure overload [40]. After that, the other study also demonstrated that miR-1 and miR-133a/b were down-regulated in physiological cardiac hypertrophy induced by two different swimming protocols, indicating that these miRNAs could be regulated by exercise regardless of exercise mode or volume (moderate and high) [41].
Unlike in pathological hypertrophy [42], the expressions of miR-208a and miR-208b were reduced in exercise group compared with sedentary group, parallel to an increase of target gene transcriptional activator protein Pur-beta (Purβ) [17, 43]. Interestingly, overexpression of Purβ inhibited β-MHC expression accompanied by increased α-MHC expression and improved ventricular compliance, suggesting that down-regulation of miR-208 may mediate the beneficial effect of exercise against CVDs [43].
It is well known that angiotensin (Ang) II is an inducer for cardiac pathological hypertrophy, while exercise-induced physiological hypertrophy is associated with increased Ang-converting enzyme 2 (ACE2) activity, which might protect against pathological hypertrophy via reducing Ang II [44]. miR-27a and miR-27b have been reported to be increased in exercise-induced physiological hypertrophy in rats and they could target ACE, while decrease of miR-143 could lead to increased ACE2 activity and reduced Ang II level [44, 45].
The phosphoinositide-3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling is critically involved in the regulation of cellular proliferation and survival, and plays a positive role in exercise-induced cardiac physiological hypertrophy [46]. Exercise could elevate cardiac miR-21 and miR-144 expressions, which were both predicted by bioinformatic analysis to target phosphatase and tensin homolog (PTEN), a negative regulator of the PI3K/Akt/mTOR pathway. Besides that, miR-145 was also found to be increased after exercise training, accompanied by a decrease in its target gene tuberous sclerosis complex 2 (TSC2, another negative regulator of the PI3K/Akt/mTOR pathway). Moreover, exercise training decreased cardiac miR-124 expression with an increase in its target gene PI3K (p110α) [46].
Cardiomyocyte hypertrophy, as well as proliferation, are two important cellular changes during physiological cardiac growth. Recently, miR-222 and miR-17-3p have been reported to be increased in exercised heart and are necessary for exercise-induced cardiac growth [47, 48]. miR-222 can directly target P27 and HIPK1 in the regulation of cardiomyocyte proliferation, while target HMBOX1 in the regulation of cardiomyocyte hypertrophy [47]. Moreover, miR-17-3p enhances cardiomyocyte proliferation via targeting TIMP3, and induces cardiomyocyte hypertrophy by inhibition of PTEN and subsequent activation Akt [48]. However, overexpression of miR-222 or miR-17-3p alone was not sufficient to recapitulate the phenotypes of physiological growth as seen in exercised heart, suggesting that these miRNAs might work together to promote exercise-induced physiological cardiac growth in vivo [47, 48].
3.2 Anti-fibrosis
miR-29c was significantly increased in cardiac physiological hypertrophy induced by swimming exercise, and its target genes including collagen IAI and collagen IIIAI were both decreased [41]. Exercise-associated increase in miR-29c was correlated with reduced collagen concentration in the heart and improved left ventricular compliance, implying an anti-fibrosis effect of miR-29c, which might also exert protective effects against pathological cardiac remodeling [49].
3.3 Angiogenesis
Vascular endothelial growth factor (VEGF) has been reviewed as an important mediator of angiogenic responses upon different stimuli, including exercise [50]. Exercise training could promote vessel growth by increasing the expression level of miR-126 and repressing its target genes including sprouty-related protein 1 (Spread-1) and phosphoinositol-3 kinase regulatory subunit 2 (PI3KR2), which are two negative regulators of VEGF by inhibiting the PI3K/Akt/endothelial nitric oxide synthase (eNOS) pathway [38, 39].
Taken together, these data indicate that exercise can promote physiological cardiac hypertrophy through regulation of miRNAs and their specific target genes (Table 15.1). These miRNA-mRNA interactions may contribute to cardiac growth, anti-fibrosis, and angiogenesis processes in the heart upon exercise, and also probably mediate the protective effect of exercise against CVDs.
4 miRNAs Mediate Protective Effects of Exercise in CVDs
Exercise-induced cardiac protection has been appreciated for many decades, and several canonical molecular mechanisms have been proposed to contribute to the benefits of exercise. As novel mechanism, incorporating miRNAs within cardiac gene regulatory networks may provide a new opportunity for developing therapeutic interventions for CVDs.
4.1 Exercise Protects Against Myocardial Infarction
Myocardial infarction (MI) occurs when blood flow stops to a part of the myocardium causing damage to the heart muscle [51]. MI is accompanied by cardiomyocyte apoptosis, and necrosis, and hypertrophy, increased collagen deposition, and new vascularization, which results in pathological cardiac remodeling and reduced ventricular compliance [52]. As we described previously, exercise training could induce miR-29a and miR-29c in the heart, leading to reduced collagen concentration and improved ventricular compliance in healthy rats [41]. Interestingly, exercise training could also restore cardiac miR-29a and miR-29c expression levels and reduce collagen type I and III expression levels in the border and remote areas of MI [53]. This suggests an anti-fibrosis effect of exercise in rats with MI through upregulating miR-29a and miR-29c, which might serve as potential therapeutic strategy to reduce infarct size and improve cardiac function in MI patients [53].
On the other hand, impairment of cardiomyocyte contractility and calcium handling are hallmarks of left ventricular contractile dysfunction in MI [54]. Aerobic intensity-controlled interval training attenuated myocardial hypertrophy and increased myocyte contractile function in post-MI rats, accompanied with upregulated sarcoplasmic reticulum Ca2 + -ATPase 2a (Serca-2a) and sacolemmal sodium/calcium exchanger (NCX) protein levels, and enhanced intracellular Ca2+ handling and Ca2+ sensitivity in cardiomyocytes from rats with MI [55]. MI could also decrease miR-1 and increase miR-214, while exercise after MI partially restored miR-1 and miR-214 by targeting NCX and Serca-2a, respectively [56]. These molecular adaptations were associated with improved left ventricular compliance in MI hearts, and thus exercise was supposed to have a positive impact on Ca2+ handling, via regulating miR-1 and miR-214, in hearts post-MI [56].
Additionally, miR-26a was shown to be increased in mouse acute MI and human acute coronary syndromes [57]. Overexpression of miR-26a, via inhibiting its target gene SMAD1, could lead to impaired tube formation of endothelial cells in vitro and reduced angiogenesis upon exercise in vivo [57]. Interestingly, inhibition of miR-26a was associated with robust angiogenesis, reduced infarct size, and improved cardiac function even within 2 days after MI [57]. This suggests miR-26a as an important miRNA regulating angiogenetic response in ischemic cardiac diseases.
4.2 Exercise Protects Against Cardiac Ischemia/Reperfusion Injury
Cardiac ischemia/reperfusion (I/R) injury refers to heart damage caused when blood supply returns to the heart after a period of ischemia or lack of oxygen, which then induces a series of pathological changes including oxidative stress, inflammatory responses, Ca2+ overload, mitochondrial dysfunction, and myocardial apoptosis [58]. Burgeoning evidence indicates that physical exercise can protect against I/R injury in both clinical patients and experimental animal models by upregulating anti-oxidative capacity, promoting angiogenesis, and decreasing cardiomyocyte apoptosis [59,60,61]. Recently, miRNAs have been reported to underline these mechanisms mediating the protective effects of exercise against I/R injury.
miR-222 is a highly conserved member of a miRNA cluster with miR-221, which is encoded on the X chromosome [62, 63]. miR-222 has been found to be increased in the plasma of athletes after both acute and chronic exercise, suggesting a potential relevance of miR-222 with exercise [64]. Interestingly, circulating miR-222 could also be elevated after acute cardiopulmonary exercise in heart failure patients, indicating a potential role of miR-222 in mediating the beneficial effect of exercise in heart failure patients [47]. More importantly, miR-222 was proved to be necessary for exercise-induced physiological growth by promoting both hypertrophy and proliferation of cardiomyocytes through targeting P27, HIPK1, and HMBOX1 [47]. Although overexpression of miR-222 was not sufficient to recapitulate the exercise phenotype at baseline, it did protect against adverse ventricular remodeling and cardiac dysfunction after I/R injury [47]. These effects were also associated with inhibition of cardiomyocyte apoptosis and a dramatic reduction of cardiac fibrosis [47].
As overexpression of miR-222 alone was not sufficient to recapitulate the phenotypes of physiological growth observed in exercised heart, we also speculated that other molecular mechanisms (including miRNAs) must be involved in this process. Recently, miR-17-3p, a passenger miRNA that belongs to the miR-17-92 cluster, was also proved to be necessary for exercise-induced cardiac growth and have protective effects against cardiac remodeling after I/R injury, which was at least in part due to enhanced proliferation and reduced apoptosis of cardiomyocytes [48].
4.3 Exercise Protects Against Heart Failure
Heart failure (HF) often refers to congestive heart failure and occurs when the heart is unable to pump enough blood to meet the body’s needs [65]. Accumulating studies indicate that exercise training has protective effect on the myocardium in patients with HF and in animal models of pathological cardiac hypertrophy and HF, which could be associated with increased exercise tolerance, improved cardiac structure and function, and reduced HF-related biomarkers during cardiac remodeling [66,67,68,69,70]. Currently, exercise training has been formally recommended by major guidelines as a safe and important strategy for patients with HF [71, 72]. However, the molecular mechanisms by which it exerts the therapeutic value for HF are far from understood.
Recently, Souza RW et al. conducted a miRNA expression profile in ascending aortic stenosis-induced HF rats randomized to either 10 weeks of exercise training or sedentary group [66]. Therapeutic effects of exercise in reducing cardiac remodeling and maintaining systolic and diastolic function were associated with differentially regulated miRNAs between exercise and sedentary group, including miR-208b-3p, miR-21-5p, miR-132-3p, and miR-212-3p [66]. Interestingly, some of these miRNAs were reported to regulate I/R injury or cardioprotection by ischemic pre- and post-conditioning [73,74,75,76,77]. Further gene-term enrichment analysis showed that these differentially regulated miRNAs between exercised or sedentary HF rats could target genes involved in programmed cell death, TGF-β signaling, cellular metabolic process, cytokine signaling, and cell morphogenesis. Among these five biological modules, programmed cell death module compromise the most enriched miRNA targets, indicating that exercise may attenuate cardiac abnormalities during HF by regulating miRNAs through apoptosis-related pathways [66].
4.4 Exercise Protects Against Diabetic Cardiomyopathy
Diabetes mellitus (DM) is a group of metabolic diseases in which there are high blood sugar levels over a long period [78]. Several recent epidemiological studies have confirmed that DM was an independent predictor for heart disease and would influence 400 million people worldwide by 2030 with prevalent cardiovascular deaths [79,80,81]. Exercise has been described as a polypill that prevents myocardial apoptosis and fibrosis, ameliorates mitochondrial biogenesis, and preserves cardiac function in diabetic cardiomyopathy in mice [82]. Furthermore, exercise can also mitigate cardiac dysfunction in diabetic patients though the molecular mechanisms still remain uncertain [83].
Exosomes are small membrane vesicles (30–100 nm) that contain various biological contents like DNA, RNA, protein, as well as miRNA, thus participating in cell-to-cell communications [84]. Extracellular vesicles derived from stem cells or even from plasma of healthy individuals have been documented to diminish cardiomyocyte apoptosis and improve cardiac function after ischemic cardiac injury, suggesting exosomes as critical agents for cardiac repair [85, 86]. Exercise training could trigger the release of exosomes that contain miRNAs (miR-455, miR-29b, miR-323-5p, and miR-466) from diabetic hearts compared to sedentary diabetes group. Interestingly, these miRNAs were proved to bind to the 3′ region of matrix metallopeptidase 9 (MMP9) and thus silence MMP9, a gene regulating extracellular matrix remodeling [87]. Thus, a close relationship has been suggested between exercise-derived exosomes, exosomal miRNAs, and the benefit of exercise for the heart, which could delineate novel strategy to cope up with diabetic cardiomyopathy.
4.5 Exercise Protects Against Atherosclerosis
Atherosclerosis (AS), a disease associated with chronic inflammation, is characterized by thickened artery wall linked to invasion and accumulation of foam cells, proliferation of intimal smooth muscle cells, and finally formation of atheromatous (fibrofatty) plaque in the arteries [88, 89]. Actually, physical exercise is also recommended as an effective way to diminish vascular injuries in patients with AS, probably by reducing triglyceride and apolipoprotein B, enhancing tissue plasminogen activator activity, and decreasing coronary artery calcium [90, 91]. Exercise was able to reduce foam cell accumulation and plaque formation, accompanied with an increase in vascular miR-146a and miR-126 expression levels, and a decrease in vascular miR-155 expression level in apolipoprotein E-null mice fed with high-fat diet [92]. Importantly, miR-146a was further demonstrated to directly target tumor necrosis factor receptor 6 (TRAF6), a gene involved in the Toll-like receptor 4 (TLR4) signaling pathway, suggesting that exercise-induced miR-146a may protect against AS by repressing vascular inflammatory injury [92].
4.6 Exercise Protects Against Hypertension
Hypertension is a long term medical condition in which the blood pressure in the arteries is persistently elevated [93]. Long term high blood pressure represents a major risk factor for CVDs [93]. Exercise training is established as a nonpharmacological tool for treatment of hypertension by improving endothelial function, attenuating microvascular rarefaction, and reducing blood pressure [94, 95]. Exercise is also effective in reducing other CVD risk factors in patients with hypertension as evidenced by improved plasma lipoprotein-lipid profiles and insulin sensitivity [96].
For further detecting the underlying mechanisms, some studies focused on the change of miRNAs in response to exercise in hypertension. Exercise training has been found to be able to significantly reduce blood pressure and heart rate in spontaneously hypertensive rats (SHR) compared to sedentary SHR group, by regulating several angiogenesis-related miRNAs [97]. Previous studies indicated that miR-16 via targeting VEGF and Bcl-2, miR-21 via targeting Bcl-2, and miR-126 via targeting sprouty-related protein 1 (Spread-1) and phosphoinositol-3 kinase regulatory subunit 2 (PI3KR2), lead to the dysregulation of angiogenesis and apoptosis processes [98,99,100,101]. Interestingly, exercise could restore the increased miR-16 and miR-21, and the decreased miR-126 expression levels in the soleus of hypertensive rats [97]. Exercise could also activate the VEGF and anti-apoptotic signaling pathways and improve endothelial nitric oxide synthase (eNOS) level as well [97]. These data provide evidence that exercise can balance angiogenic and apoptotic pathways by regulating miRNAs, and thus prevent microvascular abnormalities in hypertension [97].
4.7 Exercise Protects Against Pulmonary Hypertension
Pulmonary hypertension (PH) refers to an increase of blood pressure in the pulmonary arterial system. Pulmonary arterial hypertension (PAH) is the most common form characterized by sustained vasoconstriction, vascular remodeling of small pulmonary arteries, in situ thrombosis, and chronic inflammation, that leads to increased mean pulmonary arterial pressure and ultimately right heart failure and death [102]. Despite significant progress in treatment, the three-year survival of patients with PAH is a little bit higher than 50% and the quality of life remains severely affected [103]. More recently, a body of clinical evidence has shown the safety and efficacy of exercise training in PAH [104, 105]. Exercise training was demonstrated to be effective to enhance exercise tolerance, improve quality of life, and possibly increase survival rate in patients with PAH associated with connective tissue diseases [106]. Exercise training could also significantly lower right ventricular end diastolic pressure, reduce pulmonary artery thickness, and decrease right ventricular interstitial volume in monocrotaline-induced PAH [107]. Despite the certain beneficial effect of exercise on PAH, the mechanisms involved especially the role of miRNAs need to be further explored.
5 Circulating miRNAs in Response to Exercise
Circulating miRNAs (c-miRNAs) are the most investigated ncRNAs detected in the serum or plasma of humans and animals. c-miRNAs are usually protected from degradation as they can be packaged into membrane vesicles such as exosomes or microvesicles [108]. Additionally, c-miRNAs can be packaged into lipoproteins or Ago proteins as part of RNA-induced silencing complexes [109, 110]. As c-miRNAs can be released at rest or upon tissue injury or physiological stress such as exercise training, c-miRNAs may serve as unique biomarkers of disease states and exercise physiology [111,112,113].
The dose-response relationship between leisure-time physical activity and mortality was investigated by a pooled analysis, and it was indicated that moderate- or even vigorous-intensity physical exercise was associated with longevity benefit [114]. Noteworthy, no excess mortality risk was found even with ten times the recommended minimum level of leisure-time physical exercise [114]. Thus, leisure-time physical exercise should be highly recommended to inactive individuals [114]. Increasing number of studies reported the alteration of c-miRNAs implicated in muscle adaptations, angiogenesis, and inflammation during physical exercise. However, little is known about the effects of different type, intensity, and duration of exercise on c-miRNAs. In this section, we will summarize the potential changes of major c-miRNAs in response to different modes of exercise.
5.1 Circulating miRNAs in Acute Exercise
miR-1, miR-133, miR-206, miR-208b, and miR-499, also called muscle-enriched miRNAs (myomiRs), are highly abundant in cardiac and/or skeletal muscles while their expression levels in circulation are very low in healthy individuals [115]. An acute bout of endurance exercise (marathon) could induce the rapid increase of circulating miR-1, miR-133a, miR-206, miR-208b, miR-499, and miR-206, supporting the notion of distinct c-miRNA changes would in response to exercise [116]. Twenty-four hours after the marathon run, miR-208b and miR-499 returned to baseline levels, while other c-miRNAs still enhanced [116]. Moreover, miR-1, miR-133a, and miR-206 expression levels were positively correlated with the maximum oxygen uptake (VO2max), an indicator of exercise capacity, while no correlations were found between c-miRNAs and cardiac damage biomarkers such as troponin T and troponin I, indicating that the release of myomiRs into circulation might be used as unique biomarkers for exercise physiology rather than consequences of cell death [116]. However, another study reported that circulating miR-1 and miR-133a were increased immediately after marathon, but declined very close to baseline levels 24 hours after race completion [113]. Interestingly, circulating miR-133a has also been reported to be unchanged after an acute exhaustive exercise test, suggesting that the regulation of c-miRNAs might be closely related to exercise type, intensity, and duration [64].
miR-126 is enriched in vascular endothelium and miR-146a is an important regulator of inflammation [117, 118]. Circulating miR-146a and miR-126 were both increased immediately after a marathon run, and rapidly returned to baseline levels 24 hours later [113]. However, in another study, circulating miR-146 was down-regulated immediately after an acute exercise bout in young healthy men [119]. In addition, circulating miR-146a level has also been reported to be unchanged at 0 h, 1 h, and 24 h after an acute resistance exercise, while it began to decrease at 3 days post exercise in healthy young males [120]. These different regulatory patterns of c-miRNAs indicate that participants, as well as exercise type, intensity, and duration may affect changes of c-miRNAs in response to exercise.
Some other miRNAs have also been found to be modulated by exercise. miR-106a, miR-221, miR-30b, miR-151-5p, let-7i, miR-146a, miR-652, and miR-151-3p were robustly down-regulated immediately after an acute exercise bout. miR-338-3p, miR-330-3p, miR-223, miR-139-5p, and miR-143 were up-regulated at 1 hour after exercise and miR-1 was elevated at 3 h after exercise [119]. Additionally, a rapid decrease of muscle-enriched miR-486 in circulation after an acute exercise was found [121], indicating that exercise may reduce the release of myomiRs into circulation, or perhaps accelerate the uptake of specific c-miRNAs from circulation into certain recipient tissues and cells, though the mechanisms remain largely unknown.
5.2 Circulating miRNAs in Chronic Exercise
Little is known about the regulation and function of c-miRNAs in chronic exercise. After 12 weeks of chronic endurance training, miR-342-3p, miR-766, let-7d, miR-25, miR-148a, miR-185, and miR-21 were decreased while miR-103 and miR-107 were increased in plasma [119]. In addition, circulating miR-20a was increased after a 90 days period of rowing training, but not affected by acute exercise in healthy competitive athletes [64]. Noteworthy, the change in circulating miR-20a quantitatively correlated with the change in VO2max, indicating a potential role of miR-20a as a biomarker for chronic exercise fitness [64]. The altered circulating miRNAs in response to exercise were listed in Table 15.2.
6 Conclusions
In this chapter, we summarize the current knowledge about miRNAs responsible for cardiac adaptations to physical exercise and address their roles in mediating the protective effects of exercise against diverse CVDs, including MI, IRI, HF, diabetic cardiomyopathy, AS, hypertension, and PH. Also, we discuss changes of circulating miRNAs in response to acute and chronic exercise. These evidences highly suggest that miRNAs could serve as potential biomarkers for exercise physiology as well as novel therapeutic targets to combat CVDs.
Mounting evidence has confirmed the roles of miRNAs mediating the beneficial effects of exercise. However, limitations of these studies should be acknowledged. First, mechanistic regulations of miRNAs in response to exercise are still unclear. The dysregulation of miRNAs in the settings of acute as well as chronic exercise may rely upon either de novo miRNA transcription or post-transcriptional processing of premature miRNAs forms [122]. Circular RNAs may act as miRNA sponge by inhibiting miRNA activity, thus regulate cardiac adaptations to exercise [123]. Second, individuals may response differently to variable type, intensity, and duration of exercise. Thus, subsequent studies are needed to evaluate the regulation of miRNAs upon different modes of exercise across diverse populations. Third, the exact cellular sources as well as the secretion mechanisms of exercise-induced circulating miRNAs remain largely unknown. Despite that skeletal muscle function is closely related and contributes to circulating miRNAs upon exercise stimuli, other tissue or cell types such as myocardium, cardiac fibroblasts, and vascular endothelial cells should be explored as potential sources of circulating miRNAs [124]. Finally, the biological functions of exercise-induced miRNAs in physiological cardiac hypertrophy as well as their potential in CVD therapeutics deserve further explorations.
References
Pagidipati NJ, Gaziano TA (2013) Estimating deaths from cardiovascular disease: a review of global methodologies of mortality measurement. Circulation 127(6):749–756
Mozaffarian D, Benjamin EJ, Go AS et al (2016) Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133(4):e38–e360
Hill JA, Olson EN (2008) Cardiac plasticity. N Engl J Med 358(13):1370–1380
Ellison GM, Waring CD, Vicinanza C et al (2012) Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart 98(1):5–10
Tao L, Bei Y, Zhang H et al (2015) Exercise for the heart: signaling pathways. Oncotarget 6(25):20773–20784
Shima T, Matsui T, Jesmin S et al (2016) Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 diabetes. Diabetologia 60:597
Kwak MS, Kim D, Chung GE et al (2016) The preventive effect of sustained physical activity on incident nonalcoholic fatty liver disease. Liver Int 37:919
Kim HS, Ike A, Matthew J (2016) Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J Hepatol 66:664–665
Deijle IA, Van Schaik SM, Van Wegen EE et al (2016) Lifestyle interventions to prevent cardiovascular events after stroke and transient ischemic attack: systematic review and meta-analysis. Stroke 48:174
Sandroff BM, Motl RW, Scudder MR et al (2016) Systematic, evidence-based review of exercise, physical activity, and physical fitness effects on cognition in persons with multiple sclerosis. Neuropsychol Rev 26(3):271–294
Todoric J, Antonucci L, Karin M (2016) Targeting inflammation in cancer prevention and therapy. Cancer Prev Res (Phila) 9(12):895–905
Golbidi S, Laher I (2012) Exercise and the cardiovascular system. Cardiol Res Pract 2012:210852
Thum T (2014) Noncoding RNAs and myocardial fibrosis. Nat Rev Cardiol 11(11):655–663
Ounzain S, Pedrazzini T (2015) The promise of enhancer-associated long noncoding RNAs in cardiac regeneration. Trends Cardiovasc Med 25(7):592–602
van Rooij E, Sutherland LB, Qi X et al (2007) Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316(5824):575–579
Tao L, Bei Y, Lin S et al (2015) Exercise training protects against acute myocardial infarction via improving myocardial energy metabolism and mitochondrial biogenesis. Cell Physiol Biochem 37(1):162–175
Fernandes T, Barauna VG, Negrao CE et al (2015) Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am J Physiol Heart Circ Physiol 309(4):H543–H552
Nadruz W (2015) Myocardial remodeling in hypertension. J Hum Hypertens 29(1):1–6
Bernardo BC, Weeks KL, Pretorius L et al (2010) Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther 128(1):191–227
MacDougall JD, Tuxen D, Sale DG et al (1985) Arterial blood pressure response to heavy resistance exercise. J Appl Physiol (1985) 58(3):785–790
McMullen JR, Jennings GL (2007) Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol 34(4):255–262
Abel ED, Doenst T (2011) Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res 90(2):234–242
Volpe M (2014) Natriuretic peptides and cardio-renal disease. Int J Cardiol 176(3):630–639
Shephard RJ, Balady GJ (1999) Exercise as cardiovascular therapy. Circulation 99(7):963–972
Ooi JY, Bernardo BC, McMullen JR (2014) The therapeutic potential of miRNAs regulated in settings of physiological cardiac hypertrophy. Future Med Chem 6(2):205–222
Gullestad L, Ueland T, Vinge LE et al (2012) Inflammatory cytokines in heart failure: mediators and markers. Cardiology 122(1):23–35
Hein S, Arnon E, Kostin S et al (2003) Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 107(7):984–991
Kolwicz SC Jr, Purohit S, Tian R (2013) Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res 113(5):603–616
Mohl W, Gangl C, Jusic A et al (2015) PICSO: from myocardial salvage to tissue regeneration. Cardiovasc Revasc Med 16(1):36–46
Bergmann O, Bhardwaj RD, Bernard S et al (2009) Evidence for cardiomyocyte renewal in humans. Science 324(5923):98–102
Kocher AA, Schlechta B, Gasparovicova A et al (2007) Stem cells and cardiac regeneration. Transpl Int 20(9):731–746
Bostrom P, Mann N, Wu J et al (2010) C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 143(7):1072–1083
Kubin T, Poling J, Kostin S et al (2011) Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell 9(5):420–432
Waring CD, Vicinanza C, Papalamprou A et al (2014) The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J 35(39):2722–2731
Laughlin MH, Bowles DK, Duncker DJ (2012) The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol 302(1):H10–H23
Leone AM, Valgimigli M, Giannico MB et al (2009) From bone marrow to the arterial wall: the ongoing tale of endothelial progenitor cells. Eur Heart J 30(8):890–899
Volaklis KA, Tokmakidis SP, Halle M (2013) Acute and chronic effects of exercise on circulating endothelial progenitor cells in healthy and diseased patients. Clin Res Cardiol 102(4):249–257
Silva DA, ND J, Fernandes T, Soci UP et al (2012) Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med Sci Sports Exerc 44(8):1453–1462
Fish JE, Santoro MM, Morton SU et al (2008) miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 15(2):272–284
Care A, Catalucci D, Felicetti F et al (2007) MicroRNA-133 controls cardiac hypertrophy. Nat Med 13(5):613–618
Soci UP, Fernandes T, Hashimoto NY et al (2011) MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol Genomics 43(11):665–673
van Rooij E, Quiat D, Johnson BA et al (2009) A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 17(5):662–673
Soci UP, Fernandes T, Barauna VG et al (2016) Epigenetic control of exercise training-induced cardiac hypertrophy by miR-208. Clin Sci (Lond) 130:2005–2015
Fernandes T, Hashimoto NY, Magalhaes FC et al (2011) Aerobic exercise training-induced left ventricular hypertrophy involves regulatory MicroRNAs, decreased angiotensin-converting enzyme-angiotensin ii, and synergistic regulation of angiotensin-converting enzyme 2-angiotensin (1-7). Hypertension 58(2):182–189
Mann N, Rosenzweig A (2012) Can exercise teach us how to treat heart disease? Circulation 126(22):2625–2635
Ma Z, Qi J, Meng S et al (2013) Swimming exercise training-induced left ventricular hypertrophy involves microRNAs and synergistic regulation of the PI3K/AKT/mTOR signaling pathway. Eur J Appl Physiol 113(10):2473–2486
Liu X, Xiao J, Zhu H et al (2015) miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab 21(4):584–595
Shi J, Bei Y, Kong X et al (2017) miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics 7(3):664–676
van Rooij E, Sutherland LB, Thatcher JE et al (2008) Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A 105(35):13027–13032
Brown MD (2003) Exercise and coronary vascular remodelling in the healthy heart. Exp Physiol 88(5):645–658
Nabel EG, Braunwald E (2012) A tale of coronary artery disease and myocardial infarction. N Engl J Med 366(1):54–63
Palojoki E, Saraste A, Eriksson A et al (2001) Cardiomyocyte apoptosis and ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol 280(6):H2726–H2731
Melo SF, Fernandes T, Barauna VG et al (2014) Expression of MicroRNA-29 and collagen in cardiac muscle after swimming training in myocardial-infarcted rats. Cell Physiol Biochem 33(3):657–669
Lawler PR, Filion KB, Eisenberg MJ (2011) Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J 162(4):571–584.e572
Wisloff U, Loennechen JP, Currie S et al (2002) Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res 54(1):162–174
Melo SF, Barauna VG, Neves VJ et al (2015) Exercise training restores the cardiac microRNA-1 and -214 levels regulating Ca2+ handling after myocardial infarction. BMC Cardiovasc Disord 15:166
Icli B, Wara AK, Moslehi J et al (2013) MicroRNA-26a regulates pathological and physiological angiogenesis by targeting BMP/SMAD1 signaling. Circ Res 113(11):1231–1241
Carden DL, Granger DN (2000) Pathophysiology of ischaemia-reperfusion injury. J Pathol 190(3):255–266
Zhang KR, Liu HT, Zhang HF et al (2007) Long-term aerobic exercise protects the heart against ischemia/reperfusion injury via PI3 kinase-dependent and Akt-mediated mechanism. Apoptosis 12(9):1579–1588
Gomes EC, Silva AN, de Oliveira MR (2012) Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxidative Med Cell Longev 2012:756132
Li J, Zhang H, Zhang C (2012) Role of inflammation in the regulation of coronary blood flow in ischemia and reperfusion: mechanisms and therapeutic implications. J Mol Cell Cardiol 52(4):865–872
Felli N, Fontana L, Pelosi E et al (2005) MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc Natl Acad Sci U S A 102(50):18081–18086
Galardi S, Mercatelli N, Giorda E et al (2007) miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem 282(32):23716–23724
Baggish AL, Hale A, Weiner RB et al (2011) Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol 589(16):3983–3994
Liu SZ, Marcinek DJ (2016) Skeletal muscle bioenergetics in aging and heart failure. Heart Fail Rev 21:723
Souza RW, Fernandez GJ, Cunha JP et al (2015) Regulation of cardiac microRNAs induced by aerobic exercise training during heart failure. Am J Physiol Heart Circ Physiol 309(10):H1629–H1641
Hajdusek P, Kotrc M, Kautzner J et al (2016) Heart rate response to exercise in heart failure patients: the prognostic role of metabolic-chronotropic relation and heart rate recovery. Int J Cardiol 228:588–593
Aengevaeren VL, Hopman MT, Thijssen DH et al (2017) Endurance exercise-induced changes in BNP concentrations in cardiovascular patients versus healthy controls. Int J Cardiol 227:430–435
Giuliano C, Karahalios A, Neil C et al (2017) The effects of resistance training on muscle strength, quality of life and aerobic capacity in patients with chronic heart failure – a meta-analysis. Int J Cardiol 227:413–423
Wisloff U, Stoylen A, Loennechen JP et al (2007) Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115(24):3086–3094
Haykowsky MJ, Liang Y, Pechter D et al (2007) A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol 49(24):2329–2336
Heymans S, Corsten MF, Verhesen W et al (2013) Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation 128(13):1420–1432
Aurora AB, Mahmoud AI, Luo X et al (2012) MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca(2)(+) overload and cell death. J Clin Invest 122(4):1222–1232
Baars T, Skyschally A, Klein-Hitpass L et al (2014) microRNA expression and its potential role in cardioprotection by ischemic postconditioning in pigs. Pflugers Arch 466(10):1953–1961
Li J, Rohailla S, Gelber N et al (2014) MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol 109(5):423
Tu Y, Wan L, Fan Y et al (2013) Ischemic postconditioning-mediated miRNA-21 protects against cardiac ischemia/reperfusion injury via PTEN/Akt pathway. PLoS One 8(10):e75872
Varga ZV, Zvara A, Farago N et al (2014) MicroRNAs associated with ischemia-reperfusion injury and cardioprotection by ischemic pre- and postconditioning: protectomiRs. Am J Physiol Heart Circ Physiol 307(2):H216–H227
Masoudi FA, Inzucchi SE (2007) Diabetes mellitus and heart failure: epidemiology, mechanisms, and pharmacotherapy. Am J Cardiol 99(4a):113b–132b
Raymond T, Raymond R, Lincoff AM (2013) Management of the patient with diabetes and coronary artery disease: a contemporary review. Futur Cardiol 9(3):387–403
Cleland SJ (2012) Cardiovascular risk in double diabetes mellitus—when two worlds collide. Nat Rev Endocrinol 8(8):476–485
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87(1):4–14
Wang H, Bei Y, Lu Y et al (2015) Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1alpha and Akt activation. Cell Physiol Biochem 35(6):2159–2168
Le Douairon Lahaye S, Bekono FR, Broderick T (2014) Physical activity and diabetic cardiomyopathy: myocardial adaptation depending on exercise load. Curr Diabetes Rev 10(6):371–390
Kalani A, Tyagi A, Tyagi N (2014) Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol Neurobiol 49(1):590–600
Barile L, Lionetti V, Cervio E et al (2014) Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res 103(4):530–541
Ibrahim AG, Cheng K, Marban E (2014) Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep 2(5):606–619
Chaturvedi P, Kalani A, Medina I et al (2015) Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. J Cell Mol Med 19(9):2153–2161
Stellos K, Gatsiou A, Stamatelopoulos K et al (2016) Adenosine-to-inosine RNA editing controls cathepsin S expression in atherosclerosis by enabling HuR-mediated post-transcriptional regulation. Nat Med 22(10):1140–1150
Woollard KJ (2013) Immunological aspects of atherosclerosis. Clin Sci (Lond) 125(5):221–235
Ahmed HM, Blaha MJ, Nasir K et al (2012) Effects of physical activity on cardiovascular disease. Am J Cardiol 109(2):288–295
Kraus WE, Houmard JA, Duscha BD et al (2002) Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med 347(19):1483–1492
XD W, Zeng K, Liu WL et al (2014) Effect of aerobic exercise on miRNA-TLR4 signaling in atherosclerosis. Int J Sports Med 35(4):344–350
Munroe PB, Barnes MR, Caulfield MJ (2013) Advances in blood pressure genomics. Circ Res 112(10):1365–1379
Amaral SL, Zorn TM, Michelini LC (2000) Exercise training normalizes wall-to-lumen ratio of the gracilis muscle arterioles and reduces pressure in spontaneously hypertensive rats. J Hypertens 18(11):1563–1572
Melo RM, Martinho E Jr, Michelini LC (2003) Training-induced, pressure-lowering effect in SHR: wide effects on circulatory profile of exercised and nonexercised muscles. Hypertension 42(4):851–857
Hagberg JM, Park JJ, Brown MD (2000) The role of exercise training in the treatment of hypertension: an update. Sports Med 30(3):193–206
Fernandes T, Magalhaes FC, Roque FR et al (2012) Exercise training prevents the microvascular rarefaction in hypertension balancing angiogenic and apoptotic factors: role of microRNAs-16, -21, and -126. Hypertension 59(2):513–520
Sun CY, She XM, Qin Y et al (2013) miR-15a and miR-16 affect the angiogenesis of multiple myeloma by targeting VEGF. Carcinogenesis 34(2):426–435
Diniz MG, Gomes CC, de Castro WH et al (2012) miR-15a/16-1 influences BCL2 expression in keratocystic odontogenic tumors. Cell Oncol (Dordr) 35(4):285–291
Harmalkar M, Upraity S, Kazi S et al (2015) Tamoxifen-induced cell death of malignant glioma cells is brought about by oxidative-stress-mediated alterations in the expression of BCL2 family members and is enhanced on miR-21 inhibition. J Mol Neurosci 57(2):197–202
Salajegheh A, Vosgha H, Rahman MA et al (2016) Interactive role of miR-126 on VEGF-A and progression of papillary and undifferentiated thyroid carcinoma. Hum Pathol 51:75–85
Benza RL, Miller DP, Barst RJ et al (2012) An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest 142(2):448–456
Humbert M, Sitbon O, Chaouat A et al (2010) Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 122(2):156–163
Paolillo S, Farina S, Bussotti M et al (2012) Exercise testing in the clinical management of patients affected by pulmonary arterial hypertension. Eur J Prev Cardiol 19(5):960–971
Arena R, Cahalin LP, Borghi-Silva A et al (2015) The effect of exercise training on the pulmonary arterial system in patients with pulmonary hypertension. Prog Cardiovasc Dis 57(5):480–488
Grunig E, Maier F, Ehlken N et al (2012) Exercise training in pulmonary arterial hypertension associated with connective tissue diseases. Arthritis Res Ther 14(3):R148
Colombo R, Siqueira R, Becker CU et al (2013) Effects of exercise on monocrotaline-induced changes in right heart function and pulmonary artery remodeling in rats. Can J Physiol Pharmacol 91(1):38–44
Ajit SK (2012) Circulating microRNAs as biomarkers, therapeutic targets, and signaling molecules. Sensors (Basel) 12(3):3359–3369
Arroyo JD, Chevillet JR, Kroh EM et al (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A 108(12):5003–5008
Turchinovich A, Weiz L, Langheinz A et al (2011) Characterization of extracellular circulating microRNA. Nucleic Acids Res 39(16):7223–7233
Mitchell PS, Parkin RK, Kroh EM et al (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 105(30):10513–10518
Laterza OF, Lim L, Garrett-Engele PW et al (2009) Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem 55(11):1977–1983
Baggish AL, Park J, Min PK et al (2014) Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. J Appl Physiol (1985) 116(5):522–531
Arem H, Moore SC, Patel A et al (2015) Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 175(6):959–967
Ai J, Zhang R, Li Y et al (2010) Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun 391(1):73–77
Mooren FC, Viereck J, Kruger K et al (2014) Circulating microRNAs as potential biomarkers of aerobic exercise capacity. Am J Physiol Heart Circ Physiol 306(4):H557–H563
Hartmann D, Fiedler J, Sonnenschein K et al (2016) MicroRNA-based therapy of GATA2-deficient vascular disease. Circulation 134(24):1973–1990
Li K, Ching D, Luk FS et al (2015) Apolipoprotein E enhances microRNA-146a in monocytes and macrophages to suppress nuclear factor-kappaB-driven inflammation and atherosclerosis. Circ Res 117(1):e1–e11
Nielsen S, Akerstrom T, Rinnov A et al (2014) The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS One 9(2):e87308
Sawada S, Kon M, Wada S et al (2013) Profiling of circulating microRNAs after a bout of acute resistance exercise in humans. PLoS One 8(7):e70823
Aoi W, Ichikawa H, Mune K et al (2013) Muscle-enriched microRNA miR-486 decreases in circulation in response to exercise in young men. Front Physiol 4:80
Davis BN, Hilyard AC, Lagna G et al (2008) SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454(7200):56–61
Hansen TB, Jensen TI, Clausen BH et al (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495(7441):384–388
Lerchenmuller C, Rosenzweig A (2014) Mechanisms of exercise-induced cardiac growth. Drug Discov Today 19(7):1003–1009
Acknowledgements
This work was supported by the grants from National Natural Science Foundation of China (81570362, 91639101 and 81200169 to JJ Xiao and 81400647 to Y Bei), and the development fund for Shanghai talents (to JJ Xiao), Innovation Program of Shanghai Municipal Education Commission (2017-01-07-00-09-E00042), the grant from Science and Technology Commission of Shanghai Municipality (17010500100).
Competing Financial Interests
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bei, Y., Tao, L., Cretoiu, D., Cretoiu, S.M., Xiao, J. (2017). MicroRNAs Mediate Beneficial Effects of Exercise in Heart. In: Xiao, J. (eds) Exercise for Cardiovascular Disease Prevention and Treatment. Advances in Experimental Medicine and Biology, vol 1000. Springer, Singapore. https://doi.org/10.1007/978-981-10-4304-8_15
Download citation
DOI: https://doi.org/10.1007/978-981-10-4304-8_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4303-1
Online ISBN: 978-981-10-4304-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)