Abstract
Current study was conducted to investigation of effect of magnetic field on cell dedifferentiation and follow it callus induction derived from mature embryo culture in bread wheat genotypes. For this purpose, a factorial experiment based on completely randomized design was carried out with two wheat genotypes and three level of magnetic field strength (0.0, 8.8 and 17.6 Tesla) in three replications. Callus growth rate (CGR), relative growth rate (RGR), callus relative growth rate (CRGR), percentage of callus water content and percentage of callus induction traits were measured. To sum up, the results showed that differences between wheat genotypes and level of magnetic field strength were significant for some studied treats related to callus induction. The effect of magnetic field levels on CGR (from 0.181 to 0.175), RGR (from 1.442 to 0.655) and CRGR (from 0.052 to 0.022) were decreased with increment of magnetic field intensity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advances in plant molecular biology and biotechnology have increased interest in the differentiation and dedifferentiation processes. Plant in vitro culture is an efficient and reproducible experimental tool but many factors influence growth and development in plants. These events are under hormone control and also depend on endogenous and exogenous stimuli regulated by light and temperature cycles [1].
Plant stem cells are characterized by two individual properties: the capability to create all differentiated cell types and the ability to self-renew such that the number of stem cells is maintained [1].
In plants organogenesis, cell differentiation and dedifferentiation are fundamental processes accede to high developmental plasticity. Such plasticity involved epigenetic mechanisms but limited information is accessible concerning quantitative aspects [2].
Magnetic field as a physical phenomenon widely has been used in different aspect of biology and several effects of its application. Some of above effects including positive effects on healing of bone injuries and osteoblast activity [3], formation of blood vessels [4], having reduction role in infection rate of early blight and enhancement role in growth and yield of tomato by pre-sowing magnetic treatment [5], effect on growth and immunoreactivity of some bacteria [6], effect on proliferation, differentiation and cellular, subcellular, physiological and biochemical changes in plants [7], changes in specific gene transcription [8] and delay to senescence in Cucumis sativus [9]. Overall, Exposure to magnetic fields seems to alter the biological behavior of some important parts of several plants [10].
Embryo culture as a biotechnological method has been used in plant breeding in order to making intraspecific and intragenic hybrids [1]. Study and evaluation of these embryos against environmental stresses, the effects of ions, herbicide, pesticide and pathogens is one of the most vital goals to culture embryos in the in vitro conditions [11].
Several researches have been carried out callus induction from mature embryos and affect of many items especially various kinds and levels of hormone have been examined [12–15].
The process of plant dedifferentiation involves numerous changes in the morphology and gene expression of the cells. Many genes can be specifically induced through dedifferentiation [16].
Nonetheless, effect of magnetic field on callus induction derived of embryo culture in wheat genotypes have not been done yet and based on this issue we set this research.
Materials and methods
Embryo culture
In order to evaluate the response of the two genotypes of bread wheat (Triticum aestivum L.) to callus induction, an experiment was carried out as a factorial experiment with two factors based on completely randomized design at the Tissue Culture Laboratory, Faculty of Agriculture, Razi University, Kermanshah, Iran.
The first factor was genotype in two levels and second factor was magnetic field intensity in three levels (0.0, 8.8 and 17.6 T). The experiment was lay out based on completely randomized design with three replications.
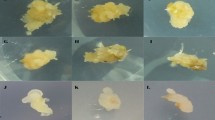
The mature seeds of two bread wheat genotypes (1 = ww33G.vee″S″.Mm.3.Atilla.Tjn and 2 = URES.3.FURY..SLN.ALDAN″S″.4.NS732.HER ICW93-0531-..) prepared from Razi University and without any pre-treatment were surface-sterilized in 70 % (v/v) ethanol for 5 min, rinsed twice with sterile distilled water, incubated in commercial bleach (5 % sodium hypochlorite) for 20 min, and rinsed several times in sterile distilled water to washout rest of previous materials. All the stages and inoculation were performed under strict aseptic conditions in a laminar airflow cabinet. In order to easily separating of mature embryos, seeds were incubated at 33 °C for 2 h in sterile distilled water for imbibition. Ten scutellums placed in the petri dishes containing basic MS medium [17]. Finally, all the dishes transferred to the growth chamber (Conviron) under darkness condition at the 25 °C temperature for 28 days and were exposure to magnetic field (Fig. 1). All petridishes and medium were autoclaved at 121 °C for 20 min before using. The following traits related to callus were measured:
-
Callus growth rate (CGR) CGR (mm/day) of cultured embryos were measured at 7, 14, 21 and 28 days after transferring calli to medium.
-
Relative growth rate (RGR) = [LnW2 − LnW1]/GP [14], where W1 = initial weight of callus, W2 = final weight of callus, GP = growth period
-
Callus relative growth rate (CRGR) RFWG = [(W2 − W1)]/W1 [18], where W1 = initial weight of callus before and after 4 weeks, W2 = final weight of callus before and after 4 weeks.
-
Percentage of callus induction (POCI) POCI was evaluated 4 weeks after embryo culture in Petri dishes.
-
Percentage of callus water content (PCWC) PCWC = [(W2-W1)/W2] × 100. Callus samples were dried in an oven set at 70 °C for 24 h [12].
Magnetic field treatment
Magnetic field in this study generated by a magnetic coil by cooper wire wound around made in tissue culture laboratory with 40 cm height and 12 cm diameter (Fig. 1). A direct current power supply offered electrical flow in the coil. This coil and the authenticity of induced three levels of desire magnetic field (0.0, 8.8 and 17.6 T).
Statistical analysis
Analysis of variance, mean comparison using Duncan’s multiple range test, were performed by MSTAT-C and SAS version 9.1.
Result and discussion
Analysis of variance
As it can be seen from Table 1, analysis of variance showed high significant differences (P < 0.01) between genotypes for all measured traits except PCWC. Depending callus induction to genotype has been shown in several studies: Raymond et al. [19] in T. aestivum L., Arzani and Mirodjagh [13] in durum wheat, Grigoryeva and Shletser [20] in durum and bread wheat, Hanzel et al. [21] in durum and bread wheat, Kahrizi and Mohammadi [22], in barley and Kahrizi and Mirzaei [23] in cereals.
In addition, we observed significant differences (P < 0.05) between magnetic field levels for CGR, RGR and CRGR while POCI and PCWC have not been affected by this factor.
In this study, the genotype × magnetic field interaction was not statistically significant except for CGR.
The highly differentiated cell in plant in vitro culture could be dedifferentiated by artificial culture, and then recover to embryonic stage of cell [23].
Alatzas and Foundouli [24] reported ubiquitinated histone distribution pattern during cell differentiation and/or dedifferentiation, particularly in Zea mays. In this study callus cultures representing the three developmental zones and subsequently second and third subcultures were developed from part of each first culture, in order to study uH2A distribution during plant cell dedifferentiation. They reported that Histone H2A ubiquitination level is also changed through plant cell dedifferentiation, according to uH2A ratio to H2A in distinct calli subcultures (Fig. 2).
These information agree with the fact that, although morphological similarity observed in calli derived from divergent developmental stages, differences in biochemical properties continue to exist during plant cell dedifferentiation [24].
The enhance in cell division rate, resulting from dedifferentiation process, was completed during the first culture stage, DNA damage during replication could be leveled and therefore, increased H2A ubiquitination level may be not essential and uH2A fraction is similar to that of meristematic cells [24].
Mean comparisons
Table 2 shows the mean comparison for the effect of different magnetic field strength levels. As it can be seen from the Table 2, the effect of magnetic field levels on CGR, CRGR and POCI were decreased with increment of magnetic field intensity. Different level of magnetic field did not effect on PCWC in two mentioned genotypes. Decreasing in these traits by introducing is may be due to decrease cell number and delaying in G1 and G2 phases in the process of cell division [7].
Mean comparisons between genotypes indicated that maximum CGR, RGR, CRGR and POCI were belonged to genotype No. 1, but there was not significantly difference between genotypes for PCWC (Table 3). As it mentioned above, response in tissue culture for several traits is highly genotype dependent.
Abbreviations
- CGR:
-
Callus growth rate
- RGR:
-
Relative growth rate
- CRGR:
-
Callus relative growth rate
- PCWC:
-
Percentage of callus water content
- POCI:
-
Percentage of callus induction
- S.O.V:
-
Source of variations
- d.f.:
-
Degree of freedom
- CV:
-
Coefficient of variation
References
Kahrizi D, Arminian A, Masumi AA (2007) In vitro plant breeding. Razi University Press, Kermanshah
Causevic A, Delaunay A, Ounnar S, Righezza M, Delmotte F, Brignolas F, Hagège D, Maury S (2005) DNA methylating and demethylating treatments modify phenotype and cell wall differentiation state in sugarbeet cell lines. Plant Physiol Biochem 43:681–691
Midura RJ, Ibiwoye MO, Powell KA, Sakai Y, Doehring T, Grabiner MD, Patterson TE, Zborowski M, Wolfman A (2005) Pulsed electromagnetic field treatments enhance the healing of fibular osteotomies. J Orthop Res 23:1035–1046
Shenzhi X, Naohide T, Ken I, Yoshito I (2006) Recovery of small-sized blood vessels in ischemic bone under static magnetic field. Evid Based Complement Alternat Med 4:59–63
De Souza A, Garcia D, Sueiro L, Gilart F, Porras E, Licea L (2006) Pre-sowing magnetic treatments of tomato seeds increase the growth and yield of plants. Bioelectromagnetics 27:247–257
Triampo W, Doungchawee G, Triampo D, Wong-Ekkabut J, Tang I (2004) Effects of static magnetic field on growth of leptospire, leptospira interrogans serovar canicola: immunoreactivity and cell division. J Biosci Bioeng 98:182–186
Belyavskaya NA (2004) Biological effects due to weak magnetic field on plants. Adv Space Res 34:1566–1574
Jerry LP, Wendy H, William JT, Tamako IJ, Adey WR (1992) Magnetic field-induced changes in specific gene transcription. Biochim Biophys Acta 1132:140–144
Albertini MC, Accorsi A, Citterio B, Burattini S, Piacentini MP, Uguccioni F, Piatti E (2003) Morphological and biochemical modifications induced by a static magnetic field on Fusarium culmorum. Biochimie 85:963–970
Germana MA, Chiancone B, Melati MR, Firetto A (2003) Preliminary results on the effect of magnetic fields on anther culture and pollen germination of Citrus clementine. Acta Hortic 625:411–418
Ghasempour HR, Kahrizi D, Mahdiah N (2007) New discussions in biotechnology. Razi University Press, Kermanshah
Al-Khayri JM, Al-Bahrany AM (2004) Growth, water content, and proline accumulation in drought-stressed callus of date palm. Biol Plant 48:105–108
Arzani A, Mirodjagh SS (1999) Response of durum wheat cultivars to immature embryo culture, callus induction and in vitro salt stress. Plant Cell Tissue Organ Cult 58:67–72
Birsin MA, Ozgen MA (2004) A comparison of callus induction and plant regeneration from different embryo explants of triticale (× Triticosecale wittmack). Cell Mol Biol Lett 9(2):353–361
Soorni J, Kahrizi D, Molsaghi M (2012) The effects of photoperiod and 2, 4-D concentrations on callus induction of Cuminum cyminum’s leaf explant: an important medicinal plant. Asian J Biol Sci (in press)
Kim SS, Choi SY, Park JH, Lee DJ (2004) Regulation of the activity of Korean radish cationic peroxidase promoter during dedifferentiation and differentiation. Plant Physiol Biochem 42:763–772
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15:473–497
Chen JJ, Yue RQ, Xu HX, Chen XJ (2006) Study on plant regeneration of wheat mature embryos under endosperm-supported culture. Agric Sci China 5:572–578
Raymond RC, Anaira C, Wahl RL (1996) Exposure to strong static magnetic field slows the growth of human cancer cells in vitro. Bioelectromagnetics 17:358–363
Grigoryeva LP, Shletser IA (2006) Screening wheat cultivars for morphogenesis ability in immature embryo culture in vitro. Biologia 3:64–66
Hanzel JJ, Miller JP, Brinkman MA, Fendos E (1985) Genotype and media effects on callus formation and regeneration in barley. Crop Sci 25:27–31
Kahrizi D, Mohammad R (2009) Study of androgenesis and spontaneous chromosome doubling in barley (Hordeum vulgare L.) genotypes using isolated microspore culture. Acta Agron Hung 57(2):155–164
Kahrizi D, Mirzaei M (2012) Induced androgenic embryogenesis in cereals. In: Ken-ichi S (ed) Embryogenesis. InTech. doi:10.5772/36646
Alatzas A, Foundouli A (2006) Distribution of ubiquitinated histone H2A during plant cell differentiation in maize root and dedifferentiation in callus culture. Plant Sci 171:481–487
Acknowledgments
The authors would like for gratefully acknowledge Mr Gholamreza Amjadian for his helpful comments and preparing the magnetic coil. Razi University has financially supported this research project No. 868. Thanks to The Cooperative No. 4407 the Knowledge Base New Ideas Co. and The Cooperative the Knowledge Base Shafa Hooraman for all supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kahrizi, D., Cheghamirza, K., Akbari, L. et al. Effects of magnetic field on cell dedifferentiation and callus induction derived from embryo culture in bread wheat (Triticum aestivum. L) genotypes. Mol Biol Rep 40, 1651–1654 (2013). https://doi.org/10.1007/s11033-012-2214-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2214-7