Abstract
Paraoxonase is an HDL-associated enzyme that plays a preventive role against oxidative stress, which is thought to contribute to cancer development. PON1 activity varies widely among individuals, which is in part related to two common nonsynonymous polymorphisms in the PON1 gene (Q192R and L55M). The polymorphisms in PON1 have been implicated in cancer risk. However, results from the studies to date have been conflicting. To clarify the association, a meta-analysis was performed for 7,073 cases and 9,520 controls from 25 published case–control studies. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to assess the strength of the association. Significant associations between PON1-L55M but not Q192R polymorphism and total cancer were observed from all the comparisons. In stratified analyses, PON1-55M allele was a risk factor for breast cancer. Similarly, increased risk was observed for prostate cancer (OR = 1.18, 95% CI: 1.01–1.36, P heterogeneity = 0.260) and Caucasian population (OR = 1.18, 95% CI: 1.02–1.38, P heterogeneity = 0.1) of the LM genotype, compared with the LL genotype. For PON1-Q192R polymorphism, PON1-192R allele was a decreased risk factor for cancer in the Asian group (RR vs QQ: OR = 0.61, 95% CI: 0.38–0.98, P heterogeneity = 0.268; QR vs QQ: OR = 0.71, 95% CI: 0.52–0.96, P heterogeneity = 0.130; RR + QR vs QQ: OR = 0.71, 95% CI: 0.53–0.95, P heterogeneity = 0.135). Although some modest bias could not be eliminated, this meta-analysis suggests that the PON1-55M allele is a risk factor for the development of cancer, in particular for breast cancer. Future studies with larger sample sizes are warranted to further evaluate these associations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxygen radicals can oxidize DNA bases, form mutagenic lesions and chromosome aberrations, and activate chemical carcinogens into highly reactive compounds. Elevated oxidative stress has been found to mediate carcinogenesis by causing metabolic malfunction and damage to biological molecules, including DNA [1]. It has been observed that oxidative stress is associated with an increased risk in various types of cancers [2, 3]. The level of oxidative stress is determined by the relative rates at which reactive oxygen species (ROS) are generated and detoxified. It has been reported that ROS may also play a role in human cancer development [4]. The detoxification of ROS is accomplished by small molecules, such as vitamins C and E, and by enzymes which cleave to the ROS to generate nontoxic products [5]. Human serum paraoxonase1 (PON1) is an esterase enzyme that has lipophilic antioxidant characteristics and that participates in the elimination of ROS. It binds to high-density lipoprotein (HDL) and contributes to the detoxification of organophosphorus compounds, such as paradoxes and carcinogenic lipid-soluble radicals from lipid peroxidation [6–8].
PON1, a member of a family of proteins including PON2 and PON3, is located on the long arm of chromosome 7q21.3 [6]. PON1 is an esterase that is widely distributed among tissues such as the liver, kidney, and intestine; but it is also present in blood plasma. There is a 10- to 40-fold inter-individual variability in serum PON1 activity, as measured by rates of hydrolysis of the exogenous substrate paraoxon [6]. One source of the variability is the polymorphism of the PON1 gene. Epidemiologic and molecular studies have identified that there are two important common functional genetic polymorphisms in the coding region of the gene at positions 55 and 192 of the PON1 gene. Substitution of glutamine (Q genotype) at position 192 in exon 6 of the PON1 gene by arginine (R genotype) leads to the first polymorphism (rs662A>G, Gln192Arg, Q192R, A192B). Similarly, substitution of leucine (L genotype) at position 55 in exon 3 by methionine (M genotype) leads to the second polymorphism (rs854560T>A, Leu55Met, L55M) [9, 10]. Studies revealed that polymorphisms of the PON1 gene may affect PON1 activity. The PON1 activity of the PON1 192 Q allele carriers was reported to be lower than that of the R carriers [7, 11, 12]. The PON1-55L allele is correlated with higher PON1 activity and mRNA levels than PON1-55M allele [12, 13]. The study deduced that the lower activity of PON1 was caused by a decreased stability of the PON1-55M protein [14]. Reduced PON1 activities have been reported in different groups of patients, including those with diabetes mellitus, cardiovascular disease, and hypercholesterolemia. Furthermore, a number of epidemiological studies have investigated the associations between these polymorphisms and different cancers, such as lung [15], breast [16], brain [17], and ovarian [18] cancers. However, the results remained controversial [16, 17, 19, 20], partially because of small sample size, the difference in the genotype distribution by ethnicity, study design, assay characteristics, and so on. In light of the extensive role of polymorphisms in PON1, a pooled analysis is needed to accumulate data from different studies and to provide better evidence for or against that association. Therefore, we performed a meta-analysis to evaluate the association of polymorphisms in PON1 with cancer susceptibility in all eligible case–control studies.
Materials and methods
Identification of eligible studies
An electronic literature search was performed with PubMed, Embase, for all relevant reports (the last search update was as of Jun 23, 2011), using the key words “paraoxonase l” or “PON1,” “polymorphism,” “tumor,” or “cancer.” The search was limited to human-associated studies. We also used the PubMed option “related articles” in each research article to search potentially relevant articles. In addition, studies were identified by a manual search of the reference lists of reviews and retrieved studies. When more than one of the same or overlapping population by different researchers or overlapping data by the same authors were found, only the most recent or complete study was used for this meta-analysis. Studies, regardless of sample size, were enrolled if they met the following inclusion criteria: (i) a study of the PON1 Q192R or L55M polymorphism and cancer risk; (ii) a case–control study; (iii) with available genotype frequency.
Data extraction
Two of the authors (Dai-Hua Fang and Qiang Ji) extracted all data from eligible publications independently that met the inclusion criteria and reached the consensus for any controversy. For each study, the following characteristics were collected: the first author’s last name, year of publication, the country and ethnicity of study population, the number of genotyped subjects in cases and controls, source of control groups (population- or hospital based), the types of cancers, and genotyping methods. Different ethnic descents were categorized as Caucasian, Asian, or mixed ethnicity, including more than one ethnic descent.
Statistical analysis
A meta-analysis was used to examine the overall association of the PON1-L55M and -Q192R polymorphisms with the risk of cancer by odds ratio (ORs) with 95% confidence intervals (CIs). As compared to the wild-type LL or QQ homozygote, the risk of carriage of the M or R allele (i.e., LM and MM or RR and QR genotypes) on cancers was estimated, followed by evaluating the risk of LM +MM versus LL or QR + RR versus QQ on cancer in the dominant model and MM versus LM + LL or RR versus QR + QQ on cancer in recessive effects, respectively. Stratified analyses were also performed by ethnicity, researched methods, and cancer types (if only one cancer type contained less than two individual studies, it was combined into the “Other Cancers” group).
The statistical significance of the pooled OR was determined with the Z test, and a P value of <0.05 was considered significant. Heterogeneity across the studies was evaluated by a χ2 test based on a Q statistic test [21], and was considered significant if P < 0.05. A fixed-effect model using the Mantel–Haenszel method and a random-effects model using the DerSimonian and Laird method were used to pool the results [22]; the fixed-effect model was used as well when there was no heterogeneity across results of the studies, or the random-effect model was used. Moreover, a sensitivity analysis was performed to assess the stability of the results, by which a single study in the meta-analysis was deleted each time to determine the influence of the individual data set to the overall pooled OR [23]. To test the publication bias, Funnel plots and the Egger’s linear regression test were applied [24]. The Hardy–Weinberg equilibrium for controls was also tested by the χ2 test for accuracy of fit, using a web-based program (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). All statistical tests for this meta-analysis were performed with STATA version 10.0 (Stata Corporation College Station, TX, USA).
Results
Characteristics of studies
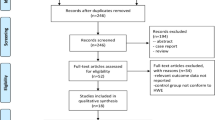
A total of 25 eligible studies were enrolled for this study according to the preset inclusion criteria (Fig. 1), in which 7,073 cases and 9,520 controls were included for the pooled analysis. For PON1-Q192R polymorphism, all 25 studies reported the available data, including five brain tumors studies, five breast cancer studies, three prostate cancer studies, and the others, which were categorized into the “Other Cancers” group. There were 15 studies of Caucasian descendents, two of Asian descendents, and eight with mixed ethnicity. Cancers were diagnosed histologically or pathologically in most studies. The polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) and TaqMan assay were performed in 12 and 11 studies, respectively, and sequencing was applied by two studies. In addition, most of the controls were sex- and age-matched for the case groups, of which 20 were population based and five were hospital based. For PON1-L55M polymorphism, 16 studies with available data from ten Caucasian descendents and one of Asian descendents, along with five with mixed ethnicity, were collected for the pooled analysis, including four breast cancer studies, three prostate cancer studies, and nine studies that were categorized into the other cancers groups (shown in Supplemental table). The genotype distributions among the controls of all studies were not deviated from the Hardy–Weinberg equilibrium, with the exception of two studies [25, 26] on PON1-L55M polymorphism and seven studies [5, 19, 25, 27–30] on PON1-Q192R polymorphism; however Hussein et al described that the distribution of Q192R genotypes of their study was also consistent with the Hardy–Weinberg equilibrium in controls (P = 0.710) (Table 1).
Meta-analysis
Overall, there was no association between PON1-R192Q polymorphism and the risk of cancer for any overall comparison. However, subgroup analysis revealed that there were significantly decreased risks of cancer in Asian population for comparison of RR versus QQ (OR = 0.61, 95% CI: 0.38–0.98, P heterogeneity = 0.268), RQ versus QQ (OR = 0.71, 95% CI: 0.52–0.96, P heterogeneity = 0.130) and dominant-model comparison (RR + RQ vs. QQ) (OR = 0.71, 95% CI: 0.53–0.95, P heterogeneity = 0.135). For the polymorphism of PON1-L55M, increased cancer risks were observed for the overall comparisons (Table 2; Fig. 2). Furthermore, in the cancer type subgroup analysis, we observed increased risks of breast cancer for all comparisons, prostate cancer for the comparison of LM versus LL (OR = 1.18, 95% CI: 1.01–1.36, P heterogeneity = 0.260), and other cancer types for the comparison of MM versus LL + LM (OR = 1.20, 95% CI: 1.02–1.42, P heterogeneity = 0.260). Similarly, in the source-of-control subgroup analysis, increased cancer risk was observed in the hospital based control group for all comparisons, and in the population based control group for the comparison of LM versus LL (OR = 1.09, 95% CI: 1.00–1.20, P heterogeneity = 0.201). In addition, increased cancer risk was also observed in the Caucasian population for the comparison of LM versus LL (OR = 1.18, 95% CI: 1.02–1.38, P heterogeneity = 0.157) and in the research method based on the polymerase chain reaction and restriction fragment length polymorphism (PCR–RFLP), as summarized in Table 2.
There was significant heterogeneity across the studies in overall comparisons for two polymorphism sites. For this, the source of heterogeneity was explored for the heterozygote comparison (RQ vs. QQ for PON1-R192Q and LM vs. LL for PON1-L55M) by between subgroups (Cancer type, ethnicity, and source of controls). For PON1-R192Q polymorphism, cancer type (χ2 = 49.66, df = 3, P = 0.000) and source of controls (χ2 = 12.89, df = 1, P = 0.000) but not ethnicity (χ2 = 5.77, df = 2, P = 0.056) contributed substantially to that heterogeneity. For PON1-L55M polymorphism, cancer type (χ2 = 9.01, df = 2, P = 0.011) but not ethnicity (χ2 = 1.92, df = 2, P = 0.373) and source of controls (χ2 = 3.54, df = 1, P = 0.060) contributed substantially to that heterogeneity.
Sensitivity analyses
For PON1-R192Q polymorphism, sensitivity analysis revealed that nine independent studies were the main source of heterogeneity; two were prostate cancer [19] and breast cancer [25] related studies, respectively. In the other cancer type subgroup, two studies were related to lung cancer [15, 31] and five studies were related to ovarian cancer [18], bladder cancer [28], osteosarcoma [29], non-Hodgkin’s lymphoma [32] and multiple myeloma [33]. The heterogeneity was decreased when these seven studies were removed (RR vs. QQ: P heterogeneity = 0.124, QR vs. QQ: P heterogeneity = 0.149, RR +QR vs. QQ: P heterogeneity = 0.060, RR vs. QQ + QR: P heterogeneity = 0.201). Significant association were observed in subgroup of breast cancer (QR vs. QQ: OR = 0.79, 95% CI: 0.66–0.95, P heterogeneity = 0.555; RR +QR vs. QQ: OR = 0.79, 95% CI: 0.66–0.94, P heterogeneity = 0.659; RR vs. QQ + QR: OR = 0.76, 95% CI: 0.58–1.00, P heterogeneity = 0.512) after the study carried by Antognelli et al [25] removed. For race subgroup analysis, seven studies [15, 18, 19, 25, 28, 29, 33] were the main source of heterogeneity of Caucasian population, and found to impact the pooled OR (data not shown). In addition, no other single study was found to impact the pooled OR as indicated by sensitivity analyses.
For PON1-L55M polymorphism, sensitivity analysis revealed that three independent studies were the main source of heterogeneity; two were prostate cancer [5, 19] and one was breast cancer [25] related studies, respectively. The heterogeneity was decreased when these three studies were removed (MM vs. LL: P heterogeneity = 0.067, MM + LM vs. LL: P heterogeneity = 0.096, MM vs. LL + LM: P heterogeneity = 0.131). In addition, no other single study was found to impact the pooled OR as indicated by sensitivity analyses.
Publication bias
Begg’s funnel plot and the Egger’s test were performed to assess the publication bias of the currently available literature. The shape of the funnel plots did not reveal any evidence for obvious asymmetry in all comparison models. Then, the Egger’s test was used to provide statistical evidence for funnel plot symmetry. The results still did not show any evidence of publication bias (t = −1.15, P = 0.263 for QR vs. QQ; t = −0.620, P = 0.545 for LM vs. LL).
Discussion
In this meta-analysis, we investigated the association between PON1-L55M and Q192R polymorphisms and risk of cancer. The results revealed that PON1-L55M but not Q192R polymorphism was associated with increased risk for developing cancers. The LM/MM genotype and M allele increased the risk of cancer occurrence, especially for breast cancer. Given the important roles of PON1 in the regulation of the oxidative stress and inflammation, which were believed to be important in carcinogenesis, we deduced that PON1-L55M polymorphism may modulate the risk of the development of cancer, especially in a type of breast cancer.
Oxidative stress and free radicals have been associated with an increased risk in various types of cancers [2, 34]. The human body has a number of endogenous free-radical scavenging systems. PON1, an antioxidant enzyme, may cause defects in the antioxidant/oxidant balance [35]. This can trigger oxidative stress and the formation of ROS. Studies have revealed that PON1 expression is depressed in human lung cancer [36], pancreatic [37], and gastric cancer [38]. In the case of PON1, PON1-55M allele has been found to have significantly lower PON1 expression than PON1-55L [13, 39], resulting in an increased risk of developing cancers [5, 18, 19, 25, 40] and suggesting PON1-55M allele was the risk of cancer. Furthermore our results were confirmed by the genetic model-free approach, which reported by Minelli et al. [41]. In the present study, significant associations were observed between the PON1-L55M polymorphism and prostate cancer for the comparison of LM versus LL and other cancer types for the comparison of MM versus LL + LM; however, a significant association was observed in breast cancer for the all comparisons, suggesting that different effects PON1-L55M polymorphism in different cancer types. The risk factors for breast cancer, including BRCA1 mutations and increased estrogen metabolism, are known to influence oxidative stress [42]. Moreover, since oxidative stress may be involved in cell proliferation and malignant conversion during the development of breast cancer [43], it is reasonable to expect that PON1, as a part of the lipid peroxidation scavenging systems, may influence breast cancer development. In the subgroup analysis, a significant association was observed between PON1-L55M polymorphism and cancer risk in that two studies based on hospital controls. In contrast, there was no association was observed in studies based on population controls. This association may be the reason that the two studies based on hospital control were the main source of heterogeneity for PON1-L55M; furthermore, the significant associations were pooled from two studies based on hospital control, and they may be affected by limited studies.
In contrast to L55M polymorphism, our data failed to observe statistical significance in the distribution of overall allele and genotype frequencies of Q192R between cancers, and the results were confirmed by the genetic model-free approach [40]. However, in the subgroup analysis, we observed that PON1-192 RR/QR or R allele acts as a decreased risk for cancer from two studies based on the Asian population. Studies revealed that PON1 192 Q allele carriers were reported to be lower than that of the R carriers [7, 11, 12], and a lower PON1 level was regarded as a risk for cancer; furthermore, allele distributions in control subjects varied significantly by ethnic group and were similar to those reported by the National Center of Biotechnology Information for Caucasian (Q: 0.668) and Asian population (Q: 0.430). Further cumulated studies would be needed to reveal the association in the Asian population, since the result presented in that study was from the data of two Asian population studies.
However, results derived from this meta-analysis should be interpreted with caution. First, when a stratified analysis of tumor type, ethnicity, or control recourse was performed, the number of each subgroup seemed to be smaller. Second, there was no further evaluation of potential gene–gene interactions and gene–environment interactions (i.e., the interaction between insecticide exposure and PON1 was deduced with higher risk for brain cancers), due to the lack of original data from the studies. Finally, the number of published studies was not enough for a comprehensive analysis, particularly for any kind of the cancers and ethnicities. In spite of these limitations, this meta-analysis had several strengths. First, no publication biases were detected, indicating that summary results may be unbiased. Second, in the sensitivity analysis, no individual study affected the pooled OR which indicated that our results were statistically trustworthy.
In conclusion, this meta-analysis suggests that the PON1-L55M polymorphism may contribute to the genetic susceptibility of cancer, particularly for breast cancer. However, further studies would be needed to clarify the possible roles of the PON1 polymorphisms in the etiology of cancer.
References
Cejas P, Casado E, Belda-Iniesta C, De Castro J, Espinosa E, Redondo A, Sereno M, Garcia-Cabezas MA, Vara JA, Dominguez-Caceres A, Perona R, Gonzalez-Baron M (2004) Implications of oxidative stress and cell membrane lipid peroxidation in human cancer (Spain). Cancer Causes Control 15(7):707–719. doi:10.1023/B:CACO.0000036189.61607.52
Ames BN (1983) Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science 221(4617):1256–1264
Klaunig JE, Kamendulis LM (2004) The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 44:239–267. doi:10.1146/annurev.pharmtox.44.101802.121851
Wiseman H, Halliwell B (1996) Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 313(1):17–29
Stevens VL, Rodriguez C, Talbot JT, Pavluck AL, Thun MJ, Calle EE (2008) Paraoxonase 1 (PON1) polymorphisms and prostate cancer in the CPS-II nutrition cohort. Prostate 68(12):1336–1340. doi:10.1002/pros.20796
Humbert R, Adler DA, Disteche CM, Hassett C, Omiecinski CJ, Furlong CE (1993) The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet 3(1):73–76. doi:10.1038/ng0193-73
Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowalla J, Furlong CE (1996) The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet 14(3):334–336. doi:10.1038/ng1196-334
Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ (1998) Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 394(6690):284–287. doi:10.1038/28406
Aynacioglu AS, Cascorbi I, Mrozikiewicz PM, Nacak M, Tapanyigit EE, Roots I (1999) Paraoxonase 1 mutations in a Turkish population. Toxicol Appl Pharmacol 157(3):174–177. doi:10.1006/taap.1999.8690
Lurie G, Wilkens LR, Thompson PJ, McDuffie KE, Carney ME, Terada KY, Goodman MT (2008) Genetic polymorphisms in the Paraoxonase 1 gene and risk of ovarian epithelial carcinoma. Cancer Epidemiol Biomarkers Prev 17(8):2070–2077. doi:10.1158/1055-9965.EPI-08-0145
Mackness B, Mackness MI, Arrol S, Turkie W, Durrington PN (1997) Effect of the molecular polymorphisms of human paraoxonase (PON1) on the rate of hydrolysis of paraoxon. Br J Pharmacol 122(2):265–268. doi:10.1038/sj.bjp.0701390
Li WF, Costa LG, Richter RJ, Hagen T, Shih DM, Tward A, Lusis AJ, Furlong CE (2000) Catalytic efficiency determines the in-vivo efficacy of PON1 for detoxifying organophosphorus compounds. Pharmacogenetics 10(9):767–779
Leviev I, Negro F, James RW (1997) Two alleles of the human paraoxonase gene produce different amounts of mRNA. An explanation for differences in serum concentrations of paraoxonase associated with the (Leu-Met54) polymorphism. Arterioscler Thromb Vasc Biol 17(11):2935–2939
Leviev I, Deakin S, James RW (2001) Decreased stability of the M54 isoform of paraoxonase as a contributory factor to variations in human serum paraoxonase concentrations. J Lipid Res 42(4):528–535
Aksoy-Sagirli P, Cakmakoglu B, Isbir T, Kaytan-Saglam E, Kizir A, Topuz E, Berkkan H (2011) Paraoxonase-1 192/55 polymorphisms and the risk of lung cancer in a Turkish population. Anticancer Res 31(6):2225–2229
Gallicchio L, McSorley MA, Newschaffer CJ, Huang HY, Thuita LW, Hoffman SC, Helzlsouer KJ (2007) Body mass, polymorphisms in obesity-related genes, and the risk of developing breast cancer among women with benign breast disease. Cancer Detect Prev 31(2):95–101. doi:10.1016/j.cdp.2007.02.004
Searles Nielsen S, Mueller BA, De Roos AJ, Viernes HM, Farin FM, Checkoway H (2005) Risk of brain tumors in children and susceptibility to organophosphorus insecticides: the potential role of paraoxonase (PON1). Environ Health Perspect 113(7):909–913
Arpaci A, Gormus U, Dalan B, Berkman S, Isbir T (2009) Investigation of PON1 192 and PON1 55 polymorphisms in ovarian cancer patients in Turkish population. In Vivo 23(3):421–424
Antognelli C, Mearini L, Talesa VN, Giannantoni A, Mearini E (2005) Association of CYP17, GSTP1, and PON1 polymorphisms with the risk of prostate cancer. Prostate 63(3):240–251. doi:10.1002/pros.20184
Martinez C, Molina JA, Alonso-Navarro H, Jimenez-Jimenez FJ, Agundez JA, Garcia-Martin E (2010) Two common nonsynonymous paraoxonase 1 (PON1) gene polymorphisms and brain astrocytoma and meningioma. BMC Neurol 10:71. doi:10.1186/1471-2377-10-71
Handoll HH (2006) Systematic reviews on rehabilitation interventions. Arch Phys Med Rehabil 87(6):875. doi:10.1016/j.apmr.2006.04.006
Midgette AS, Wong JB, Beshansky JR, Porath A, Fleming C, Pauker SG (1994) Cost-effectiveness of streptokinase for acute myocardial infarction: a combined meta-analysis and decision analysis of the effects of infarct location and of likelihood of infarction. Med Decis Making 14(2):108–117
Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127(9):820–826
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Antognelli C, Del Buono C, Ludovini V, Gori S, Talesa VN, Crino L, Barberini F, Rulli A (2009) CYP17, GSTP1, PON1 and GLO1 gene polymorphisms as risk factors for breast cancer: an Italian case-control study. BMC Cancer 9:115. doi:10.1186/1471-2407-9-115
Uyar OA, Kara M, Erol D, Ardicoglu A, Yuce H (2011) Investigating paraoxonase-1 gene Q192R and L55M polymorphism in patients with renal cell cancer. Genet Mol Res 10(1):133–139. doi:10.4238/vol10-1gmr927
Rajaraman P, Hutchinson A, Rothman N, Black PM, Fine HA, Loeffler JS, Selker RG, Shapiro WR, Linet MS, Inskip PD (2008) Oxidative response gene polymorphisms and risk of adult brain tumors. Neuro Oncol 10(5):709–715. doi:10.1215/15228517-2008-037
Ozturk O, Kagnici OF, Ozturk T, Durak H, Tuzuner BM, Kisakesen HI, Cakalir C, Isbir T (2009) 192R allele of paraoxanase 1 (PON1) gene as a new marker for susceptibility to bladder cancer. Anticancer Res 29(10):4041–4046
Ergen A, Kilicoglu O, Ozger H, Agachan B, Isbir T (2011) Paraoxonase 1 192 and 55 polymorphisms in osteosarcoma. Mol Biol Rep 38(6):4181–4184. doi:10.1007/s11033-010-0538-8
Hussein YM, Gharib AF, Etewa RL, ElSawy WH (2011) Association of L55 M and Q192R polymorphisms in paraoxonase 1 (PON1) gene with breast cancer risk and their clinical significance. Mol Cell Biochem 351(1–2):117–123. doi:10.1007/s11010-011-0718-4
Lee CH, Lee KY, Choe KH, Hong YC, Kim YD, Kang JW, Kim H (2005) Effects of oxidative DNA damage induced by polycyclic aromatic hydrocarbons and genetic polymorphism of the paraoxonase-1 (PON1) gene on lung cancer. J Prev Med Public Health 38(3):345–350
Kerridge I, Lincz L, Scorgie F, Hickey D, Granter N, Spencer A (2002) Association between xenobiotic gene polymorphisms and non-Hodgkin’s lymphoma risk. Br J Haematol 118(2):477–481
Lincz LF, Kerridge I, Scorgie FE, Bailey M, Enno A, Spencer A (2004) Xenobiotic gene polymorphisms and susceptibility to multiple myeloma. Haematologica 89(5):628–629
Sun Y (1990) Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med 8(6):583–599
Karaman E, Uzun H, Papila I, Balci H, Ozdilek A, Genc H, Yanardag H, Papila C (2010) Serum paraoxonase activity and oxidative DNA damage in patients with laryngeal squamous cell carcinoma. J Craniofac Surg 21(6):1745–1749. doi:10.1097/SCS.0b013e3181f4040a
Elkiran ET, Mar N, Aygen B, Gursu F, Karaoglu A, Koca S (2007) Serum paraoxonase and arylesterase activities in patients with lung cancer in a Turkish population. BMC Cancer 7:48. doi:10.1186/1471-2407-7-48
Akcay MN, Polat MF, Yilmaz I, Akcay G (2003) Serum paraoxonase levels in pancreatic cancer. Hepatogastroenterology 50(2):ccxxv–ccxxvii
Akcay MN, Yilmaz I, Polat MF, Akcay G (2003) Serum paraoxonase levels in gastric cancer. Hepatogastroenterology 50(2):cclxxiii–cclxxv
Garin MC, James RW, Dussoix P, Blanche H, Passa P, Froguel P, Ruiz J (1997) Paraoxonase polymorphism Met-Leu54 is associated with modified serum concentrations of the enzyme. A possible link between the paraoxonase gene and increased risk of cardiovascular disease in diabetes. J Clin Invest 99(1):62–66. doi:10.1172/JCI119134
Naidu R, Har YC, Taib NA (2010) Genetic Polymorphisms of paraoxonase 1 (PON1) Gene: association Between L55 M or Q192R with breast cancer risk and clinico-pathological parameters. Pathol Oncol Res. doi:10.1007/s12253-010-9267-5
Minelli C, Thompson JR, Abrams KR, Thakkinstian A, Attia J (2005) The choice of a genetic model in the meta-analysis of molecular association studies. Int J Epidemiol 34(6):1319–1328. doi:10.1093/ije/dyi169
Ambrosone CB (2000) Oxidants and antioxidants in breast cancer. Antioxid Redox Signal 2(4):903–917
Delimaris I, Faviou E, Antonakos G, Stathopoulou E, Zachari A, Dionyssiou-Asteriou A (2007) Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin Biochem 40(15):1129–1134. doi:10.1016/j.clinbiochem.2007.06.007
Author information
Authors and Affiliations
Corresponding author
Additional information
Dai-Hua Fang and Cong-Hai Fan are contributed equally to this work and should be considered as co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fang, DH., Fan, CH., Ji, Q. et al. Differential effects of paraoxonase 1 (PON1) polymorphisms on cancer risk: evidence from 25 published studies. Mol Biol Rep 39, 6801–6809 (2012). https://doi.org/10.1007/s11033-012-1505-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1505-3