Abstract
Diabetic retinopathy (DR) is a leading cause of blindness globally and its pathogenesis has still not been completely elucidated. Some studies show a close relation between oxidative stress and DR. This study was aimed to investigate the effects of anti-oxidant in DR and expression of vascular endothelial growth factor (VEGF) and intercellular adhesion molecule-1 (ICAM-1) from retinal blood vessels in diabetic rats. Diabetic rat models were established by intraperitoneal injection of streptozotocin (60 mg/kg) and confirmation of high serum glucose levels in the animals. Antioxidant N-acetylcysteine was given to diabetic rats to elicit antioxidative responses, and rats were sacrificed at 3 and 5 months. Ultrastructures of retinal vascular tissues were observed under transmission electron microscope, and pathology of retinal capillaries was examined using retinal vascular digest preparations. Changes in the expression of VEGF and ICAM-1 were examined by immunofluorescence; and reactive oxygen species contents in the retinas were detected using dichlorofluorescein assay. Compared with normal rats, diabetic rats displayed significant retinopathy both under electronic and light microscopy, accompanied by elevated reactive oxygen species contents in the retinas; N-acetylcysteine treatment alleviated the pathological changes and also decreased reactive oxygen species, most significantly at 5 months. VEGF and ICAM-1 expressions were significantly up-regulated in retinal blood vessels from diabetic rats, and such up-regulation was attenuated by N-acetylcysteine treatment. The expression of both factors returned to basal levels after 5-month treatment with N-acetylcysteine. Long-term N-acetylcysteine treatment exerts protective effects on the diabetic retinas, possibly through its down-regulation of the expression of VEGF and ICAM-1, and reduction of reactive oxygen species content in retinal vascular tissues in diabetic rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) is the most common and serious microvascular complication of diabetes, and also a major cause of blindness in adults. The probability of blindness is positively correlated with duration of disease course [1]. It is a duration-dependent disease that develops in stages; the incidence of retinopathy is rarely detected in the first few years of diabetes, but the incidence increases to 50% by 10 years, and to 90% by 25 years of diabetes [2].
Although pathogenesis of DR has not been fully elucidated, it is believed to be related to synergistic effects of many factors. Recent studies have shown that reactive oxidative species (ROS) play the most critical role in the pathogenesis of DR [3], which is also closely related to other diabetic abnormalities such as dyslipidemia and hyperglycemia. When ROS exceeds the antioxidant capacity of the cell, excess ROS are capable of modifying both structure and function as a result of injury to lipids loss of membrane function and increased permeability, as well as modifying nucleic acids and proteins in DR [4, 5]. The retina has high content of polyunsaturated fatty acids and has the highest oxygen uptake and glucose oxidation relative to any other tissue. This phenomenon renders retina more susceptible to oxidative stress [3]. At the same time, other studies have shown that vascular endothelial growth factor (VEGF) plays a key role in diabetic microvascular complications by promoting retinal angiogenesis and increasing vascular permeability [6]. Oxidative stress is strongly associated with increased VEGF expression in investigations of ischemic retinopathy [7]. Studies on micro-circulation in diabetes research also showed that leukocyte aggregation caused by over-expression of intercellular adhesion molecule-1 (ICAM-1) is an important contributing factor to the damage of retinal blood–retinal barrier, loss of retinal vascular perfusion, and initiation of angiogenesis [8]. There is growing evidence that leukocyte adhesion plays an important role in the pathogenesis of DR. Adjacent to the static leukocytes were areas of endothelial cell damage, capillary loss, and leukocyte extravasations [9]. Therefore, increased levels of ICAM-1 and VEGF have been commonly used to assess the oxidative stress as well as retinal vascular injury in diabetic models. Recent studies showed that diabetes-induced retinal vascular dysfunction can be prevented by inhibitors of ROS, further supporting for the role of oxidative stress in DR [10].
N-Acetylcysteine has been widely used as an antioxidant in vivo and in vitro. It is the acetylated variant of the amino acid l-cysteine, is an excellent source of sulfhydryl (SH) groups, and is converted in the body into metabolites capable of stimulating glutathione (GSH) synthesis, promoting detoxification, and acting directly as free radical scavengers. Administration of NAC has historically been as a mucolytic agent in a variety of respiratory illnesses; however, it appears to also have beneficial effects in conditions characterized by decreased GSH or oxidative stress, such as HIV infection, cancer, heart disease, and cigarette smoking [11]. And the beneficial effects of NAC on long-term diabetes observed in protecting the glomeruli from diabetes-induced disorders [12]. Most importantly, NAC could reduce oxidative stress and vascular endothelial activation, in diabetic patients, which also are the key features of DR.
Therefore, this study aimed to examine the relationship of oxidative response and the expression of VEGF and ICAM-1, and explore the roles of these factors in the onset and development of DR. We established diabetic rat models and investigated how antioxidant N-acetylcysteine affected the morphology of and expression of VEGF and ICAM-1 expression in retinal blood vessels from these rats.
Materials and methods
Establishment of diabetic rat models
Healthy adult male Sprague-Dawley (SD) rats weighting 300–350 g were provided by the Experimental Animal Center, Medical School of Xi’an Jiaotong University. After fasting for 12 h, they were intraperitoneally injected with a dose of 1% streptozotocin (STZ) at 60 mg/kg in 0.1 mol/l citric acid buffers (pH 4.5). Rats injected with the same amount of citric acid buffer served as controls. 72 h after the injection, blood was drawn from the tail vein and blood glucose level was measured. The rats showing elevated blood glucose level ≥16.7 mmol/l were defined as diabetic model animals.
Animal grouping and treatments
Eighty rats were randomly divided into two normal control groups (group C, n = 10) to receive no treatment, two groups of diabetic rats (group D, n = 15) to receive STZ alone as described above, and two groups of diabetic rats to be further treated with NAC (group N, n = 15) by feeding with NAC (N) added into the drinking water at 1.4 g/kg. The weights of rats were measured regularly. Rats were given standard pellet feed, free access to drinking water, and raised under the natural light. Rats from one group in all three treatments were sacrificed at 3 months (MON3) and the others at 5 months (MON5) to be used for the following experiments.
Retinal sample preparation for transmission electron microscopy
Rats were anesthetized after intraperitoneal injection of 10% chloral hydrate, and their eyes were rapidly removed and immediately placed in ice-cold 2.5% glutaraldehyde. After fixing at 4°C for 2 h, eyes were cut open along the edge of the cornea, all the anterior segment tissues were removed, and the eye cup was placed back to the fixative and incubated overnight. Then the eye cup was cut with a razor blade centered at the optic nerve head to small tissue pieces of 1 mm × 1 mm, and placed back to the fixative for another 2 h. The fixed tissues were washed with 0.1 M phosphate buffer for 30 min, fixed in 1% osmium tetroxide at 4°C for 2 h, washed again with 0.1 M phosphate buffer for 10 min, dehydrated by a series of ethanol solutions with increasing concentrations, and stained with 70% ethanol uranyl acetate for 2 h. After that, tissues were treated sequentially with 90% ethanol twice, 10 min each, 100% ethanol for three times, 10 min each, and propylene oxide for 10 min once. They were then soaked and embedded in Epoxy EPON812 to allow polymerization of the resin. Then, tissue block was cut into 1–2 μm semi-thin sections. Tissues in the sections were first visualized by methylene blue staining and observation under a light microscope, and then selected sections were further processed to ultrathin sections of 50–70 nm with a Sweden LKB-V microtome. Ultrathin sections were stained with Uranyl acetate and lead citrate, observed and photographed under a Hitachi H-600 transmission electron microscope.
Fluorimetric determination of ROS levels in retinal tissues
ROS levels in retinal tissues were measured using a commercial ROS kit based on the traditional dichlorofluorescein assay. The key reagent was the fluorescin derivatives dichlorofluorescin, which is membrane permeable and nonfluorescent. After entering cells and being oxidized by various intracellular oxidants, it becomes dichlorofluorescein and emits fluorescence. By quantifying the fluorescence, the level of ROS generated in tissues under oxidative stress can be qualified.
Rats were anesthetized as described above; the eyes were removed and placed into pre-cooled PBS. Eyes were cut open along the limbus, and the anterior segment was removed. The retina was detached, and rinsed in pre-cooled cleaning solution. Cleaned retinas were homogenized in dilution buffer provided by in the kit, and protein content of the homogenate was determined. After incubation with the working solution for 20 min, ROS levels were determined using a fluorescent detection kit according to the manufacture’s instruction. Fluorescence intensities were measured by a spectrophotometer under excitation wavelength 490 nm and emission wavelength 520 nm. The increase in ROS level was calculated by relative fluorescence units (RFU) of a sample over that of the blank.
Retinal vascular digest preparations
Rats were anesthetized as described above; eyes were removed, fixed in ice-cold 4% formaldehyde for 48 h, and transferred into a dry Petri-dish. The anterior segment was removed and retina was isolated. The retina was divided into four portions centered at the optic nerve, rinsed with running water for 24 h, transferred with a pipette into a dry ampoule, and digested with 5 ml 3% trypsin in 0.1 mol/l Tris–Cl (pH 7.8) at 37°C for 1–2 h. The ampoule was gently shaken every 15 min. Retina was transferred into a Petri-dish with distilled water, subjected to repeated blowing and suction with a pipette until a transparent mesh of vessels was formed. Then the blood vessel mesh was rinsed and lifted onto a glass slide and dried naturally. Cares were taken to avoid folding of the tissues.
H&E staining of retinal vascular digest preparations
Retinal vascular digest preparations were placed in double distilled water for hydration for 1 min, stained with hematoxylin for 10 min, rinsed in distilled water for 2 min, stained with eosin for 2 min, washed with distilled water, and dried naturally. Slides were cleared with xylene and mounted with neutral gum. LEICA image signal acquisition and analysis system was used to analyze the stained blood vessels.
Immunofluorescence (IF) for VEGF and ICMA-1 expression in retinal vascular digest preparations
Retinal vascular digest preparations were treated with 0.3% TritonX-100 for 1 h, washed in PBS for 3 times, 3 min each, and blocked in normal goat serum for 1 h. After the serum was removed, the vessels were incubated with primary antibodies against VEGF and ICAM-1 (Mouse anti-rat VEGF monoclonal antibody, Santa Cruz, USA, and rabbit anti-mouse ICAM-1 polyclonal antibody, Beijing BIOSS Company) (diluted 1:50 into 0.01 M PBS) at 4°C overnight. After washing for 3 times in PBS, 5 min each, the liquid was removed with a piece of filter paper. In the dark room, 1–2 drops of secondary antibody conjugated with Goat anti-mouse IgG/fluorescent and goat anti-rabbit IgG diluted 1:100 in PBS was added to cover the tissue in the slide, and incubated in a dark box at 37°C for 1–2 h. After washing with PBS for 3 times, 5 min each, the excess amount of liquid was removed by a piece of filter paper, and the slide was washed again with distilled water for 2 min, mounted with buffered glycerol, and observed under a fluorescence microscope and photographed. Applied Medical Image Analysis System (provided by Xi’an Jiaotong University, Department of Pathology) was used to process the images. Fluorescence optical densities were obtained and semi-quantitative data were generated to present the expression levels of VEGF and ICAM in the retinal vascular preparations.
Statistical analysis
All data were subjected to statistical analyses with SPSS 13.0 statistical package, and were expressed as mean ± standard deviation (±SD). One-way ANOVA test was used to compare the differences among groups and LSD test was used for comparison among multiple groups. P < 0.05 was considered statistically significant.
Results
Ultrastructure of retinal cells by transmission electron microscopy
Under a transmission electron microscope, retinal tissues from control groups displayed the typical ultrastructures of normal vessels: thin vascular wall composed of flat endothelial cells with evenly distributed chromatins in the nuclei attached to the basement membrane (Fig. 1a). At 3 months, ultrastructures of retinas from D and N groups showed no significant differences. They both showed vascular wall thickening and irregular endothelial cells bulged into the lumen, but other pathological changes characteristic of DR were not prominent (Fig. 1b, c). At 5 months, more severe pathological changes in the retinal ultrastructures were seen as vessel blockage of, infiltration of inflammatory cells around vessels, large number of platelets aggregated inside the lumen causing occlusion of capillaries (Fig. 1d–g). However, rats in the N group showed much less pathological changes compared with these in the D group, which were mainly basement membrane thickening and vascular destabilization (Fig. 1h, i).
Ultrastructure of retinal cells by transmission electron microscopy. a Normal vessels in the control group consist of flat endothelial cells evenly distributed on the basement membrane (×6,000); b vessels from diabetic rats at 3 months have some narrowed area with bulged endothelial cells (×6,000); c vessels from N group at 3 months had some bulged endothelial cells and small amount of platelet aggregation (×6,000); d–g D group at 5 months had vessel blockage (d, ×3,000), infiltration of leukocytes around capillaries (e, ×5,000), larger number of platelets aggregated inside the lumen causing blockage (f, ×6,000) or occlusion of vessel and thickening of basement membrane (g, ×10,000); h, i N group at 5 months showed the flat endothelial cells, thickening of vascular basement membrane (h, ×6,000) with small amount of red blood cells (i, ×6,000)
Morphology of retinal vessels using retinal vascular digest preparations
Retinal vascular digest preparations were made from all the rat groups, and subjected to conventional H&E staining and microscopic observation. Normal rat retinal capillaries from the control groups were composed of pericytes and endothelial cells. The endothelial cells were elongated oral cells with lightly stained nuclei. The pericytes were small, round or triangle cells with heavily stained nuclei. The numbers of endothelial cells and pericytes was roughly 1:1 (Fig. 2a). At 3 months, vessels from D group showed decreased number of pericytes but increased endothelial cells, therefore the imbalanced ratio of endothelial cells and pericytes (Fig. 2b). Those from N group also displayed increased proliferation of endothelial cells but less extensive than in the D group (Fig. 2c). At 5 months, in vessels from diabetic rats, the decrease in pericytes and increase of endothelial cells were more pronounced, which was accompanied by the aggregation and adhesion of leukocytes. In the most severe cases, segments of capillary without associated pericytes were observed (Fig. 2d). In NAC treated rats, proliferation of endothelial cells was also significant but leukocyte aggregation was not significant and no such acellular capillary segment was seen (Fig. 1f).
Morphology of retinal vascular digest preparations. Retinal vascular digest preparations were made from all the rat groups, and subjected to conventional H&E staining and microscopic observation (×1,000). a Retinal capillaries in the control groups consist of endothelial cells (red triangle) and pericytes (black triangle); b D group at 3 months had decreased number of pericytes but increased endothelial cells; c N group at 3 months displayed less extensive increase of endothelial cells; d, e D group at 5 months showed adhesion of leukocytes in the vessels (arrows, d) and segments of acellular capillaries. (Color figure online)
ROS levels in the retina of diabetic rats
Compared with the CON group, ROS levels in retinal tissues from D group were significantly higher (*P < 0.01) at 3 months and further increased at 5 months. Retinal ROS levels in the N group were lower than those of the D group but the difference was not significant at 3 months (P = 0. 228) and became significant at 5 months (*P < 0.01). At 5 months, ROS levels in the N group was not significantly different than the control group (P = 0.094, Fig. 3).
Fluorimetric determination of ROS levels in retinal tissues (a 3 months data after NAC-treated; b 5 months data after NAC-treated). Data are presented as mean ± SD from 36 measurements shown in the table (upper) and a graphic view (lower). ROS level in the retina of diabetic rats. The data in the table is transformed to statistic demonstration as a and b
Immunofluorescence for expression of VEGF and ICAM-1 from retinal vessels
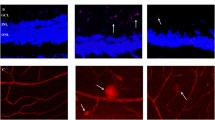
In normal control rats, the basal level expression of VEGF and ICAM-1 in retinal blood vessels were low, showing little positive staining by IF at both 3 and 5 months. At 3 months, VEGF expression in D group was significantly higher than both in CON and N groups (*P < 0.01). VEGF expression in the N group was lower than in the D group but still significantly higher than the CON group (*P < 0.01). At 5 months, VEGF expression in retinal vascular was further increased in D group (*P < 0.01) but the increase was attenuated in N group (*P < 0.01). At this later time point, the level of VEGF expression in N group showed no significant difference from that in CON group (P = 0.591) (Fig. 4).
Immunofluorescence staining for VEGF expression from retinal vessels (g 3 months data after NAC-treated; h 5 months data after NAC-treated). Upper panels: images of IF stained vessels in retinal vascular digest preparations (×1,000). a–c At 3 months, expression of VEGF was very low in normal rats (a), but significantly increased in D group (b) and also increased in N group less significantly (c); d–f at 5 months, VEGF expression in control vessels was almost undetectable (d), but further increased in D group (e); however, the increase was attenuated in N group (f). Middle panel: the table shows integrated optical density (IOD) from images in a–f. Lower panel: a graphic presentation of the IODs from above the table. Data in the table and the graphs are presented as mean ± SD from 30 images. The data in the table is transformed to statistic demonstration as g and h
At the same time, ICAM-1 expression showed similar patterns in response to different treatment at the two time points. Compared with that in the CON group, ICAM-1 expression increased significantly in D group at 3 months and further increased at 5 months (*P < 0.01 for both time points). Compared with the D group, ICAM expression in N group significantly decreased (*P < 0.01), which was still higher that that of the CON group (# P < 0.05) at 3 months, but then became insignificantly different from that in the CON group at 5 months (P = 0.94) (Fig. 5).
Immunofluorescence staining for ICAM-1 expression from retinal vessels. Upper panels: images of IF stained vessels in retinal vascular digest preparations (×1,000). a–c At 3 months, expression of ICAM-1 was very low in normal rats (a), but significantly increased in D group (b) and also increased in N group less significantly (c); d–f at 5 months, ICAM-1 expression in control vessels was almost undetectable (d), but further increased in D group (e); however, the increase was attenuated in N group (f). Middle panel: the table shows IOD from images in a–f. Lower panel: a graphic presentation of the IODs from above the table. Data in the table and the graphs are presented as mean ± SD from 30 images
Discussion
Morphologic changes of diabetic retinopathy
In this study, using STZ induced diabetic rat models and retinal vascular digest preparations, we documented pathological changes of the retinal blood vessels early in the development of DR. In contract to some previous experiments showing no significant morphological changes at 3 months [13], both transmission electron microscopy and HE staining revealed reduced pericytes and over proliferation of endothelial cells at 3 months after rats were treated with STZ. This observation suggests that the loss of pericytes is one of the early pathological features of retinal capillaries during diabetic microangiopathy [14]. Based on literature report [15], aldose reductase from vascular cells is only expressed in pericytes. High blood glucose can active the polyol pathway, causing the swelling of pericytes and ultimately cell damage and apoptosis. At the same time, we observed thickening of basement membrane, and deformation of endothelial cells breaking into the vessel lumen at 3 months. Basement membrane thickening is another earliest pathological feature in DR, which is related to VEGF-induced damages of tight junctions between endothelial cells, and activation of collagen through the PKC pathway [16].
In recent years, studies on micro-circulation in DR pathogenesis revealed that leukocyte adhesion and capillary occlusion are hallmarks of early microcirculation changes in DR [16]. Lin [13] studied vessel morphology during DR and identified leukocyte adhesion in vessels, retinal capillary occlusion, endothelial cell swelling, and infiltration of extravascular macrophages. Electron microscopic observation in this study also showed that the local vascular endothelial cells formed small protrusions inside the lumen in diabetic rats, therefore narrowing the vessels and even causing blockage. Perivascular infiltration of leukocytes, aggregation of a large number of platelets in the lumen, and adhesion of leukocytes inside the blood vessels were also seen. These are consistent with the results of previous studies. After feeding with STZ for 5 months, blocked blood vessels appeared in diabetic rats possibly as the results of combined effects of intrusion of deformed endothelial cells into the lumen, local stagnation of leukocytes, and platelet aggregation. White blood cells contribute to microcirculation disorder in DR through blocking retinal capillaries, resulting in retinal ischemia and hypoxia.
In summary, retinal ischemia and hypoxia, as well as abnormal endothelial cell metabolism can promote the expression of adhesion molecules and monocyte adhesion receptors at the membrane surface; together with changes in the local microcirculation rheology and dynamics, they all contributed to the onset and further development of DR.
In this study, based on electron microscopy and pathology examinations of the retinal vessels, we not only reported the retinopathy in diabetic rats, but also reported the morphological alleviation of retinal blood vessels in response to long-term intervention of antioxidant NAC in these rats. Antioxidant intervention significantly decreased the severity of pathological changes of retinal vascular tissues, such as leukocyte aggregation and capillary occlusion, and appearance of acellular capillaries. At 3 months, pathological changes was not significant between D and N groups but became significant at 5 months when D group showed pathological changes typical of DR but N group was similar to those in 3 months.
Oxidative stress and diabetic retinopathy
Currently, research has been focused more and more on the roles of oxidative response in the development of diabetic complications [17]. Diabetic patients show concurrent increase in plasma levels of oxidative products such as ROS and decreased antioxidants [17]. Persistent high blood sugar can increase oxidative stress, damage cell membrane integrity, and stimulate apoptosis, all leading to vascular endothelial damage and breakdown of blood–retinal barrier [18]. At the same time, oxidative stress can also induce expression of cytokines and adhesion molecules, promote the occurrence of microvascular diseases leading to retinopathy. Cross-talking and synergistic effects exist among several proposed mechanisms of diabetic complications. Oxidative stress responses accompany the production of advanced glycation end products (AGEs) and activation of polyol pathway, and also in turn lead to the activation of protein kinase C [19]. As the result, a theory on unified mechanism of chronic diabetic complications has been proposed. It explains that the four classical pathways leading to various diabetic complications are all essentially resulted from overly produced peroxides induced by high glucose; in this regard, oxidative stress is considered the key factor in the development of diabetic complications [19]. In the diabetic model rats, blood glucose elevation induced vascular endothelial cell dysfunction. Excessive production of oxygen free radicals such as O2 − and ONOO` reduced the effective circulating volume of the blood, accompanied with an increase of vascular permeability and protein leakage. All of these changes can increase VEGF, endothelial proliferation and retina angiogenesis. As a results, ROS and increased expression of VEGF and, ICAM-1 induced leukocyte adhesion in retinal vascular tissues. In our another in vitro study, Western Blot analysis showed that VEGF can induce ICAM-1 expression in retinal vascular endothelial cells, which is not affected by blocking NO or PKC, but blocking PI3K, or ROS can significantly reduce ICAM-1 expression [20].
In this study, we applied antioxidant N-acetylcysteine to diabetic rats and observed the changes in retinal tissue ROS and two important factors VEGF and ICAM-1 in response to 3–5 months treatment. At the end of 3 and 5 months treatment regimens, ROS contents increased in the retina of diabetic rats while NAC treatment significantly attenuated such increase of ROS levels, most significantly at 5 months. These observations are in agreement with results from Xi et al. [21] in their studies on the role of NAC in diabetic nephropathy. Immunofluorescence of retinal vascular digest preparations showed little vascular VEGF and ICAM-1 expression n normal rat retinas, which is consistent with immunohistochemistry results on the same tissue preparations. We further showed that retinal vascular VEGF and ICAM-1 expression was significantly increased in diabetic rats but this increase was attenuated by N-acetylcysteine treatment. The inhibitory effect of NAC on VEGF and ICAM expression was time-dependent, which is similar to its effects on ROS levels; and long term NAC treatment can bring these factors back to basal levels. These results suggest that NAC down-regulates the expression of VEGF and ICAM, possibly through inhibition of ROS production, and that the effect of NAC is persistent and most significant with long-term treatment. The changes at the molecular levels in retinal tissues are well-coordinated with the change in the retinal morphology and ultrastructures of retinal vessels. Central to the pathology of early DR are the increases in retinal ICAM-1 and neutrophil surface integrins. These molecules mediate leukocyte adhesion, a process that leads to blood–retinal barrier breakdown, capillary nonperfusion, and endothelial cell injury and death [22].
In conclusion, we demonstrated that antioxidant N-acetylcysteine decreased oxidative stress and attenuated diabetic vasculopathy. However, further investigation is needed to examine the dietary supplementation with such specific antioxidants may delay the clinical process of DR.
References
Miyamoto K, Khosrof S, Bursell SE et al (1999) Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci USA 96(19):10836–10841
Kowluru RA, Chan P-S (2007) Oxidative stress and diabetic retinopathy. Exp Diabetes Res 2007, Article ID: 43603
Kowluru RA, Chan P-S (2007) Oxidative stress and diabetic retinopathy. Exp Diabetes Res 2:1–12
Yang Y, Hayden MR, Sowers S et al (2010) Retinal redox stress and remodeling in cardiometabolic syndrome and diabetes. Oxid Med Cell Longev 3(6):392–403
Yao L, Romero MJ et al (2010) The role of RhoA/Rho kinase pathway in endothelial dysfunction. J Cardiovasc Dis Res 1(4):165–166
Talitha TR, Grammas P (2002) VEGF and VEGF receptor levels in retinal and brain-derived endothelial cells. Biochem Biophys Res Commun 293:710–713
Lee SG, Kim JL, Kee HL et al (2011) Simvastatin suppresses expression of angiogenic factors in the retinas of rats with streptozotocin-induced diabetes. Graefes Arch Clin Exp Ophthalmol 249:389–397
Hubbard A, Rothlein R (2000) Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med 28(9):1379–1386
Barouch FC, Miyamoto K, Jennifer R et al (2000) Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Investig Ophthalmol Vis Sci 41(5):1153
Sun J, Xu Y, Sun S et al (2010) Intermittent high glucose enhances cell proliferation and VEGF expression in retinal endothelial cells: the role of mitochondrial reactive oxygen species. Mol Cell Biochem 343:27–35
Kelly GS (1998) Clinical applications of N-acetylcysteine. Altern Med Rev J Clin Ther 3(2):114–127
Odetti P, Pesce C, Traverso N et al (2003) Comparative trial of N-acetyl-cysteine, taurine, and oxerutin on skin and kidney damage in long-term experimental diabetes. Diabetes 52(2):500–502
Lin P (2007) Atlas of experimental diabetes pathology. Beijing Science Press, Beijing, pp 123–124
Bai N-y, Lin X-m, Tang S-b et al (2003) The application of retinal digest preparations combined with immunofluorescence in the retinal vascular diseases. Chin J Misdiagn 13(7):977–979
Li S-l (1996) The morphologic changes of retinal blood vessel in rats with different courses of diabetes mellitus. Chin J Diabetes 4(1):13–17
Miyamoto K, Samer S et al (1999) Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci USA 9(96):10836–10841
Jakus V (2000) The role of free radicals, oxidative stress and antioxidant systems in diabetic vascular disease. Bratisl Lek Listy 101(10):541–551
Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18(2):4–25
Mamputu JC, Renier G (2004) Advanced glycation end-products increase monocyte adhesion to retinal endothelial cells through vascular endothelial growth factor-induced ICAM-1 expression: inhibitory effect of antioxidants. J Leukoc Biol 75(6):1062–1106
Zhang X-l, Liang W, Chen Y-j et al (2009) Vascular endothelial growth factor up-regulates the expression of intracellular adhesion molecule-1 in retinal endothelial cells via reactive oxygen species, but not nitric oxide. Chin Med J 122(3):338–343
Xi E, Liu T-q, Li J-j (2008) The effects of N-acetylcysteine on oxidative stress of the kidneys of diabetes rats. J Clin Med J China 15(6):843–844
Joussen AM, Poulaki V, Qin W et al (2002) Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol 160(2):506
Acknowledgments
This project was supported by a grant from the National Natural Science Foundation of China (No. 30571994).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Y., Zhang, XL., Zhu, BF. et al. Effect of antioxidant N-acetylcysteine on diabetic retinopathy and expression of VEGF and ICAM-1 from retinal blood vessels of diabetic rats. Mol Biol Rep 39, 3727–3735 (2012). https://doi.org/10.1007/s11033-011-1148-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1148-9