Abstract
Bacterial leaf streak of rice (BLS) caused by Xanthomonas oryzae pv. oryzicola (Xoc) is a widely-spread disease in the main rice-producing areas of the world. Investigating the genes that play roles in rice–Xoc interactions helps us to understand the defense signaling pathway in rice. Here we report a differentially expressed protein gene (DEPG1), which regulates susceptibility to BLS. DEPG1 is a nucleotide-binding site (NBS)-leucine rich repeat (LRR) gene, and the deduced protein sequence of DEPG1 has approximately 64% identity with that of the disease resistance gene Pi37. Phylogenetic analysis of DEPG1 and the 18 characterized NBS-LRR genes revealed that DEPG1 is more closely related to Pi37. DEPG1 protein is located to the cytoplasm, which was confirmed by transient expression of DEPG1-GFP (green fluorescent protein) fusion construct in onion epidermal cells. Semi-quantitative PCR assays showed that DEPG1 is widely expressed in rice, and is preferentially expressed in internodes, leaf blades, leaf sheaths and flag leaves. Observation of cross sections of leaves from the transgenic plants with a DEPG1-promoter::glucuronidase (GUS) fusion gene revealed that DEPG1 is also highly expressed in mesophyll tissues where Xoc mainly colonizes. Additionally, Xoc negatively regulates expression of DEPG1 at the early stage of the pathogen infection, and so do the three defense-signal compounds including salicylic acid (SA), methyl jasmonate (MeJA) and 1-aminocyclopropane-1-carboxylic-acid (ACC). Transgenic rice plants overexpressing DEPG1 exhibit enhanced susceptibility to Xoc compared to the wild-type controls. Moreover, enhanced susceptibility to Xoc may be mediated by inhibition of the expression of some SA biosynthesis-related genes and pathogenesis-related genes that may contribute to the disease resistance. Taken together, DEPG1 plays roles in the interactions between rice and BLS pathogen Xoc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is the staple food for over half the people in the world. Some devastative rice diseases, such as blast disease caused by fungal pathogen Magnaporthe grisea, bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae (Xoo) and bacterial leaf streak (BLS) caused by X. oryzae pv. oryzicola (Xoc), lead to the heavy losses of rice yield. Breeding disease-resistance varieties is an important strategy to control the diseases and to improve rice yield. To date, a number of rice blast resistance (R) genes and rice bacterial leaf blight R genes have been cloned and identified [1].Conversely, only a few quantitative trail locus (QTLs) have been reported to contribute to resistance to Xoc, but these QTLs remain to be characterized [1, 2]. In recent years, some defense-related genes have been reported to confer resistance to Xoc. For example, GH3-2 encoding an indole-3-acetic acid-amido synthetase mediates a broad-spectrum resistance to bacterial pathogens Xoo and Xoc [3]. Similarly, a pair of allelic WRKY genes that were designated as OsWRKY45-1 and OsWRKY45-2, have opposite functions in rice–bacteria interactions. OsWRKY45-1 negatively regulates resistance against Xoo and Xoc, while OsWRKY45-2 positively regulates the resistance [4]. Additionally, knock-out of OsMPK6 helps strengthen resistance to Xoo and Xoc [5]. Besides these genes, a non-host R gene Rxo1 that confers resistance to the maize bacterial stripe pathogen Burkholderia andropogonis, also regulates resistance against Xoc in rice [6]. These findings will help us to breed rice varieties with better resistance to Xoc; however, it seems that the explored genetic resources for controlling BLS of rice remain too limited to breed the rice varieties with excellent resistance to Xoc. Hence, more and continuous efforts should still be made to exploit the resistance-related genes for control of BLS.
Dissecting the molecular mechanism of the interactions between rice and Xoc, would be of great benefit in exploring the genes involved in defense response upon Xoc challenge, including the genes play pivotal roles in the defense network. Therefore, we carried out the corresponding work previously based on the above opinion. Some differentially expressed protein genes upon infection of the BLS pathogen Xoc, were obtained by analysis of rice proteomes using the technologies of two-dimensional gel electrophoresis and matrix assisted laser desorption ionization-time of flight-mass spectrometry (MALDI-TOF-MS). Among of these differentially expressed protein genes, there are several nucleotide binding site-leucine rich repeat (NBS-LRR) genes. It is reported that the NBS-LRR genes play roles in disease resistance and plant development. For example, lots of the rice blast R genes such as Pi-ta [7], Pib [8], Pi2/Piz-t [9], Pi9 [10], Pi37 [11], Pi36 [12], Pikm [13], Pit [14], Pid3 [15] and Pb1 [16], and the rice bacterial blight R gene Xa1 [17] are NBS-LRR genes. In addition, at least two studies reported that the NBS-LRR genes are involved in plant development. The NBS-LRR gene CSA1 along with other NBS-LRR genes including RPS4, contribute to the photomorphogenic development in Arabidopsis [18]. Similarly, antisense expression of a NBS-LRR sequence causes developmental abnormalities in transgenic sunflowers, and results in severe alteration in seed development of the transgenic tobacco plants and enhanced susceptibility to Phytophthora parasitic [19]. Some R genes cloned in the past few years contain two NBS-LRR gene members, no one of the two members could confer resistance independently, but do both members together. For instance, the rice blast R gene Pikm contains Pikm1-TS and Pikm2-TS, both members are required to confer Pikm-specific rice blast resistance, and no one could do that independently [13]. The other rice blast R gene Pik [20] functions in the same manner. These studies indicate that the NBS-LRR genes have diverse biological function and modes of action in plants. Thus, it would be interesting to investigate the function of the NBS-LRR genes, especially their roles in the interactions between host and pathogens.
As mentioned above, among our identified differentially expressed protein genes, there is a NBS-LRR gene designated as DEPG1. Proteomic analysis showed that the expression levels of the NBS-LRR protein DEPG1 decreased at 12 and 48 h post inoculation with Xoc as compared to 0 h post inoculation, implying that it may participate in the interaction between rice and Xoc. To investigate the role of DEPG1 in the rice–Xoc interactions, we examined its tissue-expression pattern and expression in response to Xoc and the defense-signal compounds, and assessed the resistance of the DEPG1-overexpressing rice plants to Xoc in this paper.
Materials and methods
Plant materials, growth conditions and treatments
The rice seeds of two rice varieties of Zhonghua11 and Nipponbare were germinated at 28°C, then the seedlings were cultured by aquaculture in the green house for 16 h light/8 h dark cycle at 28°C. Some seedlings at the three-leaf stage were transplanted to the soil in pots, moving to outdoors and maintaining their normal growth.
The seedlings of Zhonghua11 at the three-leaf stage were subjected to different treatments. The seedling leaves were sprayed with 2 mM salicylic acid (SA), 100 μM methyl jasmonate (MeJA), 2 mM 1-aminocyclopropane-1-carboxylic acid (ACC), respectively, while the seedlings treated with 0.1% ethanol solution were chosen as control. The leaves under these treatments were collected at different times after treatments (0, 0.5, 3, 6, 9, 12 and 24 h). The roots and leaves from the seedlings of Zhonghua11 at three-leaf stage, internodes, leaf sheaths, leaf blades, flag leaves, and inflorescences from the rice plants of Zhonghua11 at booting stage were harvested for tissue-specific expression analysis. All the collected samples were frozen in liquid nitrogen and kept at −80°C.
Pathogen inoculation
A virulent Xoc strain, RS105 [21] was used in this study. The isolate was incubated on nutrient agar adding 100 mg/l rifampicin at 28°C for 3 days. The inoculum was prepared by suspending the bacterial mass in sterile water containing 0.05% Tween 20 to a concentration of approximately 1 × 108 cells/ml. The fully expanded leaves at the booting stage were inoculated with the pathogen suspension or the sterile water containing 0.05% Tween 20 by a pinpricked method [22]. The tip, middle and end part of each leaf was pricked, respectively.
To analyze the expression of DEPG1 gene in Xoc-challenged rice, the fully expanded leaves at booting stage, which inoculated with the pathogen suspension or sterile water containing 0.05% Tween 20 (mock treatment), were collected at different time points (0–4, 6, 8, 10 days post inoculation), respectively. These collected samples were immediately frozen in liquid nitrogen, and stored at −80°C.
The disease level was scored by measuring lesion lengths at 2 weeks post inoculation. Statistic analysis of the lesion lengths was performed by using “one way ANOVA” program with Origin 8.0, and Tukey test was used for means comparison.
RNA extraction, cDNA synthesis and DNA preparation
Total RNA was extracted from the collected samples with the Trizol reagent (Invitrogen, USA) according to the manufacture’s protocol, and then was treated with RNase-free DNase I (TaKaRa, Japan) to remove the potential genomic DNA contamination. The first-strand cDNA was synthesized with PrimeScript® RT reagent Kit (TaKaRa, Japan) according to the manufacture’s protocol. Genomic DNA was isolated from rice seedlings using the cetyltrimethyl ammonium bromide (CTAB) method [23].
RNA expression analysis
Semi-quantitative reverse transcription PCR (RT-PCR) was performed using gene-specific primers (Table 1) to analyze tissue-specific expression of DEPG1. The expression level of Actin gene was used to standardize the RNA sample. For each gene, the semi-quantitative RT-PCR assays were repeated at least twice.
Quantitative real-time RT-PCR (qPCR) was conducted using SYBR® Premix Ex Taq ™ II (TaKaRa, Japan) and StepOne™ Real-Time PCR System (Applied Biosystems, USA) with the gene-specific primers (Table 2), some primers were described previously [4]. The expression level of Actin gene was used to standardize the RNA sample for each qPCR. For each gene, the qPCR assays were repeated at least twice, with each repetition having three replicates. Standard deviation was calculated for technical replicates.
Construction of plant expression vectors and transformation of rice
A 3,368 bp DNA including the open reading frame (ORF) of DEPG1 was obtained by PCR amplification with the specific primers DEPG1F and DEPG1R (Table 1), using the DEPG1 cDNA clone (http://cdna01.dna.affrc.go.jp/cDNA/, clone ID: 215008) as template. The PCR product was fused with 2× CaMV (cauliflower mosaic virus) 35S promoter, a translation enhancer and a napoline synthase polyadenylation signal (NOS) terminator to form an overexpression cassette, the cassette was inserted into the multiple cloning sites of pCAMBIA1301 vector. Then, the constructed overexpression vector of DEPG1 was introduced into Agrobacterium tumfaciens strain EHA105 by the freeze–thaw method [24].
For a green fluorescent protein (GFP) fusion expression construct, the ORF of DEPG1 without the termination codon was obtained by PCR amplification using specific primers DEGFPF and DEGFPR (Table 1). The PCR products were cloned into pENTR™/D-TOPO® vector (Invitrogen, USA), and then fused into the vector pMDC85 [25] by performing a recombination reaction using Gateway LR Clonase II Enzyme Mix (Invitrogen, USA). The target DNA of DEPG1 was fused to 5′-terminus of the GFP gene and under the control of 35S promoter in this construct. The plasmids of the fusion construct 35S::DEPG1-GFP and the empty vector 35S::GFP were introduced into A. tumfaciens strain EHA105 by the method described above, respectively.
To construct a promoter-glucuronidase (GUS) expression vector, a 1,277 bp DNA with the promoter region of DEPG1 gene was amplified by PCR with the specific primers DEPROF and DEPROR (Table 1), using the genomic DNA of Nipponbare plants as template. The PCR products were cloned into pENTR™/D-TOPO® vector, and then fused into the vector pMDC164 [25] by the method used for a GFP fusion expression construct described above. The combination construct P DEPG1 ::GUS containing the promoter of DEPG1 gene and gusA gene was introduced into A. tumfaciens strain EHA105 as described above.
Agrobacterium-mediated transformation was performed using calli derived from mature embryos of japonica variety Zhonghua11 or Nipponbare according to a published protocol [26].
Subcellular localization of DEPG1
The transient transformation of onion epidermal cells was carried out by Agrobacterium-mediation method as described by Yuan et al. [27] with the positive clones of A. tumfaciens carrying the fusion construct 35S::DEPG1-GFP and the empty construct 35S::GFP, respectively. The transformed onion epidermal cells were plasmolyzed by treating with 1 M sucrose solution for about a half hour before observation. Expression of the genes transformed into the onion epidermal cells was observed using confocal laser scanning microscope (LSM 510 META, ZEISS, Germany).
Sequences analysis
The structure of DEPG1 was analyzed by comparing the genomic DNA (GenBank accession number AC135927) and complementary DNA (GenBank accession number AK120289) sequences, and sequence alignment was done using the “MegAlign” program with DNASTAR version 7.0. Additionally, a BLAST search was performed using the deduced protein sequence of DEPG1 by the program “protein blast” online (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The online tool PSORT program (http://wolfpsort.org) was used to predicate the localization of DEPG1 protein. The phylogenetic tree of DEPG1 protein and the other 18 characterized NBS-LRR R proteins was constructed using the program with MEGA version 4 [28].
Results
Molecular characterization of DEPG1 gene
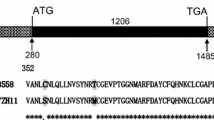
Comparative analysis of the genomic and complement DNA sequences of DEPG1 gene showed that it contains a 2,631 bp coding region, interrupted by two introns and flanked by a 499 bp 5′ and a 1,780 bp 3′ untranslated region (UTR). The first intron is located within the 5′ UTR and is 140 bp in length, while the second intron is positioned within the 3′ UTR, and is 121 bp in length. The transcript length of DEPG1 is 4,910 bp (Fig. 1). The ORF of DEPG1 gene encodes an 876-residue polypeptide with an estimated molecular mass of 100.3 kDa, and a calculated isoelectric point of 7.21. The N-terminal section of DEPG1 protein contains three typical NBS family motifs [11]. The GGMGKS sequence (beginning at residue 222) corresponds to the kinase 1a (P-loop) consensus, while the LLVLDDI (beginning at residue 297) and GATKVLVTSRS (beginning at residue 327) conform to the kinase 2, and the kinase 3a consensus motifs [11, 29], respectively. The C-terminal region of the protein is composed of about 10 irregular LRRs between residues 592 and 876 (Fig. 2). Additionally, a BLAST search showed that the deduced DEPG1 protein sequence shares about 64% identity with that of the rice blast R protein Pi37.
The rice blast resistance (R) genes such as Pi37, Pib, Pita, Pi9, Pi2, Piz-t, Pi36, Pb1, Pikm1, Pikm2-TS and rice bacterial blight R gene Xa1 are all NBS-LRR genes. Besides these genes, maize rust R gene rp1-dp3 [30], maize stripe disease R gene Rxo1, barley rust R gene Mla1 and Mla7 [31, 32], and the R genes N, RPP5 and L6 from the dicotyledonous species [33–35] also belong to this type. Therefore, the deduced amino acid sequences of these R genes were included in the phylogenetic analysis. Altogether, these 18 R proteins and DEPG1 can be clustered into 14 clades: Mla1 and Mla6 (I), Pi36 (II), Pikm-1 (III), Pita (IV), Pikm-2TS (V), Pib (VI), Piz-t, Pi2 and Pi9 (VII), Pb1 (VIII), Rxo1 (IX), Xa1 (X), rp1-kp3 (XI), DEPG1 and Pi37 (XII), L6 (XIII), and N and RPP5 (XIV) (Fig. 3). This analysis suggests that DEPG1 is more closely related to Pi37 than to any other characterized R genes.
Phylogenetic analysis of DEPG1 with the 18 NBS-LRR R genes based on their amino acid sequences. Numbers on the branches indicate bootstrap percentages. GenBank accession numbers of these proteins sequences were listed in brackets behind their names. The unit branch length is equivalent to 0.2 amino acid residue substitutions per site, as indicated by the bar in the lower left corner
Tissue-specific expression of DEPG1
The expression profile of DEPG1 in different rice organs and tissues detected by semi-quantitative RT-PCR showed that DEPG1 gene is predominantly expressed in internodes, leaf blades, flag leaves and leaf sheaths from the plants at booting stage. A basal level of expression was also detected in roots and leaves of the seedlings at the three-leaf stage, roots and inflorescences of the plants at booting stage (Fig. 4). The basal expression level in inflorescences was lower than that in roots or leaves of the seedlings. These data indicated that expression of DEPG1 is developmentally regulated, and is in a tissue and organ preferential manner.
Tissue-specific expression profiles of DEPG1 in rice. Total RNA was extracted from roots (YR) and leaf blades (SH) of the seedlings, roots (R), internodes (IN), leaf sheaths (LS), leaf blades (LB), flag leaves (FL) and inflorescences (IF) of the plants at booting stage, respectively. The Actin gene was used as the standard control to show the normalization of the amount of the templates in semi-RT-PCR reactions
The expression of the DEPG1-promoter (P DEPG1 ) and gusA (GUS) fusion gene(P DEPG1 ::GUS) in T1 transgenic plants revealed that DEPG1 is expressed in all tissues and organs of rice plants, including internodes, leaf blades, leaf sheaths, flag leaves and inflorescences of plants at booting stage and the seedlings (Fig. 5a–f). Observation of cross sections of leaves from the transgenic plant with P DEPG1 ::GUS fusion gene, we found that DEPG1 is highly expressed in the mesophyll cells, but is expressed at a relatively low level in the parenchyma cells and the vascular vessels (Fig. 5g). These results indicated that the expression of DEPG1 is of tissue preference.
Histological analysis of DEPG1 expression in transgenic plants. The tissues and organs including leaf sheaths (a), leaf blade (b), flag leaves (c), internodes (d), inflorescences (e), seedling (f) and cross section of leaves (g) from T1 P DEPG1 ::GUS transgenic plant were subjected to histological GUS analysis; M mesophyll cells; V vascular bundles; P parenchyma cells. Because similar expression profiles were obtained in three independent transgenic lines, the data from a representative line is shown
The expression of the six pathogenesis-related genes and DEPG1 in response to Xoc
Some pathogenesis-related genes (PR genes) are considered the marker genes of the defense signals such as SA and jasmonic acid (JA) signaling pathway, and inducible expression of a set of the PR genes is associated with activation of defense responses [36–38]. To determine whether the defense response is activated during Xoc attack, we examined the expression patterns of the six PR genes including PR1a, PR1#12, PR1b [36], PR10/PBZ1 [37, 39], PR5 [40], and RSPR10 [41] in rice plants of Zhonghua11 upon Xoc infection, respectively. The expression levels of the six PR genes in the leaves upon Xoc infection were higher than that of the leaves with mock treatment at the early stage of Xoc infection (1–2 days post inoculation, DPI) (Fig. 6c–h). The transcript levels of the PR genes in the leaves upon Xoc infection or with mock treatment decreased gradually over time (from 3 to 10 DPI). These results indicated that Xoc infection activates the defense response and induces the expression of the PR genes at the early stage; the defense response weakens and the expression of the PR genes recovers to the normal levels gradually over time.
The expression profiles of DEPG1 and the six pathogenesis-related genes upon Xoc challenge. The leaves inoculated with the pathogen Xoc RS105 strain (RS105) or inoculated with sterile water (Mock) were collected at different days post inoculation, respectively. RNA extracted from these samples was used for the quantitative real-time RT-PCR (qPCR) analysis. a and b The expression of DEPG1 in rice plants of Zhonghua11 and Nipponbare, respectively. c–h The expression of the six pathogenesis-related genes including PR1a, PR1#12, PBZ1/PR10, PR5, RSPR10 and PR1b in Zhonghua11 plants upon Xoc challenge. “0” represents before inoculation; “1–4, 6, 8 and 10” represents the corresponding days post inoculation. Bars represent mean (3 replicates) ± standard deviation. “*” and “**” Indicate a significant (t test, P < 0.05) and an extremely significant (t test, P < 0.01) differences detected in the average relative expression levels between Mock and Xoc-inoculation treatments, respectively
To understand whether DEPG1 is involved in the defense response triggered by Xoc, the expression patterns of DEPG1 in rice plants of Zhonghua11 and Nipponbare upon Xoc challenge were examined by qPCR. As shown in Fig. 6, the expression level of DEPG1 was lower in rice leaves of Zhonghua11 and Nipponbare inoculated with Xoc than that in leaves inoculated with water at 1 and 2 DPI. The expression of DEPG1 was induced at 6 and 10 DPI in rice plants of Zhonghua11, and at 4 and 8 DPI in rice plants of Nipponbare. In addition, the expression level of DEPG1 in leaves upon Xoc infection was lower than or comparative with that in leaves under mock treatment at the other marked time points. These data indicated that the expression of DEPG1 is suppressed at the early stage of Xoc infection (1 and 2 DPI) when the defense response is activated, and then varies with the weakened defense response over time.
The expression profiles of DEPG1 in response to the defense-signal compounds
Several phytohormones such as SA, JA and ethylene (ET), are implicated in complex signaling pathway and play pivotal roles in plant defense responses [42]. The expression pattern of DEPG1 at the transcript level in rice seedlings under the treatments of the three defense-signal compounds SA, MeJA (methyl ester of JA) and ACC (precursor of ET synthesis) was examined by qPCR to assess their effects on the expression of DEPG1. The transcript level of DEPG1 decreased quickly within 0.5 h after SA treatment, reduced to the lowest level at 12 h after treatment (HAT) and maintained a relatively low level at 24 HAT (Fig. 7a). The expression of DEPG1 was also repressed by MeJA at 0.5 HAT and maintained a relatively low level at 24 HAT (Fig. 7b). Additionally, the expression of DEPG1 in leaves with the ACC treatment was similar to that with MeJA and SA treatments (Fig. 7c). In summary, the three defense-signal compounds down regulated expression of DEPG1.
The expression profiles of DEPG1 in rice seedlings treated by the defense-signal compounds SA, JA and ACC. The seedlings of Zhonghua11 at three-leaf stage were sprayed with the defense-signal compounds 2 mM SA, 100 μM MeJA and 2 mM ACC, respectively. Total RNA extracted from the leaves collected at the successive time points after treatments was used for the qPCR analysis. Bars represent mean (3 replicates) ± standard deviation. “*” and “**” Indicate a significant (t test, P < 0.05) and an extremely significant (t test, P < 0.01) differences detected in the average relative expression levels between Mock and the different defense-signal compounds treatments, respectively
Subcellular localization of DEPG1 protein in the onion epidermal cells
DEPG1 protein was predicted to be localized in the cytoplasm by PSORT program due to the absence of the putative nuclear localization signal (PKRR) and signal peptide. To confirm the subcellular localization of DEPG1 protein, the DEPG1-GFP fusion gene and the gene encoding GFP were transiently expressed in onion epidermal cells, respectively. The fluorescence due to GFP in onion epidermal cells was monitored. As shown in Fig. 8, fluorescence signals due to DEPG1-GFP can be detected in the cytoplasm (Fig. 8a–c), while signals due to GFP can be detected in the cytoplasm and the nucleus (Fig. 8d–f). These results suggested that DEPG1 is a cytoplasm protein.
Subcellular location of DEPG1 protein in onion epidermal cells. Onion epidermal cells were transformed with vector carrying 35S::DEPG1-GFP or 35S::GFP by Agrobacterium-mediated transformation and plasmolyzed by treating with 1 M sucrose. a and d Bright-field transmission images; b expression of DEPG1-GFP in the cytoplasm; c transmission images overlaying a and b; e expression of GFP (control) in the cytoplasm and the nucleus; f transmission images overlaying d and e. Scale bars 150 μm
Overexpression of DEPG1 increases susceptibility to Xoc in transgenic plants
We over expressed DEPG1 in rice then examined resistance of the transgenic plants to Xoc to determine the roles of DEPG1 in rice–Xoc interactions. DEPG1 driven by a double CaMV 35S promoter was transformed into the susceptible rice variety Zhonghua11. Six independent transformants were obtained, and the expression of DEPG1 was significantly increased in two of them (data not shown). Six T1 transgenic plants from these transformants were inoculated with Xoc at booting stage. All of them showed increased expression of DEPG1, and the relative expression level in four of them was over 5 times that in wild-type plants (Fig. 9a). Moreover, the average lesion lengths of these transgenic plants were ranging from 6.4 to 9.6 mm, versus 5.1 mm for wild-type plants (Fig. 9b); the average lesion length of all six DEPG1-overexpressing transgenic plants (8.2 mm) was significantly (t test, P < 0.01) longer than that of wild-type plants (5.1 mm) (Fig. 9c). Therefore, these results suggested susceptibility to Xoc is enhanced in DEPG1-overexpressing transgenic plants.
The expression level of DEPG1 and performance to Xoc RS105 strain of DEPG1-overexpresssing T1 plants. a and b represent the relative expression levels of DEPG1 and average lesion lengths of the six different DEPG1-overexpresssing T1 plants, respectively; c the average lesion lengths of all the six DEPG1-overexpressing T1 plants(OE) and wild-type plants (WT). Bars represent mean ± standard deviation. “**” Indicates that an extremely significant difference (t test, P < 0.01) detected in the lesion lengths between wild type and transgenic plants
The DEPG1-GFP fusion gene was transformed into the susceptible rice variety Nipponbare, and 13 independent transformants were obtained. Ten of the 13 transformants showed significantly increased expression of DEPG1, and their relative expression level was above 10 times that of wild-type plants (Fig. 10a). Additionally, the average lesion lengths of the 13 transformants were ranging from 5.5 to 7.8 mm versus 5.1 mm for wild-type plants (Fig. 10b); the average lesion length of all 13 DEPG1-overexpressing plants (6.7 mm) was significantly (P < 0.01) longer than that of wild-type plants (5.1 mm) (Fig. 10c). These results indicated that susceptibility of DEPG1-GFP transgenic plants to Xoc is increased. The similar results were also observed in T1 DEPG1-GFP transgenic plants. The relative expression levels of all nine T1 DEPG1-GFP transgenic plants were over 10 times that of wild-type plants (Fig. 10d). More, the average lesion lengths of the nine T1 DEPG1-GFP transgenic plants were ranging from 8.5 to 12.1 mm versus 7.5 mm for wild-type plants (Fig. 10e); the average lesion length of all nine T1 plants (10.2 mm) was significantly (t test, P < 0.01) longer than that of wild-type plants (7.5 mm) (Fig. 10f). Taken together, all results showed that enhanced susceptibility to Xoc is associated with overexpression of DEPG1 in transgenic plants.
The expression level in DEPG1 and performance to Xoc RS105 strain of the T0 and T1 transgenic plants containing the DEPG1-GFP fusion gene. a and b Denote the relative expression levels of DEPG1 and the average lesion lengths of the 13 different T0 transgenic plants containing the DEPG1-GFP fusion gene, respectively; c the average lesion lengths of all the 13 T0 transgenic plants (DEGFP) and the wild type plants(WT). d and e Refer to the relative expression levels of DEPG1 and the average lesion lengths of the nine different T1 transgenic plants containing the DEPG1-GFP fusion gene, respectively; f the average lesion lengths of all the nine T1 transgenic plants (DEGFP1) and wild-type plants(WT). Bars represent mean ± standard deviation. “*” and “**” Indicate a significant (t test, P < 0.05) and an extremely significant (t test, P < 0.01) differences detected in the lesion lengths between wild-type and transgenic plants, respectively
Expression analysis of defense-related genes in the DEPG1-overexpressing transgenic plants
Lots of evidences support that SA plays key roles in disease resistance of monocotyledonous plants [43], and some PR genes are reported to contribute to the disease resistance [44]. To analyze the molecular mechanisms by which the DEPG1-overexpressing transgenic plants enhance susceptibility to Xoc, the expression of SA biosynthesis-related genes [38] including PAD4, PAL1 and ICS, and some PR genes including PR1a, PR1#12, PR5, PBZ1 and PR10, was examined by qRT-PCR. As shown in Fig. 11, the expression of ICS and PR1#12 was significantly (t test, P < 0.01) down regulated, and the transcription of PAD4 and PBZ1 was repressed in both DEPG1-overexpressing transgenic plants, compared to wild-type plants. Besides, the expression of PAD4 and PR10 was comparable with wild-type plants, while PAL1 and the other PR genes PR1a and PR5 were differently expressed in the two examined DEPG1-overexpressing transgenic plants, compared to wild-type plants. In summary, the expression of SA biosynthesis-related genes ICS and PAD4, and the PR genes PR1#12 and PBZ1 was down regulated, while the expression of the other defense-related genes was in distinct ways in the two DEPG-overexpressing transgenic plants.
Expression analysis of defense-related genes in DEPG1-overexpressing transgenic plants. RNA from the Xoc-inoculated leaves of transgenic plants and wild-type plants was used for expression analysis of defense-related genes; WT wild-type plants; 1-3 and 4-1 the two different DEPG1-overexpressing transgenic plants; “*” and “**” Indicate a significant (t test, P < 0.05) and extremely significant (t test, P < 0.01) difference detected in the average relative expression levels between wild-type and transgenic plants, respectively
Discussion
The NBS-LRR genes play pivotal roles in disease resistance [45, 46] and plant development [18, 19]. There are about 480 NBS-LRR genes in the rice genome of Nipponbare [47], and only a small number of NBS-LRR genes have been characterized and identified to mediate disease resistance [1]. Moreover, most of these known NBS-LRR genes confer resistance to the rice blast pathogen M. grisea or bacterial leaf blight pathogen Xoo. To our knowledge, the NBS-LRR genes that participate in the interaction between rice and Xoc have not been reported until now.
DEPG1 is a differentially expressed protein gene obtained by analysis of the proteomes from the rice leaves upon Xoc challenge in our previous studies (unpublished). The deduced DEPG1 protein contains a NBS and several LRR in carboxyl terminal (Fig. 2), and has approximately 64% sequence identity with the rice blast resistance gene Pi37 product. Additionally, phylogenic analysis revealed that DEPG1 is more closely related to Pi37 than to the other 17 characterized NBS-LRR genes (Fig. 3). DEPG1 protein is located in the cytoplasm, which is consistent with that of most NBS-LRR R protein including Pi37 (Fig. 8). Taken together, all these results suggest that DEPG1 is a NBS-LRR gene, and is highly similar to the rice blast R gene Pi37, and may play roles in rice–Xoc interactions.
To determine whether DEPG1 gene takes part in the interactions between rice and Xoc, we evaluated resistance of DEPG1-overexpressing plants to Xoc, and observed that both DEPG1 and DEPG1-GFP overexpressing plants show enhanced susceptibility to Xoc (Figs. 9, 10). These results suggest that DEPG1 positively regulates susceptibility to Xoc. Based on these results, we speculated that DEPG1 participates in interaction between rice and Xoc.
The activation of defense response always accompanies with induced expression of the pathogenesis-related genes such as PR1a, PR1b, and PR10/PBZ1 [36, 38]. The six PR genes (including PR1a, PR1b, PR10/PBZ1, PR5, RSPR10 and PR1#12) were induced in Xoc-inoculated rice at the first 2 days post inoculation (Fig. 6), implying that the defense response is activated at this stage. By contrast, the expression of DEPG1 in both rice plants of Zhonghua11 and Nipponbare was repressed at both 1 DPI and 2 DPI. These results suggested that DEPG1 is repressed in the defense response triggered by Xoc. The reduced expression of DEPG1 in defense response was further confirmed by analysis of the expression of DEPG1 in rice seedlings treated with the three defense-signal compounds including SA, MeJA and ACC (Fig. 7). Thus, we concluded that DEPG1 participates in the defense response triggered by Xoc through down regulating its expression.
The expression profile of DEPG1 in rice organs and tissues revealed that the expression of DEPG1 is of tissue preference, the high expression level appeared in internodes, leaf sheaths, leaf blades and flag leaves (Fig. 4). Moreover, DEPG1 was also highly expressed in the mesophyll tissues (Fig. 5g). In addition, the BLS pathogen Xoc principally infects the leaves of rice, and mainly colonizes the mesophyll tissues of rice leaves [48]. Thus, it seems that the high expression of DEPG1 in the mesophyll tissues may help rice cells to detect the effectors of the pathogen Xoc. Since the disease resistance genes belonged to the NBS-LRR gene family are thought to take part in detecting the pathogens by interacting with the effectors of the pathogens directly or through monitoring the host proteins modified by the effectors [46]. If DEPG1 functioned as the other known R gene that belonged to NBS-LRR genes in rice–Xoc interactions, the resistance phenotype would be observed. However, susceptibility to Xoc was observed in rice leaves of Nipponbare in which DEPG1 is expressed at a high level. Therefore, we inferred that DEPG1 functions in a different way. The bacterial pathogen always injects various effectors into plant cells during pathogenesis to overcome the host plant [48]. Thus, it is possible that DEPG1 product may be the targets of the effectors of the BLS pathogen Xoc, and high level of DEPG1 products in mesophyll cells may provide the pathogen effectors with enough targets, and advance colonization of Xoc. In current study, enhanced susceptibility of DEPG1-overexpressing plants to Xoc (Figs. 9, 10) supports this assumption well. In summary, the bacterial pathogen Xoc preferentially colonizes in the mesophyll tissues where DEPG1 is expressed at a high level, supporting that DEPG1 is involved in interactions between rice and Xoc.
Zhou et al. [22] reported that the non-host resistance gene Rxo1 that mediates resistance to Xoc, could specifically activate several defense pathways such as SA and ET-dependent pathways and lots of genes involved in signaling pathways leading to hypersensitivity reaction during rice and Xoc interactions. Similarly, some other researches reported enhanced resistance to bacterial blight accompanies with SA accumulation and activation of several defense related genes including pathogenesis-related genes [3–5, 38]. In this study, overexpressing DEPG1 increases susceptibility to Xoc, is the change in the expression of the components involved in SA defense signaling pathway responsible for enhanced susceptibility to Xoc? To answer this question, we examined the expression of several SA biosynthesis-related genes and some PR genes, and found that the expression of the SA biosynthesis-related genes ICS and PAD4, and the PR genes PR1#12 and PBZ1 was down regulated. Thus, inhibition of the SA biosynthesis pathway together with some PR genes might partially explain enhanced susceptibility to Xoc. However, whether the expression of the components involved in other defense signaling pathways (such as JA/ET signaling pathways) is altered in the DEPG1-overexpressing transgenic plants, and whether overexpression of DEPG1 induces silence of its highly similar NBS-LRR genes that may contribute to the disease resistance, remain to be determined.
Due to the facts that DEPG1 responds to defense signaling compounds and BLS pathogen Xoc, and enhanced susceptibility of the DEPG-overexpressing plants to Xoc, DEPG1 might play pivotal roles in the rice–Xoc interactions. In addition, Xoc preferentially colonizes in mesophyll tissues where DEPG1 is highly expressed, which may support that DEPG1 plays roles in rice–Xoc interactions. For further study, the detailed molecular mechanism of susceptibility of the DEPG1-overexpressing plants to Xoc and assessment of resistance of the DEPG1-suppressing plants or knockout plants to Xoc will be achieved to further dissect the role of DEPG1 in rice–Xoc interactions.
Abbreviations
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- CTAB:
-
Cetyltrimethyl ammonium bromide
- ET:
-
Ethylene
- GFP:
-
Green fluorescent protein
- JA:
-
Jasmonic acid
- MeJA:
-
Methyl jasmonate
- MALDI-TOF-MS:
-
Matrix assisted laser desorption ionization-time of flight-mass spectrometry
- NBS-LRR:
-
Nucleotide binding site-leucine rich repeat
- ORF:
-
Open reading frame
- QTL:
-
Quantitative trait locus
- RT-PCR:
-
Reverse transcription PCR
- SA:
-
Salicylic acid
- Xoo:
-
Xanthomonas oryzae pv. oryzae
- Xoc:
-
Xanthomonas oryzae pv. oryzicola
References
Dai LY, Liu XL, Xiao YH, Wang GL (2007) Recent advances in cloning and characterization of disease resistance genes in rice. J Integr Plant Biol 49(1):112–119. doi:10.1111/j.1672-9072.2007.00413.x
Tang D, Wu W, Li W, Lu H, Worland AJ (2000) Mapping of QTLs conferring resistance to bacterial leaf streak in rice. Theor Appl Genet 101(1):286–291. doi:10.1007/s001220051481
Fu J, Liu H, Li Y, Yu H, Li X, Xiao J, Wang S (2010) Manipulating broad-spectrum disease resistance by suppressing pathogen-induced auxin accumulation in rice. Plant Physiol. doi:10.1104/pp.110.163774
Tao Z, Liu H, Qiu D, Zhou Y, Li X, Xu C, Wang S (2009) A pair of allelic WRKY genes play opposite roles in rice–bacteria interactions. Plant Physiol 151(2):936–948. doi:10.1104/pp.109.145623
Shen X, Yuan B, Liu H, Li X, Xu C, Wang S (2010) Opposite functions of a rice mitogen-activated protein kinase during the process of resistance against Xanthomonas oryzae. Plant J 64(1):86–99. doi:10.1111/j.1365-313X.2010.04306.x
Zhao B, Lin X, Poland J, Trick H, Leach J, Hulbert S (2005) A maize resistance gene functions against bacterial streak disease in rice. Proc Natl Acad Sci USA 102(43):15383–15388. doi:10.1073/pnas.0503023102
Bryan GT, Wu KS, Farrall L, Jia Y, Hershey HP, McAdams SA, Faulk KN, Donaldson GK, Tarchini R, Valent B (2000) tA single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 12(11):2033–2046
Wang ZX, Yano M, Yamanouchi U, Iwamoto M, Monna L, Hayasaka H, Katayose Y, Sasaki T (1999) The Pib gene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes. Plant J 19(1):55–64. doi:10.1046/j.1365-313X.1999.00498.x
Zhou B, Qu SH, Liu GF, Dolan M, Sakai H, Lu GD, Bellizzi M, Wang GL (2006) The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol Plant Microbe Interact 19(11):1216–1228. doi:10.1094/Mpmi-19-1216
Qu SH, Liu GF, Zhou B, Bellizzi M, Zeng LR, Dai LY, Han B, Wang GL (2006) The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 172(3):1901–1914. doi:10.1534/genetics.105.044891
Lin F, Chen S, Que ZQ, Wang L, Liu XQ, Pan QH (2007) The blast resistance gene Pi37 encodes a nucleotide binding site-leucine-rich repeat protein and is a member of a resistance gene cluster on rice chromosome 1. Genetics 177(3):1871–1880. doi:10.1534/genetics.107.080648
Liu X, Lin F, Wang L, Pan Q (2007) The in silico map-based cloning of Pi36, a rice coiled-coil nucleotide-binding site leucine-rich repeat gene that confers race-specific resistance to the blast fungus. Genetics 176(4):2541–2549. doi:10.1534/genetics.107.075465
Ashikawa I, Hayashi N, Yamane H, Kanamori H, Wu J, Matsumoto T, Ono K, Yano M (2008) Two adjacent nucleotide-binding site-leucine-rich repeat class genes are required to confer Pikm-specific rice blast resistance. Genetics 180(4):2267–2276. doi:genetics.108.095034
Hayashi K, Yasuda N, Fujita Y, Koizumi S, Yoshida H (2010) Identification of the blast resistance gene Pit in rice cultivars using functional markers. Theor Appl Genet 121(7):1357–1367. doi:10.1007/s00122-010-1393-7
Shang J, Tao Y, Chen X, Zou Y, Lei C, Wang J, Li X, Zhao X, Zhang M, Lu Z, Xu J, Cheng Z, Wan J, Zhu L (2009) Identification of a new rice blast resistance gene, Pid3, by genome wide comparison of paired nucleotide-binding site—leucine-rich repeat genes and their pseudogene alleles between the two sequenced rice genomes. Genetics 182(4):1303–1311. doi:10.1534/genetics.109.102871
Hayashi N, Inoue H, Kato T, Funao T, Shirota M, Shimizu T, Kanamori H, Yamane H, Hayano-Saito Y, Matsumoto T, Yano M, Takatsuji H (2010) Durable panicle blast-resistance gene Pb1 encodes an atypical CC-NBS-LRR protein and was generated by acquiring a promoter through local genome duplication. Plant J 64(3):498–510. doi:10.1111/j.1365-313X.2010.04348.x
Yoshimura S, Yamanouchi U, Katayose Y, Toki S, Wang ZX, Kono I, Kurata N, Yano M, Iwata N, Sasaki T (1998) Expression of Xa1, a bacterial blight-resistance gene in rice, is induced by bacterial inoculation. Proc Natl Acad Sci USA 95(4):1663–1668
Faigon-Soverna A, Harmon FG, Storani L, Karayekov E, Staneloni RJ, Gassmann W, Mas P, Casal JJ, Kay SA, Yanovsky MJ (2006) A constitutive shade-avoidance mutant implicates TIR-NBS-LRR proteins in Arabidopsis photomorphogenic development. Plant Cell 18(11):2919–2928. doi:10.1105/tpc.105.038810
Hewezi T, Mouzeyar S, Thion L, Rickauer M, Alibert G, Nicolas P, Kallerhoff J (2006) Antisense expression of a NBS-LRR sequence in sunflower (Helianthus annuus L.) and tobacco (Nicotiana tabacum L.): evidence for a dual role in plant development and fungal resistance. Transgenic Res 15(2):165–180. doi:10.1007/s11248-005-3518-3
Zhai C, Lin F, Dong Z, He X, Yuan B, Zeng X, Wang L, Pan Q (2011) The isolation and characterization of Pik, a rice blast resistance gene which emerged after rice domestication. New Phytol 189(1):321–334. doi:10.1111/j.1469-8137.2010.03462.x
Zou LF, Wang XP, Xiang Y, Zhang B, Li YR, Xiao YL, Wang JS, Walmsley AR, Chen GY (2006) Elucidation of the hrp clusters of Xanthomonas oryzae pv. oryzicola that control the hypersensitive response in nonhost tobacco and pathogenicity in susceptible host rice. Appl Environ Microbiol 72(9):6212–6224. doi:10.1128/AEM.00511-06
Zhou YL, Xu MR, Zhao MF, Xie XW, Zhu LH, Fu BY, Li ZK (2010) Genome-wide gene responses in a transgenic rice line carrying the maize resistance gene Rxo1 to the rice bacterial streak pathogen, Xanthomonas oryzae pv. oryzicola. BMC Genomics 11:78. doi:10.1186/1471-2164-11-78
Xu RR, Song FM, Zheng Z (2006) OsBISAMT1, a gene encoding S-adenosyl-l-methionine: salicylic acid carboxyl methyltransferase, is differentially expressed in rice defense responses. Mol Biol Rep 33(3):223–231. doi:10.1007/s11033-005-4823-x
Hofgen R, Willmitzer L (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16(20):9877. doi:10.1093/nar/16.20.9877
Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133(2):462–469. doi:10.1104/pp.103.027979
Nishimura A, Aichi I, Matsuoka M (2006) A protocol for Agrobacterium-mediated transformation in rice. Nat Protoc 1(6):2796–2802. doi:10.1038/nprot.2006.469
Yuan M, Chu Z, Li X, Xu C, Wang S (2010) The bacterial pathogen Xanthomonas oryzae overcomes rice defenses by regulating host copper redistribution. Plant Cell 22(9):3164–3176. doi:10.1105/tpc.110.078022
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol Biol Evol 24(8):1596–1599. doi:10.1093/molbev/msm092
Traut TW (1994) The functions and consensus motifs of nine types of peptide segments that form different types of nucleotide-binding sites. Eur J Biochem 222(1):9–19. doi:10.1111/j.1432-1033.1994.tb18835.x
Sun Q, Collins NC, Ayliffe M, Smith SM, Drake J, Pryor T, Hulbert SH (2001) Recombination between paralogues at the rp1 rust resistance locus in maize. Genetics 158(1):423–438
Halterman D, Zhou F, Wei F, Wise RP, Schulze-Lefert P (2001) The MLA6 coiled-coil, NBS-LRR protein confers AvrMla6-dependent resistance specificity to Blumeria graminis f. sp. hordei in barley and wheat. Plant J 25(3):335–348. doi:10.1046/j.1365-313x.2001.00982.x
Zhou F, Kurth J, Wei F, Elliott C, Vale G, Yahiaoui N, Keller B, Somerville S, Wise R, Schulze-Lefert P (2001) Cell-autonomous expression of barley Mla1 confers race-specific resistance to the powdery mildew fungus via a Rar1-independent signaling pathway. Plant Cell 13(2):337–350. doi:10.1105/tpc.13.2.337
Lawrence GJ, Finnegan EJ, Ayliffe MA, Ellis JG (1995) The L6 gene for flax rust resistance is related to the Arabidopsis bacterial resistance gene RPS2 and the tobacco viral resistance gene N. Plant Cell 7(8):1195–1206. doi:10.1105/tpc.7.8.1195
Parker JE, Coleman MJ, Szabo V, Frost LN, Schmidt R, van der Biezen EA, Moores T, Dean C, Daniels MJ, Jones JD (1997) The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the toll and interleukin-1 receptors with N and L6. Plant Cell 9(6):879–894. doi:10.1105/tpc.9.6.879
Whitham S, Dinesh-Kumar SP, Choi D, Hehl R, Corr C, Baker B (1994) The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell 78(6):1101–1115. doi:10.1016/0092-8674(94)90283-6
Mitsuhara I, Iwai T, Seo S, Yanagawa Y, Kawahigasi H, Hirose S, Ohkawa Y, Ohashi Y (2008) Characteristic expression of twelve rice PR1 family genes in response to pathogen infection, wounding, and defense-related signal compounds (121/180). Mol Genet Genomics 279(4):415–427. doi:10.1007/s00438-008-0322-9
Nakashita H, Yoshioka K, Takayama M, Kuga R, Midoh N, Usami R, Horikoshi K, Yoneyama K, Yamaguchi I (2001) Characterization of PBZ1, a probenazole-inducible gene, in suspension-cultured rice cells. Biosci Biotechnol Biochem 65(1):205–208. doi:10.1271/bbb.65.205
Qiu D, Xiao J, Ding X, Xiong M, Cai M, Cao Y, Li X, Xu C, Wang S (2007) OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact 20(5):492–499. doi:10.1094/MPMI-20-5-0492
Zhao CJ, Wang AR, Shi YJ, Wang LQ, Liu WD, Wang ZH, Lu GD (2008) Identification of defense-related genes in rice responding to challenge by Rhizoctonia solani. Theor Appl Genet 116(4):501–516. doi:10.1007/s00122-007-0686-y
Datta K, Velazhahan R, Oliva N, Ona I, Mew T, Khush GS, Muthukrishnan S, Datta SK (1999) Over-expression of the cloned rice thaumatin-like protein (PR-5) gene in transgenic rice plants enhances environmental friendly resistance to Rhizoctonia solani causing sheath blight disease. Theor Appl Genet 98(6):1138–1145. doi:10.1007/s001220051178
Hashimoto M, Kisseleva L, Sawa S, Furukawa T, Komatsu S, Koshiba T (2004) A novel rice PR10 protein, RSOsPR10, specifically induced in roots by biotic and abiotic stresses, possibly via the jasmonic acid signaling pathway. Plant Cell Physiol 45(5):550–559. doi:10.1093/pcp/pch063
Bari R, Jones JD (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69(4):473–488. doi:10.1007/s11103-008-9435-0
Vlot AC, Dempsey DA, Klessig DF (2009) Salicylic Acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206. doi:10.1146/annurev.phyto.050908.135202
Song FM, Goodman RM (2001) Molecular biology of disease resistance in rice. Physiol Mol Plant Pathol 59(1):1–11. doi:10.1006/pmpp.2001.0353
DeYoung BJ, Innes RW (2006) Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol 7(12):1243–1249. doi:10.1038/ni1410
McHale L, Tan X, Koehl P, Michelmore RW (2006) Plant NBS-LRR proteins: adaptable guards. Genome Biol 7(4):212. doi:10.1186/gb-2006-7-4-212
Zhou T, Wang Y, Chen JQ, Araki H, Jing Z, Jiang K, Shen J, Tian D (2004) Genome-wide identification of NBS genes in japonica rice reveals significant expansion of divergent non-TIR NBS-LRR genes. Mol Genet Genomics 271(4):402–415. doi:10.1007/s00438-004-0990-z
Nino-Liu DO, Ronald PC, Bogdanove AJ (2006) Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol Plant Pathol 7(5):303–324. doi:10.1111/j.1364-3703.2006.00344.x
Acknowledgments
The authors acknowledge Mingfu Zhao, Fujian Academy of Agricultural Sciences for providing us with the bacterial pathogen Xanthomonas oryzae pv. oryzicola RS105 strain. We also would like to thank National 863 Project of China (Grant No. 2007AA10Z132 and 2009ZX08009-045B) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lijia Guo and Min Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, L., Li, M., Wang, W. et al. Over-expression in the nucleotide-binding site-leucine rich repeat gene DEPG1 increases susceptibility to bacterial leaf streak disease in transgenic rice plants. Mol Biol Rep 39, 3491–3504 (2012). https://doi.org/10.1007/s11033-011-1122-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1122-6