Abstract
We report the characterization of VvIAA19, an auxin/indole-3-acetic acid (Aux/IAA) protein, in grapevine (Vitis vinifera L.). VvIAA19 was expressed abundantly in berries. VvIAA19 transcription was rapidly increased at pre-anthesis and then decreased during fruit set. Before véraison, however, VvIAA19 gene expression was upregulated again and maximum expression was maintained until the end of ripening. Exogenous IAA did not induce VvIAA19 expression in grape leaves, suggesting that VvIAA19 might be auxin-nonresponsive. The overexpression of VvIAA19 in Arabidopsis thaliana had a notable effect on plant growth. Although no morphological changes were observed, transgenic Arabidopsis plants overexpressing VvIAA19 exhibited faster growth, including root elongation and floral transition, than the control plant, suggesting that the constitutive expression of VvIAA19 protein resulted in increased growth rates without any detectable harm. Taken together, we conclude that grape Aux/IAA19 protein is likely to play a crucial role as a plant growth regulator.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Berry size is one of the essential factors contributing to grape quality. Basically, small berries are preferred for winemaking to improve wine quality. It has been reported that large Cabernet Sauvignon berries exhibited poor berry composition as exemplified by decreases in total soluble solids, anthocyanin, and seed tannin contents [1]. Berry-size-dependent berry composition affects final wine quality, including wine composition and sensory score [2]. In contrast, in the case of grapes for consumption and raisin production, large berries are desired by markets and consumers. To control berry size, deficit irrigation [1, 2], light pruning [3], rootstock selection [3], and trunk girdling [4] are used as practical techniques, as well as breeding programs. For example, water status in grapevines may determine berry size [1, 2, 4]. However, the molecular mechanisms underlying berry expansion in grapevine are still poorly understood.
Cell division in the pericarp of grape berry takes place at days 5–10 after anthesis and ceases at days 7–11 after anthesis, and this is followed by cell enlargement [5]. Berry softening is necessary for berry expansion and starts at approximately day 60 after anthesis, the so-called véraison. Expansin, which plays a role in plant elongation due to cell wall loosening, is accumulated in berry at pre-véraison and véraison in relation to berry softening [6, 7]. A potassium transporter is also associated with berry size increase as well as potassium accumulation in berry [8]. Plant hormones, such as ethylene [9] and gibberellic acid [4], induce berry expansion. During ethylene-induced berry expansion, ethylene induces the expression of the water exchange gene, aquaporin, and cell wall modifying genes, cellulose synthases and expansins [9]. However, no direct evidence derived from the genetic transformation in grapevine or model plants is available so far to understand how these proteins regulate berry expansion.
Exhaustive transcriptome analyses of grape berry during development have led to the identification of a number of genes related to berry expansion [10, 11]. We have detected the transcription of auxin/indole-3-acetic acid (Aux/IAA) genes during berry development by cDNA microarray [12]. Aux/IAA proteins are transcription regulators that bind to members of the auxin response factor (ARF) family, inhibiting the auxin-responsive gene transcription activity [13]. The stimulation of plant cells with auxin promotes the degradation of Aux/IAA proteins through the ubiquitin–proteasome system and increases the expression of auxin-responsive genes, followed by the initiation of developmental processes in plant cells [13]. Accompanying Aux/IAA protein expression in berry skin, the gene expression of cullin, a component of SCF (SKP1/cullin/F-box protein) ubiquitin-protein ligase, is upregulated in berry skin at the end of ripening [12]. However, the fundamental roles of Aux/IAA proteins in grapevine are poorly understood.
Our final goal is to characterize the physiological role of Aux/IAA19 in grapevine. In the present study, we demonstrated the transcription of VvIAA19 during berry development and investigated the effect of VvIAA19 overexpression on Arabidopsis plants.
Materials and methods
Plant materials
Grapevines of Vitis vinifera cv. Chardonnay were cultivated in the experimental vineyard of The Institute of Enology and Viticulture, University of Yamanashi, Yamanashi, Japan. Berries were collected from May 25 to September 7, 2010 at one-week intervals. Flowering, fruit set, and véraison were confirmed on June 1, June 15, and August 3, respectively. Young leaves, young shoots, and stems were collected on June 2, 2010.
Measurement of berry development
The diameter at the cross-sectional plane of 30 berries was measured with a caliper. Fruit juices were obtained by hand pressing the berries. Total soluble solids of the juice were measured with a refractometer (Atago, Tokyo, Japan) and expressed as Brix. The cross sections of the berries were cut manually with a razor blade. The sections were stained with 0.5% aniline blue for 5 min, mounted on a glass slide, and covered with a cover slip. Then, the sections were observed under a light microscope (Olympus, Melville, NY) and cell number in a 500 × 500 μm2 area of mesocarp (three areas per berry) was counted from three berries.
IAA treatment
Leaf disks (8 mm diameter) were punched out from young leaves of grapevine. The disks were floated on 1 or 10 μM IAA (Wako, Osaka, Japan) for 6 h at 23°C. Control leaf disks were treated with 0.1% DMSO. After IAA treatment, the disks were subjected to RNA isolation and real-time RT-PCR analysis.
RNA isolation
Young leaves, young shoots, stems, ovaries, young berries, and berry skins were placed in a mortar containing liquid nitrogen and homogenized with a pestle. Approximately 100 mg of the pulverized tissue was transferred to a microtube. Total RNA isolation and purification were performed using Fruit-mate for RNA Purification (Takara, Otsu, Japan) and Fast Pure RNA Kit (Takara) according to the manufacturer’s instructions. Finally, total RNA was treated with RNase-free DNase I (Wako), followed by phenol–chloroform extraction and ethanol precipitation.
Real-time RT-PCR analysis
First-strand cDNA was synthesized from 500 ng of total RNA using a PrimeScript RT Reagent Kit (Takara) and real-time PCR was performed using an SYBR Premix Ex Taq II (Takara) according to the manufacturer’s instructions. Real-time RT-PCR was performed using a Thermal Cycler Dice Real Time System (Takara). Real-time RT-PCR conditions were as follows: 37°C for 15 min for RT reaction and 85°C for 5 s for cDNA synthesis, and then 40 cycles at 95°C for 5 s and at 60°C for 30 s for PCR amplification. The dissociation curves for each sample were analyzed to verify the specificity of the amplification reaction. Nucleotide sequences of the primers used in this study were as follows: V. vinifera IAA9 (VvIAA9) primers (5′-CAAGACTAGCGGGAAGAGAGG-3′ and 5′-GGTCGTTGGATGGAGGAAGA-3′, corresponding to bases 552–572 and 668–649 of GenBank accession no. HQ337788, respectively), V. vinifera IAA19 (VvIAA19) primers (5′-TCCCACCAAGGCTACTTCAATC-3′ and 5′-TACAAGCATCCAGTCCCCATC-3′, corresponding to bases 313–334 and 453–433 of GenBank accession no. HQ337790, respectively), V. vinifera HAT2 (5′-GGAGGTGCAGGAGCTGAGAA-3′ and 5′-AGAGGGTGCTGAGGATGAGG-3′, corresponding to bases 788–807 and 930–911 of GenBank accession no. XM_002272716, respectively), and β-actin primers (5′-CAAGAGCTGGAAACTGCAAAGA-3′ and 5′-AATGAGAGATGGCTGGAAGAGG-3′, corresponding to bases 409–430 and 537–516 of V. vinifera β-actin, GenBank accession no. AF369524, respectively). β-Actin was used for normalization and the expression level of each gene was expressed as a relative value.
Cluster analysis
Cluster analysis of VvIAA19 with V. vinifera Aux/IAA9 (VvIAA9), Aux/IAA16 (VvIAA16), and Arabidopsis Aux/IAA protein family was performed. Briefly, the amino acid sequences of Arabidopsis Aux/IAA proteins were collected from an NCBI database. The sequences were subjected to the neighbor-joining (NJ) method with the predicted amino acid sequence of VvIAA19 using Molecular Evolutionary Genetics Analysis software, MEGA4 (www.megasoftware.net).
Transformation of VvIAA19 in A. thaliana plants
The open reading frame of VvIAA19 was amplified by RT-PCR using total RNA isolated from berry skins. The nucleotide sequences of the primers used were as follows: 5′-AAATCTAGAATGGCCCTAGGACTCGAGAT-3′ containing an XbaI site (underline) and 5′-GGGGAGCTCCTAATCATTTATCTTCTGGA-3′ containing a SacI site (underline) for VvIAA19. PCR products were digested with XbaI and KpnI or XbaI and SacI and ligated into XbaI and KpnI or XbaI and SacI sites of the binary vector pBI121 (Clontech, Foster City, CA), resulting in plant expression plasmids. The plant expression plasmids were transformed into Agrobacterium tumefaciens strain LBA4404. A. thaliana Col-0 was transformed with the Agrobacterium by the floral dip method [14] and selected on 1× Murashige and Skoog (MS) medium plates containing 50 μg/ml kanamycin + 100 μg/ml carbenicillin. T2 seeds that propagated from the transgenic lines were obtained and used for phenotypic analysis and the IAA response test. One transgenic line transformed with pBI121 was used as the control plant in the present study.
Phenotypic analysis of Arabidopsis transgenic plants
To observe the general growth and development of Arabidopsis transgenic plants, T2 seeds were plated on MS medium or rock wool and incubated at 22°C for 10 days in an incubator (11.8 Wm−2/16 h/days). Then, the seedlings were planted in soil. To determine meristem transition from vegetative to reproductive growth, the number of rosette leaves per plant was measured before the appearance of inflorescence meristems. Plant height was measured at days 30 and 45 after seeding. Different phenotypes from control plants were photographed at each growth stage.
Statistics
Data in figures are presented as means ± standard error. Statistical analysis was performed with the non-parametric Dunnett’s multiple comparison test using Excel statistics software (Social Survey Research Information, Tokyo, Japan).
Results
Identification of genes encoding Aux/IAA19 in grapevine
We obtained a cDNA fragment encoding grape Aux/IAA19 from Chardonnay berry. The predicted amino acid sequence of Aux/IAA19 protein showed high homology to those of plant Aux/IAA proteins. Grape Aux/IAA19 protein was similar to A. lyrata subsp. lyrata IAA19 (54%, accession no. XP_002882951). Cluster analysis of the predicted amino acid sequences of grape Aux/IAA19 protein, two other V. vinifera Aux/IAA proteins, VvIAA9 and VvIAA16, and Arabidopsis Aux/IAA proteins demonstrated that grape Aux/IAA19 protein formed a cluster with Arabidopsis IAA19 (Fig. S1). Based on these results, the nucleotide sequence encoding grape Aux/IAA19 protein has been deposited in GenBank database as VvIAA19 (accession no. HQ337790).
Transcription profile of VvIAA19 during berry development
VvIAA19 transcripts were detected more abundantly in berry skins than in stems, young leaves, and shoots (Fig. S2). Therefore, as our next step, the transcription profile of VvIAA19 in berries was determined in relation to berry development.
Chardonnay berry has three growth phases, as described previously [15]. Berry diameter rapidly increased from fruit set to pre-véraison (Fig. 1a, phase I), and this was followed by a slow growth phase until the end of véraison (Fig. 1a, phase II). After véraison, the berries started to enlarge again (Fig. 1a, phase III). Total soluble solids in the berries increased dramatically after véraison. Cell number was counted in three 500 μm2 areas of the mesocarp, as shown in Fig. 1b. Cell number in berry mesocarp decreased rapidly before véraison, suggesting that the berries stopped cell division in mesocarp and started cell elongation after fruit set (Fig. 1c). These results indicate that cell elongation plays an indispensable role in berry expansion after fruit set.
Berry development and transcription profiles of VvIAA19. a Berry diameter and total soluble solids during berry development. The diameters of 30 berries were measured and then the juice was collected from the berries to measure total soluble solids. b Cross section of a berry stained with 0.5% aniline blue. Cell number in each of the three 500 × 500 μm2 areas of mesocarp (squares) per berry was counted. S seed. c Cell number in mesocarp during berry development was calculated from three berries. Bars indicate means ± standard errors. Arrows indicate the start of véraison. d Transcription profiles of VvIAA19 in ovaries, berries or berry skins during berry development. β-Actin was used as internal control. Data were calculated as gene expression relative to β-actin gene expression. Arrows indicate the start of anthesis, fruit set, and véraison, respectively. Bars indicate means ± standard errors of triplicate experiments
Because it was difficult to isolate RNA from berries according to the stage of berry development, samples for RNA isolation were prepared as follows: ovaries, from anthesis to fruit set; whole berries, from fruit set to 3 weeks after flowering; and berry skins peeled from the berries, from 4 weeks after flowering onward. VvIAA19 transcription rapidly increased before anthesis and then decreased during fruit set (Fig. 1d). Before véraison, however, VvIAA19 gene expression was upregulated again and maximum expression was maintained until the end of ripening.
Taken together, these results suggest that the VvIAA19 transcription in berries may correspond to berry development, including cell division during cell elongation from anthesis to fruit set and cell elongation after véraison.
Auxin does not induce VvIAA19 gene expression in grapevine
The gene expression of IAA-responsive Aux/IAA9 and HAT2, the latter being a member of the HD-Zip gene family [16], was induced in grape leaves by exogenous IAA treatment (Fig. 2). In contrast, the same IAA treatment did not induce VvIAA19 gene expression in grape leaves. This result suggests that VvIAA19 may be a member of the auxin-nonresponsive Aux/IAA family.
VvIAA19 expression is not induced by exogenous IAA treatment. Leaf disks were treated with 1 or 10 μM IAA for 6 h. RNA isolation and real-time RT-PCR were performed as described in “Materials and methods” section. β-Actin was used as internal control. Data were calculated as gene expression relative to β-actin gene expression. Bars indicate means ± standard errors of triplicate experiments. *P < 0.01 as compared with control. **P < 0.05 as compared with control
VvIAA19 overexpression has a notable effect on Arabidopsis plant growth
To evaluate whether VvIAA19 protein affects plant growth and development, we created transgenic Arabidopsis plants overexpressing VvIAA19. The open reading frame of VvIAA19 was placed under the control of the cauliflower mosaic virus 35S promoter. Plant expression plasmids were transformed into A. thaliana Col-0 using Agrobacterium transformation. Six lines, referred to as Col-VvIAA19, were obtained by selection on MS medium containing 50 μg/ml kanamycin. All lines constitutively expressed VvIAA19 transcripts (data not shown). One line of Col-pBI, which was transformed with a pBI121 vector, was used as the control plant in the present study.
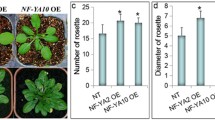
The germination rates and times of T2 seeds of Col-VvIAA19 line 1 were comparable to those of Col-pBI. Slight root elongation was observed in Col-VvIAA19 line 1 compared with Col-pBI at day 14 after plating on MS medium (Fig. 3), although no morphological differences in roots and seedlings were observed between the transgenic plants. The morphology of Col-VvIAA19 line 1 was photographed at day 30 after planting (Fig. 4a). Col-VvIAA19 showed inflorescence stem growth and flower bud formation, whereas the inflorescence stem of Col-pBI was just emerging. Although the inflorescence stem lengths of Col-VvIAA19 line 1 were significantly greater than that of Col-pBI at day 30 (Fig. 4b), no differences in inflorescence stem length were observed at day 45 between the transgenic plants (Fig. 4c). The number of rosette leaves formed before the appearance of the inflorescence meristem was 19.3 ± 1.5 in Col-pBI, whereas it was 9.8 ± 0.4 in Col-VvIAA19 line 1 (Fig. 4d). No morphological differences in flowers, seeds pods, and T3 seeds were observed between the transgenic plants. The yield of T3 seeds of Col-VvIAA19 line 1 was comparable to that of Col-pBI.
Overexpression of VvIAA19 has a notable effect on plant growth. a Phenotypes of six Col-VvIAA19 lines and a Col-pBI plant at day 30 after planting. Scale bar = 5 cm. b Length of inflorescence stem at day 30 after planting. c Length of inflorescence stem at day 45 after planting. d Number of rosette leaves formed before the appearance of inflorescence meristem. Bars indicate means ± standard errors, calculated from four independent experiments. * P < 0.01 as compared with Col-pBI
Taken together, these observations of transgenic Arabidopsis plants overexpressing VvIAA19 suggest that the constitutive expression of VvIAA19 proteins may result in the increase in growth rate, including meristem transition from vegetative to reproductive growth, without any detectable harm.
Discussion
Fruit set of grape berry is correlated to endogenous IAA concentration [17]. IAA concentration in berry reaches a maximum at prebloom or anthesis [18]. In the present study, we characterized the transcription of the grape Aux/IAA protein, VvIAA19, during berry development and in response to exogenous IAA treatment. Grape berry expressed VvIAA19 transcripts abundantly among the grapevine tissues tested (Fig. S2). VvIAA19 gene expression showed two peaks during berry development. The first peak was observed from flowering to fruit set and the second, from véraison to the end of ripening (Fig. 1). Meanwhile, VvIAA19 gene expression was not induced in grape leaves by exogenous IAA treatment (Fig. 2). Arabidopsis IAA19 was upregulated by brassinosteroid treatment [19]. Brassinosteroid accumulation also showed two peaks during berry development [20]. The concentration of brassinosteroid in grape berry was elevated from flowering to 2 weeks post flowering, and then decreased markedly by 8 weeks post flowering. The second peak of brassinosteroid was observed from véraison to the end of ripening. These findings and the transcription profile of VvIAA19 during berry development lead us to speculate that VvIAA19 is induced through brassinosteroid-mediated responses in grape berry, but not by endogenous IAA treatment. Although the mechanisms by which brassinosteroid functions as a berry growth regulator together with grape Aux/IAA proteins remain to be determined, VvIAA19 and brassinosteroid during véraison may promote an increase in berry size due to cell wall modification [20].
To understand the effects of VvIAA19 on grapevine plant growth, including berry development, we should find out why the overexpression of VvIAA19 in Arabidopsis plants had a notable effect on plant growth. Arabidopsis plants overexpressing VvIAA19 showed an increase in growth rate, including root elongation (Fig. 3) and floral transition (Fig. 4). In Arabidopsis, the IAA19-deficient mutant, massugu2 (msg2), was identified as an auxin-insensitive mutant [21]. Arabidopsis IAA19 binds to ARF7, preventing the activation of auxin-responsive genes, suggesting that IAA19 is one of the master proteins that regulate hypocotyl responses related to auxin, the formation of lateral root, and the elongation of stamen filaments [21, 22]. Hypocotyl elongation accompanying IAA19 gene expression was observed in Arabidopsis grown under low blue light condition [23]. Considering these findings and our study, VvIAA19 may be a positive regulator of plant growth, resulting in the promotion of developmental processes in Arabidopsis plants by the overexpression of VvIAA19. Further studies employing transgenic grapevine or model plants for fruit ripening [24, 25], the genome-wide analysis of Aux/IAA family in grapevine [26], and the analysis of the interactions between grape VvIAA19 and ARFs on plant growth [27] are needed to fully determine the precise biological function of VvIAA19 during fruit development in grapevine.
Abbreviations
- Aux/IAA:
-
Auxin/indole-3-acetic acid protein
References
Roby G, Harbertson JF, Adams DA, Matthews MA (2004) Berry size and vine water deficits as factors in winegrape composition: Anthocyanins and tannins. Aust J Wine Grape Res 10:100–107
Walker RR, Blackmore DH, Clingeleffer PR, Kerridge GH, Rühl ET, Nicholas PR (2005) Shiraz berry size in relation to seed number and implications for juice and wine composition. Aust J Wine Grape Res 11:2–8
Clingeleffer PR, Kristic M, Sommer K (2000) Production efficiency and relationships among crop load, fruit composition and wine quality. In: Proceedings of the ASEV 50th anniversary annual meeting. Seattle, Washington, pp 318–322
Williams LE, Ayars JE (2005) Water use of Thompson Seedless grapevines as affected by the application of gibberellic acid (GA3) and trunk girdling—practices to increase berry size. Agric For Meteorol 129:85–94
Platt C (1971) Reproductive anatomy in cultivated grapes—a review. Am J Enol Vitic 22:92–106
Ishimaru M, Smith DL, Gross KC, Kobayashi S (2007) Expression of three expansin genes during development and maturation of Kyoho grape berries. J Plant Physiol 164:1675–1682
Schlosser J, Olsson N, Weis M, Reid K, Peng F, Lund S, Bowen P (2008) Cellular expansion and gene expression in the development of grape (Vitis vinifera L.). Protoplasma 232:255–265
Davies C, Shin R, Liu W, Thomas MR, Schachtman DP (2006) Transporters expressed during grape berry (Vitis vinifera L.) development are associated with increase in berry size and berry potassium accumulation. J Exp Bot 57:3209–3216
Chervin C, Tria-umphon A, Terrier N, Zouine M, Severac D, Roustan JP (2008) Stimulation of the grape berry expansion by ethylene and effects on related gene transcripts, over the ripening phase. Physiol Plant 134:534–546
Silva FG, Iandolino A, Al-Kayal F, Bohlmann MC, Cushman MA, Lim H, Ergul A, Figueroa R, Kabuloglu EK, Osborne C, Rowe J, Tattersall E, Leslie A, Xu J, Baek JM, Cramer GR, Cushman JC, Cook DR (2005) Characterizing the grape transcriptome. Analysis of expressed sequence tags from multiple Vitis species and development of a compendium of gene expression during berry development. Plant Physiol 139:574–597
Grimplet J, Deluc LG, Tillett RL, Wheatley MD, Schlauch KA, Cramer GR, Cushman JC (2007) Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genomics 8:87
Kobayashi H, Fujita K, Suzuki S, Takayanagi T (2009) Molecular characterization of Japanese indigenous grape cultivar ‘Koshu’ (Vitis vinifera) leaf and berry skin during grape development. Plant Biotech Rep 3:225–241
Gray WM (2004) Hormonal regulation of plant growth and development. PLoS Biol 2:1270–1273
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Staudet G, Schneider A, Leidel J (1986) Phases of berry growth in Vitis vinifera. Ann Bot 58:789–800
Sawa S, Ohgishi M, Goda H, Higuchi K, Shimada Y, Yoshida S, Koshiba T (2002) The HAT2 gene, a member of the HD-Zip gene family, isolated as an auxin inducible gene by DNA microarray screening, affects auxin response in Arabidopsis. Plant J 32:1011–1022
Cawthon DL, Morris JR (1982) Relationship of seed number and maturity to berry development, fruit maturation, hormonal changes and uneven ripening of ‘Concord’ (Vitis vinifera L.) grapes. J Am Soc Hortic Sci 107:1097–1104
Costantini E, Landi L, Silvestroni O, Pandolfini T, Spena A, Mezzetti B (2007) Auxin synthesis-encoding transgene enhances grape fecundity. Plant Physiol 143:1689–1694
Nakamura A, Nakajima N, Goda H, Shimada Y, Hayashi K, Nozaki H, Asami T, Yoshida S, Fujioka S (2006) Arabidopsis Aux/IAA genes are involved in brassinosteroid-mediated growth responses in a manner dependent on organ type. Plant J 45:193–205
Symons GM, Davies C, Shavrukov Y, Dry IB, Reid JB, Thomas MR (2006) Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol 140:150–158
Tatematsu K, Kumagai S, Muto H, Sato A, Watahiki MK, Harper RM, Liscum E, Yamamoto KT (2004) MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcription activator NPH/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16:379–393
Tashiro S, Tian C, Watahiki MK, Yamamoto KT (2009) Changes in growth kinetics of stamen filaments cause inefficient pollination in massugu2, an auxin insensitive, dominant mutant of Arabidopsis thaliana. Physiol Plant 137:175–187
Pierik R, Djakovic-Petrovic T, Keuskamp DH, de Wit M, Voesenek LACJ (2009) Auxin and ethylene regulate elongation responses to neighbor proximity signals independent of gibberellin and DELLA proteins in Arabidopsis. Plant Physiol 149:1701–1712
Liu DJ, Chen J, Lu W (2011) Expression and regulation of the early auxin-responsive Aux/IAA genes during strawberry fruit development. Mol Biol Rep 38:1187–1193
Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, Chaabouni S, Latché A, Pech JC, Bouzayen M (2005) The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 17:2676–2692
Wang Y, Deng D, Bian Y, Lv Y, Xie Q (2010) Genome-wide analysis of primary auxin-responsive Aux/IAA gene family in maize (Zea mays L.). Mol Biol Rep 37:3991–4001
Wu B, Li YH, Wu JY, Chen QZ, Huang X, Chen YF, Huang XL (2011) Over-expression of mango (Mangifera indica L.) MiARF2 inhibits root and hypocotyl growth of Arabidopsis. Mol Biol Rep 38:3189–3194
Acknowledgment
We are grateful to Mr. Keisuke Sugiyama of the University of Yamanashi for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kohno, M., Takato, H., Horiuchi, H. et al. Auxin-nonresponsive grape Aux/IAA19 is a positive regulator of plant growth. Mol Biol Rep 39, 911–917 (2012). https://doi.org/10.1007/s11033-011-0816-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-0816-0