Abstract
The plant hormone auxin transcriptionally activates Aux/IAA genes. Auxin plays an important role in regulating fruit growth and ripening of strawberry and Aux/IAA genes have been extensively studied in Arabidopsis, rice and tomato, but little information is available on strawberry fruit. In the present work, two full-length of early auxin-responsive Aux/IAA genes, termed FaAux/IAA1 and FaAux/IAA2 respectively, were isolated and characterized from strawberry fruit. Moreover, the expression profiles of two FaAux/IAA genes during fruit development, and the effect of naphthalene acetic acid (NAA) on their expressions of fruits at two different developmental stages were also investigated. The results showed that the levels of FaAux/IAA1 and FaAux/IAA2 transcripts were very high at early stage of fruit development, and decreased sharply at ripening stage (after white stage). In addition, NAA applied at the stage of large green and white fruit obviously increased the accumulations of FaAux/IAA1 and FaAux/IAA2 transcripts. These data suggested that the expressions of both FaAux/IAA1 and FaAux/IAA2 genes were likely to be involved in early fruit development, and the enhancement of FaAux/IAAs transcripts might be attributed at least or partially to auxin-induced fruit growth and delayed fruit ripening of strawberry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The phytohormone auxin plays a critical role in regulating many aspects of plant growth and developmental processes. At the cellular level, auxin regulates the cell division, extension and differentiation [1]. On a whole-plant level, auxin plays an essential role in processes such as apical dominance, lateral root formation, tropic responses, fruit growth and development, vascular differentiation and embryogenesis [2]. This multiplicity of auxin regulatory activities has spurred considerable interest in mechanisms of auxin signaling and response. Previous research revealed that auxin can regulate expression of numerous genes whose products probably perform most developmental responses [3–5]. The most well characterized auxin-responsive genes are represented by the members of the Aux/IAA (auxin/indoleacetic acid), GH3 (Gretchen Hagen3), and SAUR (small auxin up RNA) gene families [3, 6–8]. Auxin induces expressions of many of these genes rapidly, specifically and without the requirement of de novo protein synthesis, and these genes are regarded as primary/early auxin-responsive genes [8, 9].
Following the initial identification of Aux/IAA genes from soybean [10, 11], members of Aux/IAA genes were isolated from pea [12], Arabidopsis [13, 14], mung bean [15], rice [16–18], and tomato [19, 20]. The Aux/IAAs are short-lived nuclear proteins that are characterized by the presence of four conserved domains (domain I, II, III, IV) [21]. It has been reported that down regulation of a tomato Aux/IAA gene from tomato, SlIAA9, has been proved to be involved in tomato fruit development and leaf morphogenesis [20]. However, the characterizations of Aux/IAA genes in relation to fruit development in other fruits remain to be elucidated.
Strawberry is considered as non-climacteric fruit of great economic importance worldwide, and its edible part is a false fruit originating mainly from the expansion of the flower base (receptacle) or pseudocarp where the real fruits (achenes) are attached. It has long been known that achene-derived auxin is the key phytohormone controlling the growth and ripening of strawberry receptacles [22–24]. Auxin stimulates receptacle expansion during fruit development, and later inhibits fruit ripening [23]. As strawberry fruit ripens, the diminution of auxin level activates the expression of ripening-related genes including many genes encoding cell wall modifying enzymes, such as expansins (FaExps), polygalacturonase (PG), β-xylosidase (FaXyl1), pectate lyases (FaPELs) and α-l-Arafases (FaAras) [25–28]. Moreover, exogenous applications of auxin delay fruit ripening and repress the expressions of these ripening-related genes [26–28], while a possible involvement of ethylene in strawberry growth and ripening have also been suggested in several reports [29–32]. Thus, the underlying biochemical mechanisms of strawberry fruit development are still unclear and little attention has been paid to characterize Aux/IAA gene during strawberry fruit development.
The objective of this work is to characterize Aux/IAA mRNA accumulations during strawberry fruit development. In addition, the effect of NAA applied at two different developmental stages, on the expression of Aux/IAA gene was also investigated.
Materials and methods
Plant materials
Strawberry fruit (Fragaria × ananassa, cv. Toyonaka) were grown under field conditions by local producers in South China Agricultural University, Guangzhou, China. Fruits at different developmental stages were harvested, transferred to the laboratory and classified according to the external coloration degree in five developmental stages as described by Civello et al. [33]: small green (SG, about 7 day after post-anthesis), large green (LG, about 15 day after post-anthesis), white (W, about 23 day after post-anthesis), turning (T, 50% red, about 26 day after post-anthesis), and full-ripe red (R, about 30 day after post-anthesis). The calyx and peduncle were removed, and the samples were washed, drained and used on the same day or frozen in liquid nitrogen and stored at −80°C prior to analysis. Other vegetative and reproductive tissues including roots, stems, leaves, flowers and calyxes were also collected, immediately frozen in liquid nitrogen, and stored at −80°C until use.
Auxin treatment
Strawberry fruit at LG and W developmental stage were randomly selected on the basis of size and absence of physical damage. The selected fruit were tagged and treated with the synthetic auxin (naphthalene acetic acid, NAA, Sigma-Aldrich Co., USA). Each fruit was dipped for 1 min in a solution containing 100 mg/l NAA. Control fruit were immersed in distilled water. After 0, 0.5, 2, 6, 12 h, 1, 2, 4, 7, 10 days of each treatment, 20 randomly selected fruit were collected, then frozen in liquid nitrogen and finally stored at−80°C until use.
Measurement of firmness
Firmness was measured using a digital force gauge pressure tester (Model Instron 5542, INSTRON Co., USA), provided with a 4 mm cylinder tip. Two measurements on each equatorial side were performed on each fruit. Twenty-five fruit per stage were measured and the mean was recorded and expressed as Newtons (N) ± standard deviation (S.D.).
RNA extraction, isolation of strawberry Aux/IAA full lengh cDNAs
Total RNA from strawberry fruit was extracted using the hot borate method of Wan and Wilkins [34]. Frozen tissues (10 g) were ground to a fine powder in a mortar using a pestle in the presence of liquid nitrogen. The extracted total RNA was used as templates for RT-PCR. The product (the first-strand cDNA) was subjected to PCR amplification. Degenerate primers of Aux/IAAs (i.e., sense: 5′-GGTGGTGCGCTGGCGNCCNRT-3′ and antisense: 5′-CGATCGCCTCGGACCGYTTNATDAT-3′, where D is A, G or T; Y is C or T, R is A or G, and N is all four nucleotides) were designed with reference to the conserved amino acids sequences of Aux/IAAs. Reactions for the RT-PCR were subjected to one cycle of 94°C for 3 min, 35 cycles each at 94°C for 1 min, 45°C for 2 min and 72°C for 2 min, and then one cycle of 72°C for 10 min. PCR products of the predicted size (about 500 bp in length) were purified and cloned into pGEM-T easy vector (Promega, USA). The nucleotide sequences of the cDNA inserts were determined using the thermo sequenase dye terminator cycle sequencing kit and a 3730 DNA sequencer (PerkinElmer Applied Biosystems).
Consequently, 3′- or 5′-rapid amplification of cDNA ends (3′- or 5′-RACE-PCR) was performed using cDNA amplification kits (Takara, Shiga, Japan) according to the manufacturer’s protocol. In order to amplify 3′-end and 5′-end fragments, the specific primers for FaAux/IAA1 (3′-RACE: outer, CAACCTTGAAGCCACCGAGC, and inner CCTCCTTCCAAAGCACAACT; 5′-RACE: outer, TTGAGTTCTTGCGGGGCGTTCTT, middle, CATCCTTGTCCTCATAAGTTGG, and inner, CTCCATCCACAGCCACCTTTACG) and FaAux/IAA2 (3′-RACE: outer, GCCAAAAGAAGAGCACCGAT and inner, GAGTATGGATGGGGCACCTT; 5′-RACE: outer, TCCCACAAGCATCCAATCTCCAT middle, TAGGGCAGTAGAAAGCTCTTGGT, and inner, GTAGATAAGGTGCCCCATCCATA) were designed based on the nucleotide sequences of the cDNA fragments already cloned by RT-PCR. The 3′- and 5′-RACE-PCR products were cloned and sequenced as described above.
DNA sequence analysis, alignment, and comparisons
Identification of nucleotide sequences from RT-PCR clones were established using the NCBI Blast program [http://www.ncbi.nlm.nih.gov/BLAST]. Alignment and comparison of sequence were made using the ClustalW program (http://www.ebi.ac.uk/clustalw). Open reading frame and protein prediction were made using NCBI ORF Finder [http://www.ncbi.nlm.nih.gov/gorf/gorf.html]. The theoretical isoelectric point (pI) and mass values for mature peptides were calculated using the PeptideMass program [http://us.expasy.org/tools/peptide-mass.html]. The phylogenetic tree was generated from the deduced amino acid sequences for FaAux/IAAs and 29 Aux/IAA homologues from other species using CLUSTAL W and PHYLIP with the PROTPARS programs. The 29 Aux/IAA homologues sequences registered in GenBank are Arabidopsis thaliana , AtIAA1 (P49677), AtIAA2 (P49678), AtIAA3 (Q38822), AtIAA4 (P33077), AtIAA6 (Q38824), AtIAA7 (Q38825), AtIAA8 (Q38826), AtIAA9 (Q38827), AtIAA11 (Q38829), AtIAA12 (Q38830), AtIAA14 (Q38832), AtIAA17 (P93830), AtIAA20 (O24410), AtIAA27 (Q9ZSY8), AtIAA28 (Q9XFM0), AtIAA29 (Q93WC4), AtIAA30 (Q9M1R4), AtIAA31 (Q8H174), AtIAA32 (Q8RYC6), AtIAA33 (Q9FKM7), AtIAA34 (Q9C5X0); tomato (Lycopersicon esculentum) LeIAA4 (AAZ20313); grape (Vitis vinifera), VvIAA (AAL92850); potato (Solanum tuberosum), StIAA (AAM29182), cucumber (Cucumis sativus), CsIAA2 (BAA85821); deep water rice (Oryza sativa), OsIAA1 (CAC80823.1) and OsIAA18 (BAA99424.1).
Northern blot analysis
Total RNA (10 μg) was separated on a 1.2% agarose–formaldehyde gel and capillary blotted onto positively charged nylon membrane (Biodyne® B, 0.45 μm, PALL Co. Sarasota, FL). The RNA was fixed to the membrane by baking for 2 h at 80°C and then cross-linked to the membranes using an ultraviolet cross linker (Amersham Biosciences, Piscataway, NJ). The membranes were prehybridized for more than 3 h in SDS buffer [50% deionized formamide (v/v), 5 × SSC, 7% SDS, 2% blocking reagent (Roche Diagnostics, Mannheim, Germany), 50 mM sodium-phosphate (pH 7.0) and 0.1% N-lauroylsarcosine (w/v)] and hybridization was then performed overnight in the same buffer containing the gene-specific digoxin (DIG)-labeled probes at 45°C. Probes were prepared with a DIG probe synthesis kit (Roche Applied Science, Mannheim, Germany) according to the manufacturer’s instructions. All probes were synthesized from the 3′-untranslated regions of the genes. Following hybridization, membranes were washed twice for 10 min with 2 × SSC containing 0.1% SDS at 25°C, followed by washing twice for 30 min in 0.1 × SSC containing 0.1% SDS at 62°C. The signals were detected with chemiluminescence using CDP-Star™ (Roche Diagnostics) as described by the manufacturer. The specific primers used for synthesis of two FaAux/IAAs DIG-labeled probes were listed in supplementary Table 1.
Results and discussion
Isolation and characterization of FaAux/IAA cDNAs
The Aux/IAA genes are present as multigene families in nearly all plants examined, including soybean [11], mung bean [15], pea [35], tobacco [36], tomato [19], Populus [37], Arabidopsis [14], loblolly pine [38], and rice [18, 39]. In this study, two fragments of different Aux/IAA homologues of approximately 500 bp, were cloned from strawberry fruit by RT-PCR using degenerate primers, and their corresponding full length sequences, designated FaAux/IAA1 and FaAux/IAA2, were subsequently amplified by RACE-PCR. FaAux/IAA1 cDNA (1007 bp) and FaAux/IAA2 cDNA (1264 bp) consisted of a 5′-untranslated region of 165 bp, an ORF of 546 bp and a 3′-untranslated region of 296 bp, a 5′-untranslated region of 148 bp, an ORF of 768 bp and a 3′-untranslated region of 348 bp, respectively. They encoded the predicted polypeptides of 182 and 256 amino acids, with the predicted molecular weights of 20.41 and 27.89 kDa, respectively. A BLAST search of GenBank revealed that FaAux/IAA1 shared 53 or 50% identity with that of PtAux/IAA (ABH01143) from poplar or StAux/IAA (ABB55368) from potato, while FaAux/IAA2 shared 66 or 50% identity with that of PtAux/IAA or VvAux/IAA from grape (AAL92850), at the protein level.
The optimal multiple sequence alignment of FaAux/IAA proteins with other homologies was presented in supplementary Fig. 1. The predicted amino acid sequence of FaAux/IAA2 and FaAux/IAA9 contained all four domains (I, II, III and IV) conserved among Aux/IAA proteins. Previous studies indicated that Domain I has been assigned a repressor function [40], domain II is responsible for rapid degradation of the Aux/IAA proteins, while domains III and IV are responsible for homo- and heterodimerization among the various members of the Aux/IAA and auxin response factor (ARF) proteins [41]. Moreover, the comparison showed high degree of identity within the four consensus domains: 100% for domain I, 81–100% for domain II, 87–100% for domain III, and 88–100% for domain IV. The N-termini and interdomain regions were more divergent in size and sequence (Supplementary Fig. 1). In addition, the basic amino acids located in between domains I and II (.KR.RSYR..) constitute a bipartite nuclear localization signal (NLS) [20, 42, 43] and a basic cluster KRLRIMK, resembling SV40 [13] and a MAT α2-like NLS presented at the end of domain IV [44, 45] were also found in the predicted amino acid sequence of FaAux/IAA1and FaAux/IAA2 (Supplementary Fig. 1). Overall, these observations indicated that these two FaAux/IAAs may be nuclear-localized proteins and both types of NLS sequences present are functional, and shared common features with Aux/IAAs obtained from other plants.
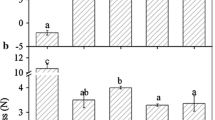
Changes of FaAux/IAA1 and FaAux/IAA2 transcripts (a), and fruit firmness (b) during strawberry fruit development. Fruit were harvest at the following stages: small green (SG), large green (LG), white (W), turning (T), ripe (R). In a total RNA (10 μg per lane) was used for northern blot analysis and hybridized with DIG-labeled probes, and ethidium bromide-stained rRNA was shown as the loading control. In b vertical bars represented standard deviations (S.D.) of means. Different letters indicate a statistical difference at 5% level among data groups according to Duncan’s multiple range test
To investigate the evolutionary relationships among FaAux/IAA genes and other homologies, a phylogenetic analysis was performed using the CLUSTAL W program (Supplementary Fig. 2). The phylogenetic tree shown in supplementary Fig. 2 suggested that Aux/IAA proteins could be grouped into four distinct subfamilies (I, II, III, and IV), as was previously reported by Wang et al. [20]. FaAux/IAA2 felled into subfamily II, along with sequences from Arabidopsis thaliana including AtIAA17 which involved in root hair development [46], while FaAux/IAA1 showed the highest homology to the sequence in subfamily III, including OsIAA1 which appeared to be correlated with the elongation of excised coleoptile segments [16].
Expression analysis of FaAux/IAA1and FaAux/IAA2 genes during fruit development and in different tissues
To investigate whether the expressions of the FaAux/IAA genes in strawberry were associated with fruit development, their spatial and temporal expression patterns in Fragaria × ananassa, cv. Toyonaka was analysed by northern blotting analysis. Thus, the transcript level of FaAux/IAA genes in fruit at five different developmental stages according to the external coloration degree, size and fruit firmness, and in root, stem, leaf, flower and calyx tissues were examined. As shown in Fig. 1a, both FaAux/IAA1 and FaAux/IAA2 transcripts showed higher levels in the fruit of small green (SG), large green (LG) and white (W), which represented the early phase of fruit development [33], showing higher levels in fruit firmness (Fig. 3b). When ripening initiated, at turning (T) and full-ripe red (R) developmental stage, with remarkable decrease in fruit firmness (Fig. 1b), transcripts of FaAux/IAA1 and FaAux/IAA2 sharply decreased to hardly detected levels (Fig. 1a). These results indicated that expressions of FaAux/IAA1 and FaAux/IAA2 genes were associated with early strawberry fruit development, and might play a negative role in regulating fruit ripening. Similar results were also obtained in tomato that down regulation a single Aux/IAA family member (IAA9) resulted in early fruit development, giving rise to parthenocarpy [20]. However, another Aux/IAA family member in tomato, SlIAA3, exhibited lower expression level in immature green fruit, and higher levels in mature green, breaker ripe and red ripe fruit [4]. These observations suggest that members of Aux/IAA gene families may play different roles in regulating fruit development. It has been reported that physiological function of Aux/IAAs was determined by both the pattern of gene expression and the properties of gene products, but that gene expression may have a primary role [47–49]. In addition, properties of Aux/IAAs were also likely determined by the binding constants for ARFs through domains III and IV [48]. Therefore, quantitative determination of the interaction between Aux/IAAs and ARFs will be needed to further understand functional differentiation of the Aux/IAA gene families [49].
The expression patterns of the two FaAux/IAAs in different strawberry tissues were presented in Fig. 2. It was interesting to observe that neither of the two FaAux/IAA genes showed fruit-specific expression. Both FaAux/IAA1 and FaAux/IAA2 expressions were detected at relatively lower levels in root. On the contrary, their transcript levels were higher in the stem, leaf, flower and calyx (Fig. 2). These results indicated that both the two Aux/IAA genes, whose expression related to the fruit development, might be also correlated with vegetative growth of ‘Toyonaka’ strawberry, which were consistent with previous reports that Aux/IAA proteins mediate several distinct cellular processes at virtually all stages of development processes [20, 50, 51].
Expression analysis of two FaAux/IAA genes in vegetative tissues. Total RNA (10 μg per lane) from roots (R), stems (S), leaves (L), flowers (FL), and calyxes (C) was electrophoresed and then hybridized with DIG-labeled FaAux/IAA probes. Ethidium bromide staining of the gel, labelled rRNA, is shown below the blots as a loading control
Regulation of FaAux/IAAs by NAA treatment
Auxin plays an important role in fruit development and growth, and NAA can facilitate fruit growth in litchi and longan [52]. In strawberry, the fruit growth and ripening are regulated mainly by auxin produced in the achenes. Auxins stimulate receptacle expansion during fruit development, and later inhibit fruit ripening [23], furthermore, the expression of most of ripening-related genes are negatively regulated by auxin [26–28]. To examine the role of NAA in stimulating growth and delaying ripening of strawberry fruit in relation to FaAux/IAAs expressions, strawberry fruit were treated with NAA at two different stages of fruit development and the levels of two FaAux/IAA transcripts were evaluated. As shown in Fig. 3a, when NAA was applied to the strawberry fruit at large green (LG) fruit stage, the accumulations of FaAux/IAA1 and FaAux/IAA2 were obviously induced within 2 h and 6 h after NAA treatment, rose to a peak at about 12 h and 2 days respectively, and FaAux/IAA2 transcripts remained relatively higher levels until 10 days. While, when treated the fruit at white fruit (W) stage, NAA increased the accumulation of FaAux/IAA1 within 0.5 h, and kept at higher levels until 2 days, meanwhile, the accumulation of FaAux/IAA2 was enhanced at 1 day after NAA treatment and FaAux/IAA2 transcripts kept at relatively higher levels until 10 days (Fig. 3b). The results exhibited that FaAux/IAA1 and FaAux/IAA2 were auxin-inducible, similar to the previous report that NAA increased the Aux/IAA accumulations in wheat [45], cestrum cut flowers [53], Arabidopsis [54], rice [18, 39, 55] and tomato [20]. Thus, it could be speculated that enhancement of FaAux/IAA1 and FaAux/IAA2 may be attributed at least or partially to auxin-induced fruit growth and delayed fruit ripening of strawberry.
Regulations of FaAux/IAA1 and FaAux/IAA2 by NAA treatment at the developmental stage of large green (LG) (a) and white (W) fruit (b). Fruit were dipped for 1 min in a solution containing 0 (control) or 100 mg/l NAA, and then sampled at 0, 0.5, 2, 6, 12 h, 1, 2, 4, 7, 10 days. Total RNA (10 μg per lane) was used for northern blot analysis and hybridized with DIG-labeled probes, and ethidium bromide-stained rRNA was shown as the loading control
In conclusion, we cloned and characterized two strawberry early auxin-responsive Aux/IAA genes. Preliminary results suggest that the two auxin-inducible FaAux/IAAs may be involved in the regulation of growth and ripening of strawberry fruit, however, further studies are needed to fully discovery the biofunction of FaAux/IAA genes in regulating fruit development by transgenic research.
References
Friml J (2003) Auxin transport-shaping the plant. Curr Opin Plant Biol 6:7–12
Quint M, Gray WM (2006) Auxin signaling. Curr Opin Plant Biol 9:448–453
Guilfoyle TJ (1999) Auxin-regulated genes and promoters. In: Hooykaas PJJ, Hall M, Libbenga KL (eds) Biochemistry and molecular biology of plant hormones. Elsevier, Leiden, pp 423–459
Zhang JH, Chen RG, Xiao JH, Zou LP, Li HX, Ouyang B, Ye ZB (2007) Isolation and characterization of SlIAA3, an Aux/IAA gene from tomato. Mitochondrial DNA 18:407–414
Zhang JH, Chen RG, Xiao JH, Qian CJ, Wang TT, Li HX, Ouyang B, Ye ZB (2007) A single-base deletion mutation in SlIAA9 gene causes tomato (Solanum lycopersicum) entire mutant. J Plant Res 120:671–678
Benjamins R, Scheres B (2008) Auxin: the looping star in plant development. Annu Rev Plant Biol 59:443–465
Mockaitis K, Estelle M (2008) Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol 24:55–80
Wang H, Tian CE, Duan J, Wu KQ (2008) Research progresses on GH3 s, one family of primary auxin-responsive genes. Plant Growth Regul 56:225–232
Tian Q, Uhlir NJ, Reed JW (2002) Arabidopsis SHY2/IAA3 inhibits auxin-regulated gene expression. Plant Cell 14:301–319
Walker JC, Key JL (1982) Isolation of cloned cDNAs to auxin responsive poly (A) RNAs of elongating soybean hypocotyls. Proc Natl Acad Sci USA 79:7185–7189
Ainley WM, Walker JC, Nagao RT, Key JL (1988) Sequence and characterization of two auxin-regulated genes from soybean. J Biol Chem 263:10658–10666
Theologis A, Huynh TV, Davis RW (1985) Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol 183:53–68
Abel S, Theologis A (1995) A polymorphic bipartite motif signals nuclear targeting of early auxin-inducible proteins related to PS-IAA4 from pea (Pisum sativum). Plant J 8:87–96
Dharmasiri N, Estelle M (2004) Auxin signaling and regulated protein degradation. Trends Plant Sci 9:302–308
Yamamoto KT, Mori H, Imaseki H (1992) cDNA cloning of indole-3-acetic acid regulated genes: Aux22 and SAUR from mung bean (Vigna radiata) hypocotyls tissue. Plant Cell Physiol 33:93–97
Thakur JK, Tyagi AK, Khurana JP (2001) OsIAA1, an Aux/IAA cDNA from rice, and changes in its expression as influenced by auxin and light. DNA Res 8:193–203
Thakur JK, Jain M, Tyagi AK, Khurana JP (2005) Exogenous auxin enhances the degradation of a light down-regulated and nuclear-localized OsiIAA1, an Aux/IAA protein from rice, via proteasome. Biochim Biophys Acta 1730:196–205
Jain M, Kaur N, Garg R, Thakur JK, Tyagi AK, Khurana JP (2006) Structure and expression analysis of early auxin-responsive Aux/IAA gene family in rice (Oryza sativa). Funct Integr Genomics 6:47–59
Nebenfuhr A, White TJ, Lomax TL (2000) The diageotropica mutation alters auxin induction of a subset of the Aux/IAA gene family in tomato. Plant Mol Biol 44:73–84
Wang H, Jones B, Li ZG, Frasse P, Delalande C, Regad F, Chaabouni S, Latche A, Pech JC, Bouzayen M (2005) The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 17:2676–2692
Abel S, Oeller PW, Theologis A (1994) Early auxin-induced genes encode short-lived nuclear proteins. Proc Natl Acad Sci USA 91:326–330
Nitsch JP (1950) Growth and morphogenesis of the strawberry as related to auxin. Am J Bot 37:211–215
Given NK, Venis MA, Grierson D (1988) Hormonal regulation of ripening in the strawberry, a non-climacteric fruit. Planta 174:402–406
Medina-Escobar N, Cárdenas J, Valpuesta V, Muñoz-Blanco J, Caballero JL (1997) Cloning and characterization of cDNAs from genes differentially expressed during the strawberry fruit ripening process by a MAST-PCR-SBDS method. Anal Biochem 248:288–296
Manning K (1994) Changes in gene expression during strawberry fruit ripening and their regulation by auxin. Planta 194:62–68
Manning K (1998) Isolation of a set of ripening-related genes from strawberry: their identification and possible relationship to fruit quality traits. Planta 205:622–631
Bustamante CA, Civello PM, Martínez GA (2009) Cloning of the promoter region of β-xylosidase (FaXyl1) gene and effect of plant growth regulators on the expression of FaXyl1 in strawberry fruit. Plant Sci 177:49–56
Rosli HG, Civello PM, Martínez GA (2009) a-L-Arabinofuranosidase from strawberry fruit: cloning of three cDNAs, characterization of their expression and analysis of enzymatic activity in cultivars with contrasting firmness. Plant Physiol Biochem 47:272–281
Wills RBH, Kim GH (1995) Effect of ethylene on postharvest life of strawberries. Postharvest Biol Technol 6:249–255
Tian MS, Prakash S, Elgar HJ, Young H, Burmeister DM, Ross GS (2000) Responses of strawberry fruit to 1-methylcyclopropene (1-MCP) and ethylene. Plant Growth Regul 32:83–90
Trainotti L, Pavanello A, Casadoro G (2005) Different ethylene receptors show an increased expression during the ripening of strawberries: does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? J Exp Bot 56:2037–2046
Iannetta PPM, Laarhoven LJ, Medina-Escobar N, James EK, McManus MT, Davies HV, Harren FJM (2006) Ethylene and carbon dioxide production by developing strawberries show a correlative pattern that is indicative of ripening climacteric fruit. Physiol Plant 127:247–259
Civello PM, Powell ALT, Sabehat A, Bennett AB (1999) An expansin gene expressed in ripening strawberry fruit. Plant Physiol 121:1273–1279
Wan CY, Wilkins TA (1994) A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Anal Biochem 223:7–12
Oeller PW, Keller JA, Parks JE, Silbert JE, Theologis A (1993) Structural characterization of the early indoleacetic acid-inducible genes PS-IAA4/5 and PS-IAA6 of pea (Pisum sativum L). J Mol Biol 233:789–798
Dargeviciute A, Roux C, Decreux A, Sitbon F, Perrot-Rechenmann C (1998) Molecular cloning and expression of the early auxin responsive Aux/IAA gene family in Nicotiana tabacum. Plant Cell Physiol 39:993–1002
Moyle R, Schrader J, Stenberg A, Olsson O, Saxena S, Sandberg G, Bhalerao RP (2002) Environmental and auxin regulation of wood formation involves members of the Aux/IAA gene family in hybrid aspen. Plant J 31:675–685
Goldfarb B, Lanz-Garcia C, Lian Z, Whetten R (2003) Aux/IAA gene family is conserved in the gymnosperm, loblolly pine (Pinus taeda). Tree Physiol 23:1181–1192
Song Y, Wang L, Xiong L (2009) Comprehensive expression profiling analysis of OsIAA gene family in developmental processes and in response to phytohormone and stress treatments. Planta 229:577–591
Tiwari SB, Hagen G, Guilfoyle TJ (2004) Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16:533–543
Ouellet F, Overvoorde PJ, Theologis (2001) A IAA17/AXR3: biochemical insight into an auxin mutant phenotype. Plant Cell 13:829–841
Robbins J, Dilworth SM, Laskey RA, Dingwall C (1991) Two interdependent basic domains in nucleoplasmin nuclear targeting sequence. Cell 64:615–623
Gorlich D, Mattaj IW (1996) Nucleocytoplasmic transport. Science 271:1513–1518
Raikhel NV (1992) Nuclear targeting in plants. Plant Physiol 100:1627–1632
Singla B, Chugh A, Khurana JP, Khurana P (2006) An early auxin-responsive Aux/IAA gene from wheat (Triticum aestivum) is induced by epibrassinolide and differentially regulated by light and calcium. J Exp Bot 57:4059–4070
Knox K, Grierson CS, Leyser O (2003) AXR3 and SHY2 interact to regulate root hair development. Development 130:5769–5777
Wang Y, Deng D, Bian Y, Lv Y, Xie Q (2010) Genome-wide analysis of primary auxin-responsive Aux/IAA gene family in maize (Zea mays L.). Mol Biol Rep. doi:10.1007/s11033-010-0058-6
Wu B, Li YH, Wu JY, Chen QZ, Huang X, Chen YF, Huang XL (2010) Over-expression of mango (Mangifera indica L.) MiARF2 inhibits root and hypocotyl growth of Arabidopsis. Mol Biol Rep. doi:10.1007/s11033-010-9990-8
Muto H, Watahiki MK, Yamamoto KT (2007) What makes each Aux/IAA gene unique in its gene family, expression pattern or properties of the gene product? Plant Signal Behav 2:390–392
Hamann T, Mayer U, Jurgens G (1999) The auxin-insensitive bodenlos mutation affects primary root formation and apical-basal patterning in the Arabidopsis embryo. Development 126:1387–1395
Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99:463–472
Feng HL, Zhong YX, Xie H, Chen JY, Li JG, Lu WJ (2008) Differential expression and regulation of longan XET genes in relation to fruit growth. Plant Sci 174:32–37
Abebie B, Lers A, Philosoph-Hadas S, Goren R, Riov J, Meir S (2008) Differential effects of NAA and 2, 4-D in reducing floret abscission in cestrum (Cestrum elegans) cut flowers are associated with their differential activation of Aux/IAA homologous genes. Ann Bot 101:249–259
Sato A, Yamamoto KT (2008) Overexpression of the non-canonical Aux/IAA genes causes auxin-related aberrant phenotypes in Arabidopsis. Physiol Plant 133:397–405
Song Y, You J, Xiong L (2009) Characterization of OsIAA1 gene, a member of rice Aux/IAA family involved in auxin and brassinosteroid hormone responses and plant morphogenesis. Plant Mol Biol 70:297–309
Acknowledgement
This work was supported in part by the Guangdong Science Foundation (Grant 06200670).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Dj., Chen, Jy. & Lu, Wj. Expression and regulation of the early auxin-responsive Aux/IAA genes during strawberry fruit development. Mol Biol Rep 38, 1187–1193 (2011). https://doi.org/10.1007/s11033-010-0216-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0216-x