Abstract
Selenoprotein S (SelS), a member of selenoprotein family, plays important regulatory function in inflammation and metabolic diseases. SelS expression is up-regulated response to the inflammatory stimulus in many mammal cells, animal models as well as patients. In order to further understand the function of SelS gene, molecular characterization and transcriptional regulation of SelS from a Bama mini-pig were analyzed in the present study. The results showed that pig SelS encoded a protein of 190 amino acid with estimated molecular weight of 21.23 kDa and pI of 9.526. The genomic structure, promoter and deduced amino acid sequence were analyzed and found to share high similarity with those of human SelS. Pig SelS fusion protein was demonstrated to localize in the cytoplasm by fluorescence microscopy. Real-time PCR revealed the ubiquitous expression pattern of pig SelS in diverse tissues, a high level expression was observed in the liver and lung, relatively low expression in other tissues, especially in muscle. Promoter deletion analysis further suggests that an NF-κB binding site within the SelS promoter is responsible for the up-regulation of SelS transcription.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium is an essential trace element for animals and humans and is best known for its role in the antioxidant function mediated by selenoproteins. Within selenoproteins, selenium is incorporated into the polypeptide cotranslationally by means of the amino acid selenocysteine (Sec) in response to an in-frame UGA codon. At least 25 selenoproteins have been identified in humans [1], but the function of most selenoproteins remains obscure.
Selenoprotein S (SelS) is a newly identified membrane protein and its gene expression is up-regulated in the liver of Psammomys obesus after fasting [2]. Glucose deprivation and endoplasmic reticulum (ER) stress inducers increase SelS gene expression and protein content in HepG2 cells [3]. SelS also protects macrophage cells against ER stress-induced apoptosis [4] and modifies ER stress during Z variant alpha1-antitrypsin deficiency [5]. In contrast, SelS protein overexpression reduces glucose intake in H4IIE cells and may be contribute to insulin resistance in the liver [6]. There is increasing evidence that SelS expression is strongly associated with inflammation [7], coronary heart disease [8], arthritis [9, 10], type 2 diabetes mellitus [11, 12], and may also be related to lipoprotein metabolism [13] and spermatogenesis [14]. One SelS promoter variant, −105G → A, showed a significant association with inflammatory response [15, 16], preeclampsia [17] and gastric cancer [18]. However, other studies suggested that there was lack of association between the SelS gene and autoimmune inflammatory diseases or cerebrovascular disease [19, 20].
Mini-pig has been considered as an important model organism widely used for human health research because the metabolic and immune similarity between mini-pig and human. In this study, we isolated SelS sequence of S. scrofa and investigated its potential biological role by analyzing its subcellular localization, tissue expression profile and NF-κB-regulated transcription utilizing a Bama mini-pig. The knowledge of SelS gained in this study will contribute useful information for further research.
Materials and methods
Isolation of pig SelS
Homologous ESTs of pig were available from GenBank using a BLASTN program based on human SelS mRNA sequence. Pig SelS EST contig was assembled using SEQMAN software (LASERGENE6.0). Total RNA was extracted from liver tissues of Bama mini-pig using Trizol reagent (Invitrogen, Beijing, China) and treated with RNase-free DNase (Promega, Madison, WI, USA). First strand cDNA synthesis was performed using MMLV reverse transcriptase (Promega) according to the manufacturer’s instructions. The specific primers (CDS-F/R) were designed to amplify pig SelS full-length coding sequence (CDS) (Online Resource Table 1).
Sequence analysis of pig SelS gene
The cDNA sequence was blasted against the S. scrofa draft genome sequence (database in http://www.sanger.ac.uk) to determine the SelS gene exon–intron architecture. The SelS amino acid sequences of pig and those of other species retrieved from GenBank were also compared. Multiple sequence alignments were performed using Clustal X2. The deduced protein structure was annotated using STRAP. Prediction of putative transcriptional binding sites was performed using Transcriptional Element Search Software (TESS) and TFSEARCH program.
Tissue expression patterns of pig SelS gene
Tissue distribution of pig SelS relative to that of pig glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was analyzed by real-time PCR. Brain, heart, lung, liver, stomach, spleen, kidney, small intestine, lymph nodes and longissimus dorsi tissues were collected from mature Bama mini-pigs. The primer pairs (RT-F/R and GAPDH-F/R, Online Resource Table 1) were designed. First strand cDNA was prepared as described above. The PCR reactions were performed on a 7500 real-time PCR System (Applied Biosystems) using SYBR Premix Ex Taq™ with ROX (TaKaRa, Dalian, China) and primers listed in Online Resource Table 1. Reaction conditions were denaturation for 2 min at 95°C, followed by 40 cycles of 15 s at 95°C, 30 s at 60°C, and 30 s at 72°C. Gene expression levels are reported relative to GAPDH expression using the comparative Ct (ΔΔCt) value method.
Plasmid construction
Genomic DNA template was prepared from Bama mini-pig ear tissue. For deletion analyses, an 1840 bp amplicon (−1822 to +17, with the first nucleotide of the “ATG” start codon designated as +1) was cloned using LA Taq DNA Polymerase and ligated into the pMD18-T vector (TaKaRa, Dalian, China) for subsequent manipulation as described previously [21]. Briefly, constructs containing variable length of truncated pig SelS promoter were individually amplified using four different forward primers and a common reverse primer. The forward primers and reverse primer contained MluI and XhoI recognition sequences, respectively, in order to facilitate plasmid ligation (Online Resource Table 1). The amplified fragments were then inserted into the multiple cloning site of the pGL3-basic vector in order to generate luciferase reporter constructs. For NF-κB binding site mutagenesis, four primers and three PCR reactions were conducted to create a site-specific mutation by overlap extension. The NF-κB mutation plasmid was constructed as described above. For mammalian cell expression, a pair of primers were designed with BamHI and XhoI restriction site at 5′ ends of the forward and reverse primer, respectively (Online Resource Table 1), and used to clone the pig SelS ORF. Within the reverse primer, one adenine was replaced with cytosine, which changed the Sec codon (TGA) into a cysteine codon (TGC). This change ensured ORF read-through without changing the amino acid skeleton. PCR-amplified products were then inserted into the pEGFP-N3 plasmid (Clontech) to generate pS-GFP. All plasmids were sequenced to confirm proper insertion prior to transfection experiments.
Cell culture, cell transfection and luciferase assays
Porcine kidney cells (PK-15) were utilized to investigate the cellular localization of the SelS protein and to perform luciferase reporter assays. Cells were seeded on coverslips in six-well plates for localization studies or in 24-well plates for luciferase assays and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, USA) supplemented with 10% (v/v) fetal bovine serum under humidified air containing 5% CO2 at 37°C. When the cells had reached 70% confluence, transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. At 24 h after transfection, the cells were treated with 10 μM PDTC (pyrrolidine dithiocarbamate, Sigma, St. Louis, MO) and cultured for another 24 h before assaying for luciferase activity. Luciferase assays were performed using Dual Luciferase Assay kits from Promega and a Luminometer TD-20/20. Firefly luciferase levels were normalized against Renilla luciferase levels.
Subcellular localization
The procedure of subcellular localization referred to [22]. The fluorescent images were captured using an IX 71 microscope (Olympus) at different excitation wavelengths, and the overlay images demonstrating the relative distribution of the fusion protein were generated using the software DPController2.
Results
Cloning of pig SelS full-length CDS
The pig SelS gene sequence was assembled using SEQMAN following a BLASTN search against human sequence. A 570 bp fragment exactly covering the full-length CDS was obtained by amplification of pig liver cDNA using the specific primer pair listed in Online Resource Table 1. The obtained pig SelS cDNA sequence was deposited in GenBank with accession no. GU983865.
Sequence analysis
A detailed pig SelS gene structure was developed based on comparison of the pig SelS cDNA and genomic DNA sequences (Online Resource Fig. 6). This sequence included a partial exon 1, 5 other exons and 5 introns. All of the exon–intron splice junctions conformed to the GT/AG rule.
Multiple sequence alignments were performed by pairwise comparisons using Clustal X2 (Online Resource Fig. 7), revealed that the deduced pig SelS amino acid demonstrated similarities with those of human (88%), chimpanzee (87%), dog (90%), cattle (92%), mouse (82%), rat (82%), platypus (61%), chicken (60%) Xenopus tropicalis (47%), zebrafish (46%). Pig SelS encoded a protein of 190 amino acids. The calculated molecular mass was 21.23 kDa and the estimated pI of this protein was 9.526. Secondary structure including helices and beta sheets and transmembrane domain were showed in Online Resource Fig. 7. An N-linked glycosylation site was also present in pig SelS.
To further understand pig SelS transcriptional regulation via its cis-regulatory elements, approximately 1.8 kb of DNA sequence located 5′ of the start codon was scanned using prediction software and a putative NF-κB binding site (−457 to −448 bp) was found (Fig. 1).
SelS was localized in cytoplasm and expressed in different tissues
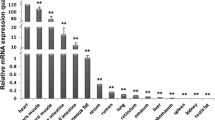
Pig SelS fusion protein was found to be mostly distributed in cytoplasm of PK15 cells (Fig. 2). The results of real-time PCR showed that pig SelS was expressed ubiquitously in all tissues examined. The high pig SelS expression was observed in the liver and lung, very low expression was detected in muscle tissues such as the longissimus dorsi. Pig SelS mRNA levels were very similar in the other tissues examined (Fig. 3).
The cellular localization of the pS-GFP fusion protein in PK15 cells. pS-GFP fusion proteins were mainly distributed in the cell cytoplasm (a); mitochondria stained with the mitochondrial-specific dye Mito-Tracker Red (b); nuclei stained with Hoechst33342 (c); and a merged overlay of the three signals (d). The arrows represented localization of pS-GFP fusion proteins
NF-κB regulated pig SelS promoter activity in PK15 cells
In order to verify the transcriptional regulation of pig SelS by NF-κB as predicted above, we undertook a 5′ deletion analysis of the pig SelS promoter sequence and tested the ability of these truncated sequences to drive the expression of a luciferase reporter. Five luciferase reporter plasmids with or without the conserved NF-κB binding site from the truncated SelS promoter sequence (pS-1616/17, pS-1117/17, pS-601/17, pS-398/17 and pS-601/17mut) were constructed and transfected PK15 cells. After co-transfection and luciferase assays, we observed that the pS-601/17 construct had the largest promoter activity, despite having a shorter sequence than the pS-1616/17 and pS-1117/17 constructs. Further deletion at position −398 (pS-398/17) resulted in a significant decrease in promoter activity. An NF-κB mutant form of pS-601/17 (pS-601/17mut) attenuated transcriptional initiation in a similar degree (Fig. 4).
Luciferase assays of pSelS promoter activity. Five luciferase reporter plasmids expressing successive truncations of the pSelS promoter sequence were constructed and transfected into PK15 cells. The resulting firefly luciferase activity was normalized to Renilla luciferase activity and the relative values are presented as fold induction over the activity of the pGL3-basic vector. The location of each 5′-deletion sequence is indicated at the left of each bar. Boxes represent the location of the putative NF-κB binding site within the promoter regions. The mutated putative NF-κB binding site is labeled with an “×”. Statistical differences in luciferase activity were assessed using the Student’s t-test. *P < 0.05
To further confirm NF-κB regulation of pig SelS expression, we transfected two shorter plasmids pS-601/17 and pS-398/17 into PK15 cells and 24 h later treated the cells with pyrrolidine dithiocarbamate (PDTC), which specifically inhibits NF-κB activation. The results showed that the pS-601/17 promoter activity decreased approximately 30% following PDTC-induced inhibition of NF-κB activation, however, no effect occurred on the activity of pS-398/17 or the control vector (Fig. 5).
The effects of PDTC on the activity of pSelS promoter fragments harbouring 5′ deletions. PK15 cells were transfected with different luciferase reporter constructs. After 24 h, cells were treated with PDTC (10 μM), incubated an additional 24 h, and then firefly luciferase activity was assessed and normalized to Renilla luciferase activity. For each construct, statistical differences in luciferase activity between basal transcription and following PDTC exposure were analyzed using the Student’s t-test. *P < 0.05
Discussion
Selenoproteins are a unique class of proteins found in many organisms from all three domains of life. Several of selenoprotein-encoding genes identified in human are known to be important for human health and disease [23]. SelS is a newly identified membrane protein and it has become clear that SelS plays an important role in inflammation and metabolic diseases [7, 11–13, 15, 16].
A new full-length 570 bp CDS encoding 190 amino acids of SelS from Bama mini-pig was cloned in the present study. Pig and human SelS genes showed high homology (88%) and similar genomic structures, which all harbor six exons and five introns. Human SelS gene have two transcript variants with different 3′ UTRs (GenBank accession no. NM_018445.4 and NM_203472.1, respectively), encoding a same protein (GenBank accession no. NP_060915.2). Common properties of SelS proteins included localization of the Sec at residue number 189 and two cysteines (Cys) located at residues 41 and 175, with the relative positions numbered according to pig SelS. Interestingly, residue number 40 was a continuous Cys in pig SelS, with other organisms as the exceptions, which justly appeared at the conjunction of helices and beta sheets and the center of the transmembrane domain (Fig. 2). Sec is often present at the catalytic sites of selenoprotein oxidoreductases and exhibits a higher activity than its Cys mutant form [24], which should execute important biological function for members of the selenoprotein family. In the case of SelW, glutathione probably bound to a Cys residue and acted as an effective reducing substance [25, 26]. However, the significance of these different Cys residues and the possible different forms of SelS has not been established.
WoLFPSORT software predicted that pig SelS protein was mostly distributed in the ER and peroxisome membranes. Cell fractionation indicated that SelS was localized in the plasma membrane and microsomes [6]. In this study we found pig SelS fusion protein was mostly distributed in cytoplasm. It has become apparent that ER stress plays a central role in development of some human metabolic diseases such as obesity, insulin resistance, and type 2 diabetes [27, 28]. Considering that cytoplasm distribution of pig SelS fusion protein and the evidences that this gene associated with ER stress, it is possible that SelS could attenuate damages arisen from ER stress by maintenance of redox homeostasis. The ubiquitous expression of pig SelS strongly suggested that SelS may play an essential role in various tissues.
There is a putative NF-κB binding site in the promoter of pig SelS gene, just as in the human SelS gene. The eukaryotic transcription factor NF-κB have a broad role in inducibly and coordinately controlling genes of significant biomedical importance, such as those encoding inflammatory factors [29, 30]. NF-κB binding sites (with the general consensus sequence: GGGRNNYYCC, R = purine, Y = pyrimidine) have been identified in promoters and enhancers of a number of inducible genes. Results of promoter activity analysis indicate that potential positive regulatory elements seem to be located in the region −601 to −398, which was essential for a high level of expression of the SelS gene, and potential negative regulatory elements seem to be located in the region −1616 to −601, which contributed to a low level expression of the SelS gene. Site-directed mutagenesis and PDTC treatments indicated that transcription factor NF-κB might play an important role in transcriptional regulation of pig SelS gene. The present results suggested that pig SelS was a regulatory target gene of NF-κB, and shed insight on further investigations into the function of pig SelS, especially via NF-κB relevant signaling pathways.
References
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300:1439–1443
Walder K, Kantham L, McMillan JS, Trevaskis J, Kerr L, De Silva A, Sunderland T, Godde N, Gao Y, Bishara N, Windmill K, Tenne-Brown J, Augert G, Zimmet PZ, Collier GR (2002) Tanis: a link between type 2 diabetes and inflammation? Diabetes 51:1859–1866
Gao Y, Feng HC, Walder K, Bolton K, Sunderland T, Bishara N, Quick M, Kantham L, Collier GR (2004) Regulation of the selenoprotein SelS by glucose deprivation and endoplasmic reticulum stress—SelS is a novel glucose-regulated protein. FEBS Lett 563:185–190
Kim KH, Gao Y, Walder K, Collier GR, Skelton J, Kissebah AH (2007) SEPS1 protects RAW264.7 cells from pharmacological ER stress agent-induced apoptosis. Biochem Biophys Res Commun 354:127–132
Kelly E, Greene CM, Carroll TP, McElvaney NG, O’Neill SJ (2009) Selenoprotein S/SEPS1 modifies endoplasmic reticulum stress in Z variant alpha1-antitrypsin deficiency. J Biol Chem 284:16891–16897
Gao Y, Walder K, Sunderland T, Kantham L, Feng HC, Quick M, Bishara N, de Silva A, Augert G, Tenne-Brown J, Collier GR (2003) Elevation in tanis expression alters glucose metabolism and insulin sensitivity in H4IIE cells. Diabetes 52:929–934
Zeng J, Du S, Zhou J, Huang K (2008) Role of SelS in lipopolysaccharide-induced inflammatory response in hepatoma HepG2 cells. Arch Biochem Biophys 478:1–6
Alanne M, Kristiansson K, Auro K, Silander K, Kuulasmaa K, Peltonen L, Salomaa V, Perola M (2007) Variation in the selenoprotein S gene locus is associated with coronary heart disease and ischemic stroke in two independent Finnish cohorts. Hum Genet 122:355–365
Bos SD, Kloppenburg M, Suchiman E, van Beelen E, Slagboom PE, Meulenbelt I (2009) The role of plasma cytokine levels, CRP and Selenoprotein S gene variation in OA. Osteoarthritis Cartilage 17:621–626
Marinou I, Walters K, Dickson MC, Binks MH, Bax DE, Wilson AG (2009) Evidence of epistasis between interleukin 1 and selenoprotein-S with susceptibility to rheumatoid arthritis. Ann Rheum Dis 68:1494–1497
Du JL, Sun CK, Lü B, Men LL, Yao JJ, An LJ, Song GR (2008) Association of SelS mRNA expression in omental adipose tissue with Homa-IR and serum amyloid A in patients with type 2 diabetes mellitus. Chin Med J 121:1165–1168
Karlsson HK, Tsuchida H, Lake S, Koistinen HA, Krook A (2004) Relationship between serum amyloid A level and Tanis/SelS mRNA expression in skeletal muscle and adipose tissue from healthy and type 2 diabetic subjects. Diabetes 53:1424–1428
Gao Y, Pagnon J, Feng HC, Konstantopolous N, Jowett JB, Walder K, Collier GR (2007) Secretion of the glucose-regulated selenoprotein SEPS1 from hepatoma cells. Biochem Biophys Res Commun 356:636–641
Windmill K, Tenne-Brown J, Bayles R, Trevaskis J, Gao Y, Walder K, Collier GR (2007) Localization and expression of selenoprotein S in the testis of Psammomys obesus. J Mol Histol 38:97–101
Seiderer J, Dambacher J, Kühnlein B, Pfennig S, Konrad A, Török HP, Haller D, Göke B, Ochsenkühn T, Lohse P, Brand S (2007) The role of the selenoprotein S (SELS) gene −105G > A promoter polymorphism in inflammatory bowel disease and regulation of SELS gene expression in intestinal inflammation. Tissue Antigens 70:238–246
Curran JE, Jowett JB, Elliott KS, Gao Y, Gluschenko K, Wang J, Abel Azim DM, Cai G, Mahaney MC, Comuzzie AG, Dyer TD, Walder KR, Zimmet P, MacCluer JW, Collier GR, Kissebah AH, Blangero J (2005) Genetic variation in selenoprotein S influences inflammatory response. Nat Genet 37:1234–1241
Moses EK, Johnson MP, Tømmerdal L, Forsmo S, Curran JE, Abraham LJ, Charlesworth JC, Brennecke SP, Blangero J, Austgulen R (2008) Genetic association of preeclampsia to the inflammatory response gene SEPS1. Am J Obstet Gynecol 198:336.e1-5
Shibata T, Arisawa T, Tahara T, Ohkubo M, Yoshioka D, Maruyama N, Fujita H, Kamiya Y, Nakamura M, Nagasaka M, Iwata M, Takahama K, Watanabe M, Hirata I (2009) Selenoprotein S (SEPS1) gene −105G > A promoter polymorphism influences the susceptibility to gastric cancer in the Japanese population. BMC Gastroenterol 9:2
Hyrenbach S, Pezzini A, del Zotto E, Giossi A, Lichy C, Kloss M, Werner I, Padovani A, Brandt T, Grond-Ginsbach C (2007) No association of the −105 promoter polymorphism of the selenoprotein S encoding gene SEPS1 with cerebrovascular disease. Eur J Neurol 14:1173–1175
Martínez A, Santiago JL, Varadé J, Márquez A, Lamas JR, Mendoza JL, de la Calle H, Díaz-Rubio M, de la Concha EG, Fernández-Gutiérrez B, Urcelay E (2008) Polymorphisms in the selenoprotein S gene: lack of association with autoimmune inflammatory diseases. BMC Genomics 9:329
Wang H, Yang S, Yang E, Zhu Z, Mu Y, Feng S, Li K (2007) NF-kappaB mediates the transcription of mouse calsarcin-1 gene, but not calsarcin-2, in C2C12 cells. BMC Mol Biol 8:19
Wang HL, Wang H, Zhu ZM, Wang CF, Zhu MJ, Mo de L, Yang SL, Li K (2006) Subcellular localization, expression patterns, SNPs and association analyses of the porcine HUMMLC2B gene. Mol Genet Genomics 276:264–272
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9:775–806
Bar-Noy S, Gorlatov SN, Stadtman TC (2001) Overexpression of wild type and SeCys/Cys mutant of human thioredoxin reductase in E. coli: the role of selenocysteine in the catalytic activity. Free Radic Biol Med 30:51–61
Gu QP, Beilstein MA, Barofsky E, Ream W, Whanger PD (1999) Purification, characterization, and glutathione binding to selenoprotein W from monkey muscle. Arch Biochem Biophys 361:25–33
Beilstein MA, Vendeland SC, Barofsky E, Jensen ON, Whanger PD (1996) Selenoprotein W of rat muscle binds glutathione and an unknown small molecular weight moiety. J Inorg Biochem 61:117–124
Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140:900–917
Marciniak SJ, Ron D (2006) Endoplasmic reticulum stress signaling in disease. Physiol Rev 86:1133–1149
Wu Y, Zhou BP (2010) TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer 102:639–644
Di J, Pang J, Sun Q, Zhang Y, Fang Y, Liu X, Zhou J, Ruan X, Gao X (2009) Toll-like receptor 9 agonists up-regulates the expression of cyclooxygenase-2 via activation of NF-κB in prostate cancer cells. Mol Biol Rep 37(4):1849–1855
Acknowledgments
This work was supported by The National Natural Science Foundation of China (30800779, 30628019), The Ministry of Science and Technology of China (2008AA10Z143, 2009CB941604) and The Funding of State Key Laboratory of Animal Nutrition of China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, N., Jing, W., Cheng, J. et al. Molecular characterization and NF-κB-regulated transcription of selenoprotein S from the Bama mini-pig. Mol Biol Rep 38, 4281–4286 (2011). https://doi.org/10.1007/s11033-010-0551-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0551-y