Abstract
Fatty acid binding protein 3 (FABP3) is a member of the FABP family which bind fatty acids and have an important role in fatty acid metabolism. A large number of studies have shown that the genetic polymorphisms of FABP3 are positively correlated with intramuscular fat (IMF) content in domestic animals, however, the function and transcriptional characteristics of FABP3 in cattle remain unclear. Real-time PCR analysis revealed that bovine FABP3 was highly expressed in cardiac tissue. The 5′-regulatory region of bovine FABP3 was cloned and its transcription initiation sites were identified. Sequence analysis showed that many transcriptional factor binding sites including TATA-box and CCAAT-box were present on the 5′-flanking region of bovine FABP3, and four CpG islands were found on nucleotides from −891 to +118. Seven serial deletion constructs of the 5′-regulatory region evaluated in dual-luciferase reporter assay indicated that its core promoter was 384 base pairs upstream from the transcription initiation site. The transcriptional factor binding sites RXRα, KLF15, CREB and Sp1 were conserved in the core promoter of cattle, sheep, pigs and dogs. These results provide further understanding of the function and regulation mechanism of bovine FABP3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatty acid binding proteins (FABPs) belong to a small molecular weight (14–15 kDa) and highly conserved cytoplasmic protein family that bind fatty acids with high affinity [1]. They have a crucial role in intracellular fatty acid transport and metabolism [2]. In mammals, there are at least nine members in the FABP family which consists of FABP1 to FABP9 [3, 4].
Fatty acid binding protein 3 (FABP3), also known as H-FABP, mainly expresses in mature cardiomyocytes and skeletal muscle, but also exists in other tissues [5]. FABP3 plays a role in fatty acid transport and utilization in cardiac or skeletal muscle [6]. In addition, FABP3 is essential for cold tolerance and efficient fatty acid oxidation in the brown adipose tissue of mice [7]. Over-expression of the FABP3 gene can promote adipogenesis in 3T3-L1 preadipocytes [8]. Recently, FABP3 has been considered as a candidate gene associated with intramuscular fat (IMF) content in domestic animals.
A large number of studies have shown that the genetic polymorphism of FABP3 is positively correlated with IMF content in pigs [9–16]. In goats, the expression level of FABP3 is significantly correlated with IMF content [17]. Furthermore, the expression level of FABP3 is significantly different between low- and high-marbled groups in Korean cattle [18]. It is therefore important to explore the transcriptional regulation mechanism of FABP3, which helps to improve IMF content in domestic animals.
To date, there are many reports about the expression regulation of FABP3 [19–22]. However, the information about transcriptional regulation of FABP3 in cattle is limited. To better understand the function and the transcriptional characteristics of bovine FABP3, we analyzed the spatial expression pattern and identified the transcription initiation sites of the bovine FABP3. In addition, the 5′-regulatory region of bovine FABP3 was cloned and investigated in skeletal muscle cells and adipocytes.
Materials and methods
Ethics statement
All animal procedures were performed according to the guidelines of the China Council on Animal Care and the protocols were approved by the Experimental Animal Management Committee (EAMC) of Northwest A&F University.
Molecular cloning and sequence analysis
Genomic DNA was extracted from Qinchuan cattle blood, as described previously [23]. To obtain the 5′-regulatory region, the FABP3 gene was searched in the UCSC genome database (http://genome.ucsc.edu/cgi-bin/hgGateway). The primers (FABP3-PF/R, Supplemental Table 1) were designed based on the bovine genomic sequence (bosTau8_refGene_NM_174313 range = chr2:122719225-122783830) to amplify approximately 2 kb of 5′-regulatory sequence. The PCR product was purified from an agarose gel and subsequently cloned into the pGEM-T-Easy vector (Promega Corp.) prior to sequencing. The 5′-regulatory region was analyzed for potential transcription factor binding sites using the TESS (http://www.cbil.upenn.edu/cgi-bin/tess), Genomatix suite (http://www.genomatix.de/) and TFSEARCH (http://www.cbrc.jp/research/db/TFSEARCH.html). CpG islands were predicted using MethPrimer (http://www.urogene.org/methprimer/).
The 4 kb 5′-regulatory region of FABP3 from eight species (Supplemental Table 2) were obtained from the genome database at the National Center for Biotechnology Information (NCBI). Multi-alignments of 5′-regulatory region among the eight species were performed using ClustalX 2.0 [24].
5′-Rapid amplification of cDNA ends (5′-RACE)
To identify the transcription initiation sites, 5′-RACE from total RNA of longissimus dorsi muscle of adult Qinchuan cattle was performed according to the manufacturer’s protocol using the SMART™ RACE Kit (Clontech Inc, Palo Alto, CA, USA). The PCR primers (FABP3-GSP1 and FABP3-GSP2, Supplemental Table 1) were adopted to acquire the 5′-end of the FABP3 gene. Reaction conditions were performed in the first PCR as described previously [25, 26]. The second PCR template was a 20-fold dilution of the first PCR products.
Real-time PCR analysis of spatial expression pattern
Sixteen tissues (longissimus dorsi muscle, kidney, heart, biceps femoris muscle, liver, subcutaneous fat, spleen, lung, cecum, rumen, reticulum, omasum, abomasum, testis fat, large intestine and small intestine) were obtained from three adult Qinchuan cattle. Total RNA was extracted from the samples using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) and treated with RNase-free DNase Ι (MBI Fermentas, St. Leon-Rot, Germany). RNA quality was assessed with agarose gel electrophoresis and a ND-1000 Spectrophotometer (NanoDrop Technologies, USA). If the 260/280 ratio of the RNA was outside the range 1.8–2.1, the sample was discarded. The RNA was then reverse-transcribed into cDNA using M-MLV reverse transcriptase (Promega Corp., Madison, WI, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was selected as the endogenous reference. The primers of FABP3-RT-F/R and GAPDH-F/R (Supplemental Table 1) [27] were used for this assay. The cycling conditions were a single cycle of 95 °C for 10 s, 50 cycles of 95 °C for 3 s, 60 °C for 25 s, then a single cycle of 95 °C for 15 s, 60 °C for 60 s and 95 °C for 15 s. Other steps were followed with SYBR Premix Ex Taq™II (TaKaRa, Biotechnology Co. Ltd, Dalian, P.R.C.) using the ABI 7500 RT-PCR system (Applied Biosystems, Foster City, Calif, USA). The results were analyzed using the \(2^{{ - \Delta \Delta {\text{C}}_{\text{t}} }}\) method.
Cell culture, transfection and dual-luciferase reporter assay
Mouse myoblasts of the C2C12 and 3T3-L1 cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (FBS) (Gibco-Invitrogen, Carlsbad, CA, USA) under humidified air containing 5 % CO2 at 37 °C. Seven serial deletion subclones were amplified with 5′-nested PCR primers (FABP3-F1 ~ F7, Supplemental Table 1) and a common 3′ primer (FABP3-R, Supplemental Table 1), which were cloned into the SacI-XhoI sites of pGL3-basic vector (Promega Corp., Madison, WI, USA). After sequencing verification, the plasmids were extracted with an Endo-free Plasmid Mini Kit (Omega Biotek, Norcross, GA, USA). The plasmids (0.8 μg) and the lipofectamine 2000 (2 μl) were co-transfected into C2C12 or 3T3-L1 cells grown in 24-well plates. To normalize the transfection efficiency, the cells were co-transfected with 10 ng of Renilla luciferase reporter plasmids (pRL-TK vector, Promega Corp.). The pGL3-basic vector was used as the external control. At 5 h after transfection, the media was replaced by DMEM with 2 % horse serum (HS) (Gibco-Invitrogen, Carlsbad, CA, USA) to induce differentiation of C2C12 myoblasts to myotubes for 40 h. At 45 h after transfection, the cells were lysed and analysed with a dual-luciferase reporter assay according to the manufacturer’s instructions [25, 26].
Statistical analysis
All values are expressed as the mean ± standard deviation (SD). Differences between groups (gene expression levels of real-time PCR between tissues, relative luciferase activities between different constructs) were tested with the Student’s T test (two-tailed). The result was considered significant when the p-value was less than 0.05. The result was considered highly significant when the p-value was less than 0.01.
Results and discussion
Spatial expression pattern of bovine FABP3 gene
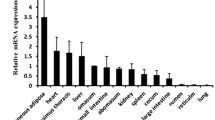
The results of real-time PCR analysis showed the highest expression of bovine FABP3 in heart, followed by biceps femoris muscle, longissimus dorsi muscle, large intestine, small intestine, subcutaneous fat, cecum, rumen, lung, reticulum, omasum, liver and abomasum (Fig. 1). Fluorescence signals were not detected in spleen, kidney or testis fat. The expression level of FABP3 in heart was highly significantly different from others. The results were generally consistent with that of FABP3 in rats [28].
Tissue distribution analysis of bovine Fatty acid binding protein 3 (FABP3) mRNA. The values of FABP3 mRNA were normalized to housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Error bars indicate the SD (n = 3). The significance levels of T tests in comparison to the expression level of heart are indicated with **P < 0.01
Transcription initiation sites of the bovine FABP3 gene
To date, there are no published reports about transcription initiation sites in the bovine FABP3 gene. In the present study, 524 and 402 bp fragments were amplified in the first and second PCR, respectively (Fig. 2). Three different 5′ ends were discovered in the first exon based on sequencing results of 13 positive clones. There were four, three and six positive clones extended to the 62, 60 and 59 bp respectively upstream from the translational start site (Fig. 4). The guanine residue (G) in the 5′-flanking sequence, 62 bp upstream from the translational start site, was designated as +1. After comparison with the Bos Taurus Assembly (BTA) reference AC_000159.1 (range from 122723225 to 122783830) and NC_007300.5 (range from 127602177 to 127609785), the identified transcription initiation site (+1) extended 16 bp to 5′-end (5′- GCTGGTCCCAGAGTCC-3′).
Characterization of the bovine FABP3 gene 5′-regulatory region
A 1998 bp fragment of the bovine FABP3 gene 5′-regulatory region spanning nucleotides from −1822 to +176 was cloned and submitted to GenBank database (GenBank No. KJ649748). Several transcription factor binding sites were recognized by sequence analysis of 5′-flanking sequences (Fig. 3). FABP3 mRNA was expressed most highly in heart, consistent with the hypothesis that promoters of tissue-specific expression genes usually contain a TATA-box [29]. In addition, four CpG islands were predicted in the bovine FABP3 gene promoter using MethPrimer (Fig. 4), which indicated that the transcription activity might be dependent on the CpG islands methylation level. In human, the methylation status of FABP3 is associated with phenotypes of cardiovascular disease [30].
Nucleotide sequences of the 5′-regulatory region of the bovine Fatty acid binding protein 3 (FABP3) gene. Arrows indicate the transcription initiation sites. The guanine residue (G) is designated as +1, other nucleotides are numbered relative to it. The transcription factor binding sites are indicated with boxes. The PCR primers are underlined. Four CpG islands are indicated in red. (Color figure online)
Compared with the 5′-regulatory sequence (GenBank No. KJ649748) of bovine FABP3, the 5′-flanking sequences of cattle, sheep, pigs and dogs shared 99.95, 91.73, 77.11 and 29.58 % sequence similarity. However, the 5′-flanking sequences of human, rats, mice and chickens did not show significant sequence similarity with that of bovine FABP3. The result indicates that the function and transcription mechanism of bovine FABP3 might be similar to that of sheep.
Transcriptional activity of the bovine FABP3 gene promoter
To identify the transcriptional activity of the bovine FABP3 gene 5′-regulatory region, seven serial deletion constructs in pGL3-basic containing −1822/+ 176, −1584/+ 176, −1284/+ 176, −984/+ 176, −684/+ 176, −384/+ 176 and −84/+ 176 were generated. The transcriptional activities of the different plasmids constructs were determined by transfecting them into C2C12 cells. The results of dual-luciferase reporter assay indicated that transcriptional activity of the construct −384/+ 176 was approximately ten-fold greater than pGL3-basic without promoter (Fig. 5a). The activities of the other constructs were lower than that of the construct −384/+ 176 and higher than that of pGL3-basic. When the C2C12 cells were induced by HS after transfection, the transcriptional activity of the construct −384/+ 176 was significantly increased and highest in the seven constructs (Fig. 5a). While the construct plasmids were transfected into 3T3-L1 cells, the transcriptional activity of the construct −384/+ 176 was also highest in the seven constructs (Fig. 5b). These results indicated that a core functional promoter was present in the upstream region of 384 bp from the transcription initiation site (+1), consistent with previous findings that the core promoter was generally near the transcription initiation site [31, 32].
Analysis of bovine Fatty acid binding protein 3 (FABP3) gene promoter activities. 7-serial deletion constructs in pGL3-basic were transfected into C2C12 myoblasts or myotubes (a) and 3T3-L1 cells (b). Error bars indicate the SD (n = 3). The significance levels of T-tests in comparison to the construct −384/+ 176 are indicated with *P < 0.05 or **P < 0.01
To identify positive regulatory elements among the eight species, the region from nucleotides −384 to +64 was analyzed by transcription factor binding sites prediction software. The result of multi-alignments showed that retinoid X receptor alpha (RXRα), Kruppel-like factor 15 (KLF15), specificity protein 1 (Sp1), cAMP responsive element-binding protein (CREB), translation elongation factor EF-1 alpha (TEF-2), TATA-box and CCAAT-box sites were conservative in domestic animals including cattle, sheep, pigs and dogs in this region (Fig. 6). RXRα is a nuclear receptor that can form a heterodimer with PPARs to regulate the transcription of genes involved in fatty acid storage and glucose metabolism [33]. It is also reported that peroxisome proliferator activated receptor alpha (PPARα) is required but not sufficient for transcriptional activation of FABP3 in the muscles of mice [20]. In the present study, three PPARα transcription factor binding sites were found on the 5′-flanking sequences of the bovine FABP3 gene.
KLF15 is a member of the Kruppel-like factors (KLFs) family that regulate diverse arrays of biological processes including cell stemness, proliferation, differentiation, apoptosis and energy metabolism [34]. KLF15, as a critical regulator, cooperates with PPARα to regulate cardiomyocyte lipid gene expression [35] and plays an important role through regulating the expression of PPARγ in transcriptional regulation of adipogenesis [36].
Sp1 is a zinc finger transcription factor that binds to GC-rich motifs of many promoters; it is involved in multiple cellular processes such as cell differentiation, cell growth and apoptosis [37, 38].
CREB is a member of the leucine zipper family of DNA binding proteins, which can form both homo- and heterodimers with related factors to regulate the transcription of adipocyte-specific genes for C/EBPβ, FABP4, and ADIPOQ in 3T3-L1 cells [39–41].
In addition, it is reported that Sirt1 can also regulate the expression of FABP3 gene partly through the PPARγ in pig adipocytes [21]. Taken together, we speculate that RXRα cooperated with PPARα to regulate the expression of FABP3 in muscle, while KLF15 and CREB interact with PPARα or PPARγ to regulate the expression of FABP3 in adipocytes.
Conclusions
In summary, this study provided important information about the expression and regulation of bovine FABP3. The promoter sequence of bovine FABP3 gene was highly similar to the homologs of sheep. It was highly expressed in heart and muscles. Its core promoter was located on the sequence from +384 to +1, where transcription factor binding sites RXRα, KLF15, CREB and Sp1 were conservative among cattle, sheep, pigs and dogs. These results provide further understanding of the function and transcriptional regulation mechanism of the FABP3 gene.
References
Chmurzynska A (2006) The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet 47(1):39–48. doi:10.1007/BF03194597
Kusudo T, Hashida Y, Ando F, Shimokata H, Yamashita H (2015) Asp3Gly polymorphism affects fatty acid-binding protein 3 intracellular stability and subcellular localization. FEBS Lett. doi:10.1016/j.febslet.2015.07.007
Furuhashi M, Hotamisligil GS (2008) Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7(6):489–503. doi:10.1038/nrd2589
Ishimura S, Furuhashi M, Watanabe Y, Hoshina K, Fuseya T, Mita T, Okazaki Y, Koyama M, Tanaka M, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Miura T (2013) Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS One 8(11):e81318. doi:10.1371/journal.pone.0081318
Binas B, Danneberg H, McWhir J, Mullins L, Clark AJ (1999) Requirement for the heart-type fatty acid binding protein in cardiac fatty acid utilization. FASEB J 13(8):805–812
Zhang J, Rickers-Haunerland J, Dawe I, Haunerland NH (1999) Structure and chromosomal location of the rat gene encoding the heart fatty acid-binding protein. Eur J Biochem 266(2):347–351
Vergnes L, Chin R, Young SG, Reue K (2011) Heart-type fatty acid-binding protein is essential for efficient brown adipose tissue fatty acid oxidation and cold tolerance. J Biol Chem 286(1):380–390. doi:10.1074/jbc.M110.184754
Yi B, Wang J, Wang S, Yuan D, Sun J, Li Z, Mao Y, Hou Q, Liu W (2014) Overexpression of Banna mini-pig inbred line fatty acid binding protein 3 promotes adipogenesis in 3T3-L1 preadipocytes. Cell Biol Int 38(8):918–923. doi:10.1002/cbin.10285
Gerbens F, van Erp AJ, Harders FL, Verburg FJ, Meuwissen TH, Veerkamp JH, te Pas MF (1999) Effect of genetic variants of the heart fatty acid-binding protein gene on intramuscular fat and performance traits in pigs. J Anim Sci 77(4):846–852
Gerbens F, Verburg FJ, Van Moerkerk HT, Engel B, Buist W, Veerkamp JH, te Pas MF (2001) Associations of heart and adipocyte fatty acid-binding protein gene expression with intramuscular fat content in pigs. J Anim Sci 79(2):347–354
Li X, Kim SW, Choi JS, Lee YM, Lee CK, Choi BH, Kim TH, Choi YI, Kim JJ, Kim KS (2010) Investigation of porcine FABP3 and LEPR gene polymorphisms and mRNA expression for variation in intramuscular fat content. Mol Biol Rep 37(8):3931–3939. doi:10.1007/s11033-010-0050-1
Serao NV, Veroneze R, Ribeiro AM, Verardo LL, Neto JB, Gasparino E, Campos CF, Lopes PS, Guimaraes SE (2011) Candidate gene expression and intramuscular fat content in pigs. J Anim Breed Genet 128(1):28–34. doi:10.1111/j.1439-0388.2010.00887.x
Cho KH, Kim MJ, Jeon GJ, Chung HY (2011) Association of genetic variants for FABP3 gene with back fat thickness and intramuscular fat content in pig. Mol Biol Rep 38(3):2161–2166. doi:10.1007/s11033-010-0344-3
Tyra M, Ropka-Molik K, Terman A, Piorkowska K, Oczkowicz M, Bereta A (2013) Association between subcutaneous and intramuscular fat content in porcine ham and loin depending on age, breed and FABP3 and LEPR genes transcript abundance. Mol Biol Rep 40(3):2301–2308. doi:10.1007/s11033-012-2311-7
Hong J, Kim D, Cho K, Sa S, Choi S, Kim Y, Park J, Schmidt GS, Davis ME, Chung H (2015) Effects of genetic variants for the swine FABP3, HMGA1, MC4R, IGF2, and FABP4 genes on fatty acid composition. Meat Sci 110:46–51. doi:10.1016/j.meatsci.2015.06.011
Sweeney T, O’Halloran AM, Hamill RM, Davey GC, Gil M, Southwood OI, Ryan MT (2015) Novel variation in the FABP3 promoter and its association with fatness traits in pigs. Meat Sci 100:32–40. doi:10.1016/j.meatsci.2014.09.014
Wang L, Li L, Jiang J, Wang Y, Zhong T, Chen Y, Wang Y, Zhang H (2015) Molecular characterization and different expression patterns of the FABP gene family during goat skeletal muscle development. Mol Biol Rep 42(1):201–207. doi:10.1007/s11033-014-3759-4
Lim D, Chai HH, Lee SH, Cho YM, Choi JW, Kim NK (2015) Gene expression patterns associated with peroxisome proliferator-activated receptor (PPAR) Signaling in the longissimus dorsi of hanwoo (Korean Cattle). Asian-Aust J Anim Sci 28(8):1075–1083. doi:10.5713/ajas.14.0811
Gerbens F, Rettenberger G, Lenstra JA, Veerkamp JH, te Pas MF (1997) Characterization, chromosomal localization, and genetic variation of the porcine heart fatty acid-binding protein gene. Mamm genome 8(5):328–332
Kawabe K, Saegusa H, Seto K, Urabe H, Motojima K (2005) Peroxisome proliferator-activated receptor alpha and its response element are required but not sufficient for transcriptional activation of the mouse heart-type fatty acid binding protein gene. Int J Biochem Cell Biol 37(7):1534–1546. doi:10.1016/j.biocel.2005.02.011
Shan TZ, Ren Y, Wu T, Liu CX, Wang YZ (2009) Regulatory role of Sirt1 on the gene expression of fatty acid-binding protein 3 in cultured porcine adipocytes. J Cell Biochem 107(5):984–991. doi:10.1002/jcb.22203
Holst D, Luquet S, Nogueira V, Kristiansen K, Leverve X, Grimaldi PA (2003) Nutritional regulation and role of peroxisome proliferator-activated receptor delta in fatty acid catabolism in skeletal muscle. Biochim et Biophys Acta 1633(1):43–50
Mullenbach R, Lagoda PJ, Welter C (1989) An efficient salt-chloroform extraction of DNA from blood and tissues. Trends Genet 5(12):391
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948. doi:10.1093/bioinformatics/btm404
Li A, Chen Y, Zhao X, Niu Y, Cong P, Zhang Z, Chen W, Jiang W, Mo D (2010) Characterization and transcriptional regulation analysis of the porcine TNFAIP8L2 gene. Mol Genet Genom 284(3):185–195. doi:10.1007/s00438-010-0558-z
Li A, Zhao Z, Zhang Y, Fu C, Wang M, Zan L (2015) Tissue expression analysis, cloning, and characterization of the 5′-regulatory region of the bovine fatty acid binding protein 4 gene. J Anim Sci 93(11):5144–5152. doi:10.2527/jas.2015-9378
Wang H, Cheng G, Fu C, Wang H, Yang W, Wang H, Zan L (2014) Sequence analysis of bovine C/EBPdelta gene and its adipogenic effects on fibroblasts. Mol Biol Rep 41(1):251–257
Heuckeroth RO, Birkenmeier EH, Levin, Gordon JI (1987) Analysis of the tissue-specific expression, developmental regulation, and linkage relationships of a rodent gene encoding heart fatty acid binding protein. J Biol Chem 262(20):9709–9717
Hochheimer A, Tjian R (2003) Diversified transcription initiation complexes expand promoter selectivity and tissue-specific gene expression. Genes Dev 17(11):1309–1320. doi:10.1101/gad.1099903
Zhang Y, Kent JW 2nd, Lee A, Cerjak D, Ali O, Diasio R, Olivier M, Blangero J, Carless MA, Kissebah AH (2013) Fatty acid binding protein 3 (fabp3) is associated with insulin, lipids and cardiovascular phenotypes of the metabolic syndrome through epigenetic modifications in a Northern European family population. BMC Med Genom 6:9. doi:10.1186/1755-8794-6-9
Frith MC, Valen E, Krogh A, Hayashizaki Y, Carninci P, Sandelin A (2008) A code for transcription initiation in mammalian genomes. Genome Res 18(1):1–12. doi:10.1101/gr.6831208
Megraw M, Pereira F, Jensen ST, Ohler U, Hatzigeorgiou AG (2009) A transcription factor affinity-based code for mammalian transcription initiation. Genome Res 19(4):644–656. doi:10.1101/gr.085449.108
Lemkul JA, Lewis SN, Bassaganya-Riera J, Bevan DR (2015) Phosphorylation of PPARgamma affects the collective motions of the PPARgamma-RXRalpha-DNA complex. PLos One 10(5):e0123984. doi:10.1371/journal.pone.0123984
Prosdocimo DA, Sabeh MK, Jain MK (2015) Kruppel-like factors in muscle health and disease. Trends Cardiovasc Med 25(4):278–287. doi:10.1016/j.tcm.2014.11.006
Prosdocimo DA, John JE, Zhang L, Efraim ES, Zhang R, Liao X, Jain MK (2015) KLF15 and PPARalpha cooperate to regulate cardiomyocyte lipid gene expression and oxidation. PPAR Res 2015:201625. doi:10.1155/2015/201625
Mori T, Sakaue H, Iguchi H, Gomi H, Okada Y, Takashima Y, Nakamura K, Nakamura T, Yamauchi T, Kubota N, Kadowaki T, Matsuki Y, Ogawa W, Hiramatsu R, Kasuga M (2005) Role of Kruppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J Biol Chem 280(13):12867–12875. doi:10.1074/jbc.M410515200
Huong PT, Soung NK, Jang JH, Cha-Molstad HJ, Sakchaisri K, Kim SO, Jang JM, Kim KE, Lee KS, Kwon YT, Erikson RL, Ahn JS, Kim BY (2013) Regulation of CEP131 gene expression by SP1. Gene 513(1):75–81. doi:10.1016/j.gene.2012.10.074
Deniaud E, Baguet J, Mathieu AL, Pages G, Marvel J, Leverrier Y (2006) Overexpression of Sp1 transcription factor induces apoptosis. Oncogene 25(53):7096–7105. doi:10.1038/sj.onc.1209696
Reusch JE, Colton LA, Klemm DJ (2000) CREB activation induces adipogenesis in 3T3-L1 cells. Mol cell biol 20(3):1008–1020
Kim HB, Kim WH, Han KL, Park JH, Lee J, Yeo J, Jung MH (2010) cAMP-response element binding protein (CREB) positively regulates mouse adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun 391(1):634–639. doi:10.1016/j.bbrc.2009.11.111
Ling F, Li J, Chen Y, Du H, Mei Y, Mo D, Wang C (2009) Cloning and characterization of the 5′-flanking region of the pig adiponectin gene. Biochem Biophys Res Commun 381(2):236–240. doi:10.1016/j.bbrc.2009.02.031
Acknowledgments
This research was funded by the National Natural Science Foundation of China (Grant No. 31402042), the Technical Innovation Engineering Project of Shaanxi Province (Grant No. 2016KTCL02-15) and the Northwest A&F University Special Funds of Central Colleges Basic Scientific Research Operating Expenses (Grant Nos. 2014YB009 and 2452015296).
Author information
Authors and Affiliations
Corresponding author
Additional information
Anning Li and Lijuan Wu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, A., Wu, L., Wang, X. et al. Tissue expression analysis, cloning and characterization of the 5′-regulatory region of the bovine FABP3 gene. Mol Biol Rep 43, 991–998 (2016). https://doi.org/10.1007/s11033-016-4026-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-016-4026-7